Table 1.
Molecular docking scores and PASS prediction of all the designed compounds.
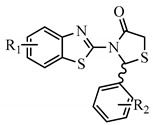
| N | R1 | R2 | Free Binding Energy (kcal/mol) S-(−) |
Free Binding Energy (kcal/mol) R-(+) |
Pa | Ν | R1 | R2 | Free Binding Energy (kcal/mol) -(−) |
Free Binding Energy (kcal/mol) R-(+) |
Pa |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a1 (1) | 7-Cl | 2,6-di-F | −10.21 | −13.37 | 0.651 | i7 | 6-Br | 2,3-di-Cl | −3,25 | −4.07 | 0.352 |
| a2 (2) | 7-Cl | 2-F, 6-Cl | −11.42 | −14.10 | 0.704 | i8 | 6-Br | 2,4-di-Cl | −5,37 | −6.43 | 0.341 |
| b1 (3) | 6-F | 4-F | −10.95 | −13.21 | 0.472 | k1 | 6-CN | 3-Cl | −5.29 | −6.57 | 0.353 |
| b2 (4) | 6-F | 4-NO2 | −8.02 | −9.11 | 0.374 | k2 | 6-CN | 3-Br | −4.18 | −5.66 | 0.393 |
| b3 (5) | 6-F | 4-Cl | −8.15 | −9.02 | 0.398 | k3 | 6-CN | 3-F | −6.32 | −703 | 0.287 |
| b4 (6) | 6-F | 4-OCH3 | −7.14 | −8.70 | 0.360 | k4 | 6-CN | 2,6-di-Cl | −6.58 | −7.84 | 0.498 |
| b5 (7) | 6-F | 4-OH | −8.27 | −9.27 | 0.415 | k5 | 6-CN | 2,3-di-Cl | −6.45 | −7.16 | 0.447 |
| b6 | 6-F | 4-Br | −6.17 | −6.54 | 0.401 | k6 | 6-CN | 2,4-di-Cl | −6.93 | −7.18 | 0.366 |
| b7 | 6-F | 2,3-di-Cl | −6.53 | −6.71 | 0.353 | l1 | 6-CF3 | 2,6-di-Cl | −6.44 | −7.12 | 0.448 |
| b8 | 6-F | 2,4-di-Cl | −6.05 | −6.41 | 0.349 | l2 | 6-CF3 | 3-Cl | −5.41 | −6.27 | 0.332 |
| c1 (8) | 6-Cl | 4-F | −10.13 | −12.30 | 0.426 | l3 | 6-CF3 | 3-Br | −5.07 | −5.71 | 0.254 |
| c2 | 6-Cl | 2-Cl | −6.28 | −6.85 | 0.372 | l4 | 6-CF3 | 4-Br | −5.09 | −5.82 | 0.378 |
| c3 | 6-Cl | 3-Cl | −6.55 | −6.93 | 0.285 | l5 | 6-CF3 | 2,3-di-Cl | −4.05 | −4.70 | 0.336 |
| c4 | 6-Cl | 3-Br | −6.01 | −7.11 | 0.402 | l6 | 6-CF3 | 2,4-di-Cl | −6.41 | −6.91 | 0.332 |
| c5 (9) | 6-Cl | 4-OH | −11.08 | −13.63 | 0.492 | m1 | 6-Ad | 3-Cl | −5.12 | −6.74 | 0.273 |
| c6 | 6-Cl | 4-Br | −6.01 | −7.11 | 0.419 | m2 | 6-Ad | 3-Br | −5.96 | −6.89 | 0.280 |
| c7 | 6-Cl | 2,3-di-Cl | −6.25 | −7.59 | 0.411 | m3 | 6-Ad | 2,6-di-F | −6.25 | −7.04 | 0.428 |
| c8 | 6-Cl | 2,4-di-Cl | −5.92 | −6.42 | 0.419 | m4 | 6-Ad | 2,3-di-Cl | −7.14 | −8.05 | 0.289 |
| d1 (10) | 4-Cl | 4-F | −11.16 | −14.59 | 0.469 | m5 | 6-Ad | 2,4-di-Cl | −7.02 | −7.94 | 0.285 |
| d2 (11) | 4-Cl | 4-NO2 | −8.45 | −9.28 | 0.393 | m6 | 6-Ad | 4-F | −6.82 | −7.88 | 0.325 |
| d3 (12) | 4-Cl | 4-Cl | −8.73 | −10.26 | 0.480 | m7 | 6-Ad | 4-NO2 | −5.17 | −6.75 | 0.226 |
| d4 (13) | 4-Cl | 4-OCH3 | −9.11 | −11.69 | 0.378 | m8 | 6-Ad | 4-Cl | −6.79 | −7.55 | 0.319 |
| d5 (14) | 4-Cl | 4-OH | −10.58 | −13.72 | 0.436 | m9 | 6-Ad | 4-OCH3 | −6.49 | −7.28 | 0.295 |
| d6 | 4-Cl | 4-Br | −6.17 | −7.76 | 0.422 | m10 | 6-Ad | 4-OH | −4.18 | −5.94 | 0.333 |
| d7 | 4-Cl | 2,3-di-Cl | −6.63 | −7.15 | 0.424 | n1 | 4-CH3, 6-Ad | 3-Cl | −4.26 | −5.97 | 0.226 |
| d8 | 4-Cl | 2,4-di-Cl | −7.14 | −7.93 | 0.404 | n2 | 4-CH3, 6-Ad | 3-Br | −4.13 | −5.62 | 0.280 |
| e1 (15) | 4-OCH3 | 4-F | −9.93 | −11.51 | 0.353 | n3 | 4-CH3, 6-Ad | 2-F, 6-Cl | −7.03 | −8.12 | 0.507 |
| e2 (16) | 4-OCH3 | 4-NO2 | −10.05 | −11.58 | 0.374 | n4 | 4-CH3, 6-Ad | 2,3-di-Cl | −6.58 | −7.89 | 0.280 |
| e3 (17) | 4-OCH3 | 4-Cl | −9.92 | −11.23 | 0.347 | n5 | 4-CH3, 6-Ad | 2,4-di-Cl | −6.93 | −7.91 | 0.276 |
| e4 (18) | 4-OCH3 | 4-OCH3 | −8.76 | −10.97 | 0.380 | n6 | 4-CH3, 6-Ad | 4-F | −6.74 | −7.59 | 0.313 |
| e5 (19) | 4-OCH3 | 4-OH | −10.15 | −11.02 | 0.379 | n7 | 4-CH3, 6-Ad | 4-NO2 | −5.08 | −6.65 | 0.202 |
| e6 | 4-OCH3 | 4-Br | −6.58 | −7.46 | 0.329 | n8 | 4-CH3, 6-Ad | 4-Cl | −6.83 | −7.94 | 0.307 |
| e7 | 4-OCH3 | 2,3-di-Cl | −5.14 | −6.03 | 0.317 | n9 | 4-CH3, 6-Ad | 4-OCH3 | −6.37 | −7.17 | 0.287 |
| e8 | 4-OCH3 | 2,4-di-Cl | −5.79 | −6.48 | 0.322 | n10 | 4-CH3, 6-Ad | 4-OH | −4.16 | −5.93 | 0.321 |
| f1 (20) | 6-OCH3 | 4-F | −10.87 | −12.17 | 0.393 | o1 | 5,6-di-CH3 | 4-F | −6.95 | −7.56 | 0.433 |
| f2 (21) | 6-OCH3 | 4-NO2 | −7.14 | −8.96 | 0.346 | o2 | 5,6-di-CH3 | 4-NO2 | −5.18 | −6.05 | 0.328 |
| f3 (22) | 6-OCH3 | 4-Cl | −9.88 | −11.00 | 0.365 | o3 | 5,6-di-CH3 | 4-Cl | −5.21 | −6.17 | 0.432 |
| f4 (23) | 6-OCH3 | 4-OCH3 | −9.91 | −11.49 | 0.420 | o4 | 5,6-di-CH3 | 4-OCH3 | −3.28 | −4.56 | 0.327 |
| f5 | 6-OCH3 | 3-Br | −5.03 | −6.11 | 0.302 | o5 | 5,6-di-CH3 | 4-OH | −4.19 | −5.13 | 0.351 |
| f6 | 6-OCH3 | 4-Br | −5.17 | −6.13 | 0.367 | o6 | 5,6-di-CH3 | 4-Br | −3.05 | −4.27 | 0.355 |
| f7 | 6-OCH3 | 2,3-di-Cl | −5.61 | −6.92 | 0.303 | o7 | 5,6-di-CH3 | 2,3-di-Cl | −6.17 | −7.01 | 0.275 |
| f8 | 6-OCH3 | 2,4-di-Cl | −6.24 | −7.32 | 0.325 | o8 | 5,6-di-CH3 | 2,4-di-Cl | −6.14 | −6.86 | 0.286 |
| g1 (24) | 6-OCH2CH3 | 4-F | −6.97 | −8.35 | 0.373 | p1 | 6-NO2 | 4-F | −6.84 | −6.92 | 0.390 |
| g2 (25) | 6-OCH2CH3 | 4-NO2 | −7.14 | −9.02 | 0.339 | p2 | 6-NO2 | 4-NO2 | −5.11 | −6.01 | 0.225 |
| g3 (26) | 6-OCH2CH3 | 4-Cl | −9.84 | −11.35 | 0.376 | p3 | 6-NO2 | 4-Cl | −5.20 | −6.15 | 0.382 |
| g4 (27) | 6-OCH2CH3 | 4-OCH3 | −9.10 | −10.98 | 0.356 | p4 | 6-NO2 | 4-OCH3 | −3.15 | −4.42 | 0.348 |
| g5 (28) | 6-OCH2CH3 | 4-OH | −10.05 | −11.56 | 0.387 | p5 | 6-NO2 | 4-OH | −3.47 | −4.18 | 0.398 |
| g6 | 6-OCH2CH3 | 4-Br | −4.28 | −5.43 | 0.358 | p6 | 6-NO2 | 4-Br | −3.01 | −4.15 | 0.284 |
| g7 | 6-OCH2CH3 | 2,3-di-Cl | −5.84 | −6.47 | 0.286 | p7 | 6-NO2 | 2,3-di-Cl | −6.18 | −6.81 | 0.341 |
| g8 | 6-OCH2CH3 | 2,4-di-Cl | −5.33 | −6.21 | 0.320 | p8 | 6-NO2 | 2,4-di-Cl | −6.29 | −6.98 | 0.337 |
| h1 (29) | 6-OCF3 | 2,6-di-Cl | −9.87 | −11.03 | 0.388 | q1 | 4-CH3 | 4-F | −7.01 | −7.82 | 0.345 |
| h2 | 6-OCF3 | 2,6-di-F | −7.77 | −8.85 | 0.424 | q2 | 4-CH3 | 4-NO2 | −5.10 | −6.12 | 0.326 |
| h3 | 6-OCF3 | 3-Cl | −5.96 | −6.88 | 0.315 | q3 | 4-CH3 | 4-Cl | −5.03 | −5.85 | 0.335 |
| h4 (30) | 6-OCF3 | 2,3-di-Cl | −7.23 | −9.31 | 0.300 | q4 | 4-CH3 | 4-OCH3 | −4.85 | −5.16 | 0.290 |
| h5 | 6-OCF3 | 3-Br | −5.17 | −6.33 | 0.242 | q5 | 4-CH3 | 4-OH | −4.08 | −5.49 | 0.353 |
| h6 | 6-OCF3 | 4-F | −6.94 | −8.74 | 0.345 | q6 | 4-CH3 | 4-Br | −3.17 | −4.55 | 0.338 |
| h7 (31) | 6-OCF3 | 4-NO2 | −8.45 | −9.07 | 0.315 | q7 | 4-CH3 | 2,3-di-Cl | −6.14 | −6.80 | 0.390 |
| h8 (32) | 6-OCF3 | 4-Cl | −6.89 | −8.71 | 0.332 | q8 | 4-CH3 | 2,4-di-Cl | −6.33 | −6.86 | 0.382 |
| h9 | 6-OCF3 | 3-F | −5.22 | −6.19 | 0.330 | r1 | 6-CH3 | 4-F | −7.00 | −7.81 | 0.337 |
| h10 | 6-OCF3 | 4-OH | −7.97 | −8.51 | 0.442 | r2 | 6-CH3 | 4-NO2 | −5.02 | −5.93 | 0.315 |
| i1 | 6-Br | 4-F | −7.31 | −8.10 | 0.301 | r3 | 6-CH3 | 4-Cl | −5.01 | −5.83 | 0.327 |
| i2 | 6-Br | 4-NO2 | −6.88 | −7.95 | 0.326 | r4 | 6-CH3 | 4-OCH3 | −4.77 | −5.12 | 0.284 |
| i3 | 6-Br | 4-Cl | −7.19 | −8.02 | 0.393 | r5 | 6-CH3 | 4-OH | −4.05 | −5.40 | 0.345 |
| i4 | 6-Br | 4-OCH3 | −5.35 | −6.42 | 0.285 | r6 | 6-CH3 | 4-Br | −3.12 | −4.52 | 0.330 |
| i5 | 6-Br | 4-OH | −7.22 | −8.02 | 0.409 | r7 | 6-CH3 | 2,3-di-Cl | −6.13 | −6.78 | 0.377 |
| i6 | 6-Br | 4-Br | −5.11 | −5.84 | 0.405 | r8 | 6-CH3 | 2,4-di-Cl | −6.10 | −6.64 | 0.372 |
| Etravirine | −11.25 | Nevirapine | −11.95 | ||||||||
