Abstract
Better understanding of cerebral blood flow (CBF) perfusion in stroke recovery can help inform decisions about optimal timing and targets of restorative treatments. In this study, we examined the relationship between cerebral perfusion and recovery from stroke‐induced reading deficits. Left stroke patients were tested with a noninvasive CBF measure (arterial spin labeling) <5 weeks post‐stroke, and a subset had follow up testing >3 months post‐stroke. We measured blood flow perfusion within the left and right sides of the brain, in areas surrounding the lesion, and areas belonging to the reading network. Two hypotheses were tested. The first was that recovery of reading function depends on increased perfusion around the stroke lesion. This hypothesis was not supported by our findings. The second hypothesis was that increased perfusion of intact areas within the reading circuit is tightly coupled with recovery. Our findings are consistent with this hypothesis. Specifically, higher perfusion in the left reading network measured during the subacute stroke period predicted better reading ability and phonology competence in the chronic period. In contrast, higher perfusion of the right homologous regions was associated with decreased reading accuracy and phonology competence in the subacute and chronic periods. These findings suggest that recovery of reading and language competence may rely on improved blood flow in the reading network of the language‐dominant hemisphere.
Keywords: arterial spin labeling, ASL, MRI, phonology, reading, reading network, recovery, semantics, stroke
1. INTRODUCTION
Acquired reading deficits represent a significant handicap for millions of stroke survivors. People living with this disability encounter challenges effectively returning to work and enjoying leisure activities. Only a handful of studies have looked at how recovery from acquired reading deficits unfolds in the weeks to months after a stroke and how this recovery is related to changes in cerebral blood flow (Fridriksson et al., 2002; Marsh & Hillis, 2005). This study focuses on a group of left‐brain stroke survivors, in whom we tested for an association of resting cerebral blood flow with concurrent reading ability, and with recovery of reading ability several months after stroke.
The importance of cerebral blood flow (CBF) in stroke recovery is rooted in aspects of stroke biology. Stroke occurs when CBF to an area of the brain is cut off, resulting in hypoperfusion, or decreased delivery of oxygen and nutrients to brain tissues by the blood supply. The effect of hypoperfusion on brain function affects the stroke core, peri‐infarct tissue, and the associated functional network. In the stroke core, which includes areas of extremely limited perfusion (<10% of baseline blood flow; <6 ml/100 g per min), neurons are likely to die (Baron & Marchal, 1999). Loss of neural tissue in a given component of the distributed network for reading will result in a deficit affecting the function or functions of the lost component. Surrounding the stroke core are peri‐infarct areas where perfusion is reduced, but neurons can remain alive (~20% of baseline blood flow, 7–22 ml/100 g per min; Baron & Marchal, 1999; Hillis, 2007a). Cells within the peri‐infarct area will die in roughly 2 days if blood flow is not restored (Carmichael, 2016), but sub‐baseline hypoperfusion in the peri‐infarct area can linger for weeks after stroke (Brumm et al., 2010; Hillis, 2007a). When the hypoperfused areas include the reading network, this contributes to reading deficits (Cloutman, Newhart, Davis, Heidler‐Gary, & Hillis, 2011). Beyond the peri‐infarct areas, the relayed effects of stroke can affect the entire functional network for reading. This may be an example of diaschisis (Crinion, Holland, Copland, Thompson, & Hillis, 2013), defined as electrical, metabolic, or blood flow dysfunction in functionally connected areas remote from the lesion, or can occur through other signals leading to a widespread disconnection of brain networks (Carmichael, 2016). Hypoperfusion of anatomically intact areas can be seen up to a year after stroke (Brumm et al., 2010). Among the three zones—infarct core, peri‐infarct tissue, and the extended functional network—as the cells in the stroke core die within hours of the infarct‐tissue repair and recovery depend largely on what happens in the peri‐infarct areas and in the larger functional neural network (Carmichael, 2016; Ohab & Carmichael, 2008; Ohab, Fleming, Blesch, & Carmichael, 2006).
Return of blood circulation and coherent neural activity is an integral part of stroke recovery. In the first few days after an infarct, restoration of blood supply to the peri‐infarct areas via collateral flow can alleviate behavioral deficits. For example, Hillis and Heidler (2002) and Hillis et al. (2005) reported that re‐perfusion of Wernicke's area and BA 37 seen on perfusion magnetic resonance imaging (MRI) was associated with an improvement of naming and word comprehension. In contrast, either prolonged hypoperfusion or re‐perfusion injury, that is, injury that occurs due to the return of blood circulation to a brain area, may cause cell death (Ten & Starkov, 2012), exacerbating behavioral deficits. The process of recovery begins within days to weeks after the infarct, when signals from the peri‐infarct areas trigger angiogenesis, neurogenesis, cell migration, and axonal sprouting (Carmichael, 2016; Krupinski, Kaluza, Kumar, Kumar, & Wang, 1994; Lichtenwalner & Parent, 2006; Macas, Nern, Plate, & Momma, 2006; Ohab & Carmichael, 2008). During this period, processes arising in the vasculature play a causal role in promoting neural repair (Chopp, Zhang, & Jiang, 2007; Ohab et al., 2006; Ohab & Carmichael, 2008). Although stroke‐induced tissue repair may be more limited in humans, compared to rodents and primates, there are patient studies showing both an upregulation of angiogenesis in the peri‐infarct cortex and increased neurogenesis in the ipsilesional subventricular zone (SVZ), with some evidence of neuron migration to the site of injury (Krupinski et al., 1994; Macas et al., 2006). Whether such migration is possible to areas remote from the SVZ and what proportion of the nascent neurons survive and integrate into existing circuits is not yet known. Nonetheless, findings from human and animal studies suggest that collateral flow can be augmented by stroke‐induced angiogenesis, which brings more blood flow to an ischemic area and triggers neural migration. Thus, cerebral blood flow is one of the earliest markers of stroke‐related injury and subsequent recovery.
At the level of the functional neural network, the processes associated with ischemic injury and neural repair may result in reorganization of structure–function relationships (Fridriksson, 2011; Fridriksson, Richardson, Fillmore, & Cai, 2012). Damage to a primary node in the reading network will alter connectivity dynamics by reducing inhibitory and excitatory projections from the affected area to other components of the network. This will likely result in behavioral deficits, but may eventually lead to altered patterns of network activation with strengthening of connections, which yield improved behavioral performance, a phenomenon known as neural plasticity (for a relevant review see Crosson et al., 2017). In aphasia recovery, evidence of transient re‐organization of the language network and recruitment of the right hemisphere was observed in a previous study of 14 aphasia patients assessed longitudinally. FMRI‐indexed brain activity showed early decreases in the frontal language areas. This was followed by increases in activation of the right‐hemisphere language homologs and subsequent normalization of the left‐lateralized brain activity as stroke survivors recovered their language ability (Saur et al., 2006; Stockert, Kummerer, & Saur, 2016). Although the study showed an improvement of aphasia symptoms over time, it remains unclear if transient reliance on right brain areas reflects a necessary step in recovery or represents a maladaptive change detrimental to ultimate functional attainment (Price & Crinion, 2005; Saur et al., 2006; Thompson & den Ouden, 2008).
While much has been done to understand the brain bases of aphasia, little is known about reading recovery after stroke, despite the fact that reading impairments affect up to 60% of aphasia patients (Brookshire, Wilson, Nadeau, Gonzalez Rothi, & Kendall, 2014). For example, it is not yet known if recovery of reading ability is supported primarily by perfusion and neural activation in the peri‐infarct areas, or by functional reorganization/neural plasticity within the left reading circuit (or both). The peri‐infarct region may support functional recovery because its proximity to the lesioned area suggests it may have similar function. Similarly, activation of secondary centers of the ipsilateral functional network may lead to incomplete but satisfactory behavioral improvement (Heiss, 2009). The current study tested these two potential recovery mechanisms (functional compensation due to either (a) peri‐infarct perfusion or (b) reorganization of neural function suggested by increased perfusion within the ipsilesional neural network) in their ability to predict improved reading accuracy. We also considered the role of the right hemisphere analogues of the left reading network in recovery. We hypothesized that increased blood flow within (a) the peri‐infarct areas and (b) the left reading network will signal increased neural activation and will be closely coupled with an improvement of reading‐related performance accuracy.
To examine the effects of cerebral hemodynamics on reading recovery, we tested stroke patients at two time points during post‐stroke recovery: the subacute period (<5 weeks after stroke) and the chronic period (>3 months after stroke), assessing reading ability and brain perfusion. Perfusion was measured using arterial spin labeling (ASL) MRI, which provides a noninvasive absolute (ml (blood volume)/100 g (tissue volume)/min (time)) measure of CBF. Thus, ASL allows a direct comparison of CBF across individuals. Moreover, ASL is suitable even for patients who may have impaired kidney function and cannot undergo contrast‐based perfusion MRI (Zaharchuk, 2014). It can provide a more complete understanding of the negative effects of stroke beyond the core lesioned areas. In ASL, water molecules are labeled with a magnetic pulse before they enter the brain through the arterial blood supply. The effect of labeling can be quantified by characterizing the interaction of blood flow and label decay (Detre et al., 1998; Detre, Rao, Wang, Chen, & Wang, 2012). CBF measurements obtained with ASL have been shown to be lower in patients with significant large artery stenosis and chronic stroke (Brumm et al., 2010; Roc et al., 2006). In the same patients, baseline CBF correlated with functional brain activation, specifically, with task‐induced time‐to‐peak (TTP) of initial dip in the Blood Oxygen Level Dependent (BOLD) response, and marginally correlated with positive BOLD response magnitude (Roc et al., 2006). In a previous study of six patients with chronic stroke and agrammatic aphasia, ASL perfusion correlated with BOLD TTP during an auditory picture‐sentence verification task, and therapy‐induced increases in BOLD signal coincided with increased ASL perfusion (Thompson, den Ouden, Bonakdarpour, Garibaldi, & Parrish, 2010). These studies indicate that ASL can capture changes in baseline cerebral circulation that coincide with changes in BOLD‐indexed neural activity and functional recovery. Moreover, given that cerebrovascular patients often have altered blood flow (Brumm et al., 2010; Detre et al., 1998), it is imperative that we consider a CBF measure when studying brain recovery after stroke. This is important, because BOLD signal magnitude in these patients may be susceptible to under‐ or over‐estimation (Roc et al., 2006). It is also difficult to interpret longitudinal changes in BOLD magnitude in the presence of a changing CBF baseline. ASL offers an opportunity to better understand the role of cerebral perfusion in stroke‐induced deficits and recovery.
We defined reading ability in terms of cognitive components specified by a well‐supported computational cognitive model (Plaut, McClelland, Seidenberg, & Patterson, 1996; Seidenberg & McClelland, 1989). The single‐process triangle model defines reading as an interactive co‐activation of orthography (visual word form), phonology (auditory word form), and semantics (word meaning). This model has been applied to fMRI (Binder, Medler, Desai, Conant, & Liebenthal, 2005; Fiez, Balota, Raichle, & Petersen, 1999; Frost et al., 2005; Graves, Desai, Humphries, Seidenberg, & Binder, 2010), and has been validated in patients with acquired reading disorders (Woollams, Ralph, Plaut, & Patterson, 2007). According to the model, the links between orthographic representations and phonology are formed and updated with reading experience. The strength of these links depends on the frequency of exposure to each orthography–phonology correspondence and is modulated by semantic processing. Consistent with previous studies, we expected that stroke patients would predominantly show phonological deficits in reading (Boukrina, Barrett, Alexander, Yao, & Graves, 2015; Cloutman, Newhart, Davis, Kannan, & Hillis, 2010; Rapcsak et al., 2009), and may rely on semantics to support impaired phonological processing, as was shown in a recent study of residual reading aloud abilities in patients with chronic left hemisphere stroke (Pillay et al., 2018). To assess the integrity of the cognitive components necessary for successful reading and to test these hypotheses, we measured semantic, phonological, and orthographic processing in choice tasks that did not require a verbal response. We also assessed reading skill using a reading aloud task.
2. METHODS
2.1. Participants
Thirty‐one stroke survivors in the subacute epoch (>1 and <5 weeks post stroke) participated in the first phase of the study. They included 16 women and 15 men, aged 34–82 (M = 64; SD = 10). Fifteen returned for follow‐up testing (M = 6.67 months post‐stroke, SD = 2.99). Patients were recruited from the Kessler Institute for Rehabilitation if they met inclusion criteria: left‐hemisphere supratentorial stroke (ischemic or hemorrhagic), 18–100 years old, right‐handed, fluent and literate in English prior to stroke, no prior neurological disorders or clinical stroke event, <5 weeks post‐stroke; ability to undergo an MRI and to complete the study tasks. The study was approved by the Kessler Foundation Institutional Review Board. All participants completed a written informed consent prior to study participation.
2.2. Materials and procedure
All patients were screened with a bedside neuropsychological scoring procedure using the Florida Mental Status Exam (FMSE; Doty et al., 1990). This procedure includes the Hopkins Verbal Learning Test (HVLT; Brandt, 1991) and Boston Naming Test‐Short Form (BNT; Kaplan, Goodglass, & Weintraub, 1983). Participants were tested using the Geriatric Depression Scale (GDS; Yesavage et al., 1982) and an in‐house test of motor and cognitive function. Refer to Table 1 for detailed characteristics of the participant sample with respect to these measures.
Table 1.
Patient sample characteristics at the time of initial assessment
| Measure (maximum score) | N = 31 | ||
|---|---|---|---|
| Mean | SD | Range | |
| Age | 63.68 | 10.28 | 34–82 |
| Years of education (∼years) | 15.16 | 2.56 | 10–20 |
| Days post‐stroke | 15.19 | 4.83 | 8–29 |
| Lesion size (∼cm3) | 25.15 | 22.82 | 0.83–83.91 |
| Boston Naming Test (15) | 12.55 | 3.12 | 1–15 |
| Geriatric Depression Scale (30) | 6.42 | 5.43 | 0–18 |
| Hopkins Verbal Learning Test Immediate Recall (36) | 16.40 | 8.25 | 0–30 |
| Hopkins Verbal Learning Test Delayed Recall (12) | 3.47 | 3.64 | 0–12 |
| Hopkins Verbal Learning Test Recognition Index: True Positives – False Positives (12) | 7.03 | 3.70 | 0–12 |
| Motor Function Proximal Right Upper Extremity Strength (5) | 2.81 | 1.92 | 0–5 |
| Motor Function Distal Right Upper Extremity Strength (5) | 2.66 | 1.88 | 0–5 |
| Motor Function Proximal Right Lower Extremity Strength (5) | 3.21 | 1.74 | 0–5 |
| Motor Function Distal Right Lower Extremity Strength (5) | 2.84 | 1.92 | 0–5 |
| Word Reading accuracy (100%) | 78.00 | 29.84 | 0–100 |
| Nonword Reading accuracy (100%) | 59.06 | 30.48 | 0–100 |
| Orthographic task accuracy (100%) | 78.99 | 15.90 | 40–100 |
| Phonological task accuracy (100%) | 72.45 | 18.10 | 45–100 |
| Semantic word task accuracy (100%) | 79.66 | 21.41 | 0–100 |
| Semantic picture task accuracy (100%) | 85.54 | 11.75 | 53–100 |
To determine the nature of the acquired reading deficits, patients completed touch‐screen computer tests of semantics, phonology (Binder et al., 2016; Pillay, Stengel, Humphries, Book, & Binder, 2014), and orthography (Figure 1a). To test semantic processing, patients were asked to respond using their finger by touching one of the options at the bottom of the screen (e.g., sweater vs. watch) that best matched the example at the top of the screen (e.g., coat). The stimuli were either words or images of concrete objects. Similarly, in the phonology task, participants selected one of two nonword strings that rhymed with a target at the top (e.g., boak). To discourage use of orthographic strategies for this phonological task, one of the strings was a close rhyme of the target with nonmatching letters (e.g., groke) and the other was a nonrhyming string (spreak). In the orthography task, the stimuli were nonword strings that appeared in pairs on the computer screen (task and stimuli adopted from Cassar and Treiman (1997). The strings contained consonant or vowel doubles in a position that is either possible or impossible in English. For example, double “b” never occurs in the beginning of English words. Participants were asked to touch the letter string that looked more like it could be an English word. Accurate completion of this test requires sensitivity to statistical properties of English orthography. There were 60 trials of each task, and accuracy on each trial was recorded by a computer.
Figure 1.
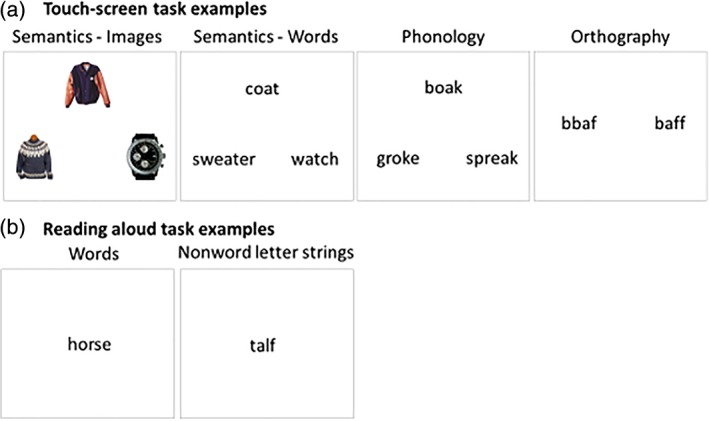
Behavioral tasks. (a) Touch‐screen tests of semantics, phonology, and orthography processing. (b) Self‐paced reading aloud task [Color figure can be viewed at http://wileyonlinelibrary.com]
Patients also completed a reading aloud task. They read 120 words and 60 nonword letter strings. The nonword strings were matched to the words in length and position‐constrained bigram (2‐letter combination) and biphone (2‐sound combination) frequency (Figure 1b). The words varied systematically in orthography‐phonology consistency, word frequency, and imageability (Jared, 2002; Monsell, 1991; Strain et al., 1995; Taraban & McClelland, 1987) and each condition created from a factorial combination of these variables was matched on length, orthographic and phonological neighborhood size, and log‐transformed bigram and biphone frequencies. Although word frequency, imageability and consistency were included as predictors in the mixed linear effects models, a detailed analysis of the effects of these variables is not presented here as it is beyond the scope of the current report. Patients were asked to read aloud each string of letters on the screen to the best of their ability. The stimulus presentation was self‐paced. Responses were recorded using a portable recording device. Accuracy was scored manually by a single rater, and scores were verified by an independent rater. The interrater agreement was 90% and all disagreements were resolved through discussion prior to analysis.
2.3. MRI acquisition
MRI scans were collected on a 3.0 T Skyra Magnetom scanner (Siemens) at the Kessler Foundation Rocco Ortenzio Neuroimaging Center located in the same building as the rehabilitation facility and patient rooms. To help segment different tissue types (permanent lesion vs. salvageable penumbra vs. healthy brain) and to assess recovery, patients underwent ASL, T2 Fluid Attenuated Inversion Recovery (FLAIR), and high‐resolution T1 structural MRI scans during subacute and chronic post‐stroke periods (See Table 2 for Acquisition Parameters, Figure 2a,b).
Table 2.
MRI sequence parameters used in the study
| Parameter | MRI sequence | ||
|---|---|---|---|
| T1‐weighted MPRAGE (magnetization‐prepared rapid gradient‐Echo) | T2‐weighted FLAIR (fluid attenuated inversion recovery) | Pulsed ASL (arterial spin Labeling) perfusion | |
| Voxel dimensions | 1 mm3 isotropic | 1 × 1 × 3 mm | 3.4 × 3.4 × 4 mm |
| Field of view | 256 mm | 256 mm | 220 mm |
| Number of slices | 176 | 50 | 24 |
| Distance factor | 50% | 0% | 25% |
| TR (repetition time) | 2,100 ms | 9,000 ms | 3,000 ms |
| TE (Echo time) | 3.43 ms | 91 ms | 30 ms |
| Flip angle | 9° | 150° | 90° |
| N of measurements | 1 | 1 | 105 |
| Bolus duration | NA | NA | 1,500 ms |
| Perfusion mode | NA | NA | PICORE Q2T |
Figure 2.
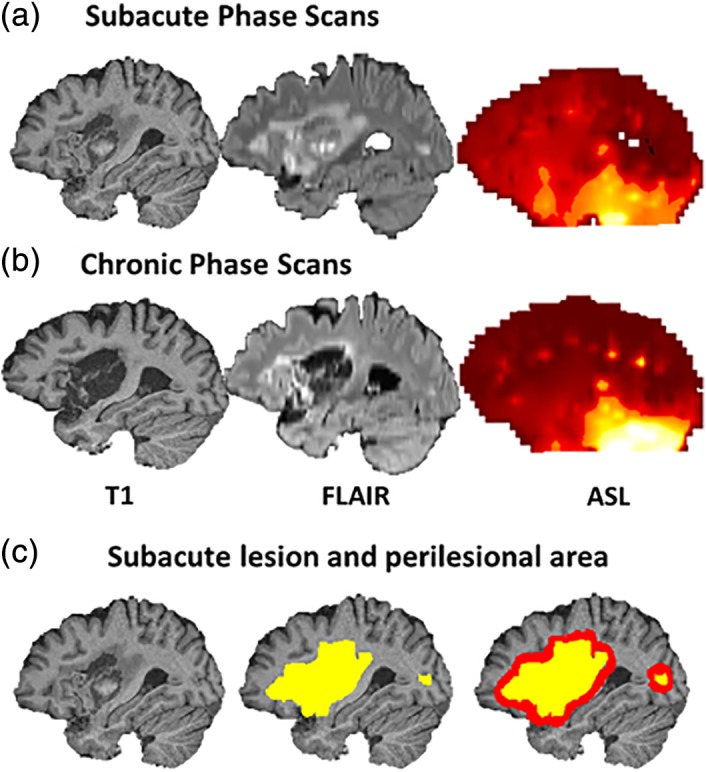
Sample scans from a patient during subacute (a) and chronic (b) post‐stroke period; (c) sample lesion and perilesional area
2.4. MRI analysis
2.4.1. Structural data
Structural scans were skull stripped using the FSL Brain Extraction Tool, BET (Smith, 2002), with robust brain center estimation. The resulting images were registered to the MNI152 1 mm voxel brain template. We used cost‐function masking of the lesion volume to avoid warping undamaged tissue into the lesioned area. To enable group‐level analysis and comparisons between multiple scans of a single participant, the transformation matrices obtained during registration were applied to lesion masks and ASL data.
2.4.2. Lesion mapping
Lesion mapping was done manually in FSLView software available as part of FSL library of analysis tools (Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). Approximate lesion location was identified from the review of hospital records and clinical scans. Research‐grade high resolution T1‐weighted structural and FLAIR images (Table 2) were overlaid onto each other to assist in identification of voxels with abnormal intensity. Areas surrounding stroke lesions that appeared hyperintense on the FLAIR scans were included as part of the lesion map. Lesion maps were created by author OB, and verified by WG. Cases of unclear lesion boundaries were discussed with a licensed neurologist (AMB). Any bilateral, subclinical lacunar lesions that appeared on both the T1‐weighted and FLAIR scans, and contained more than 15 voxels, were mapped as part of the lesion‐weighting mask. No participant had a clinically defined stroke in the right hemisphere (Figure 4a). Lesions were re‐traced for brain scans collected in the chronic period using the same procedure.
Figure 4.
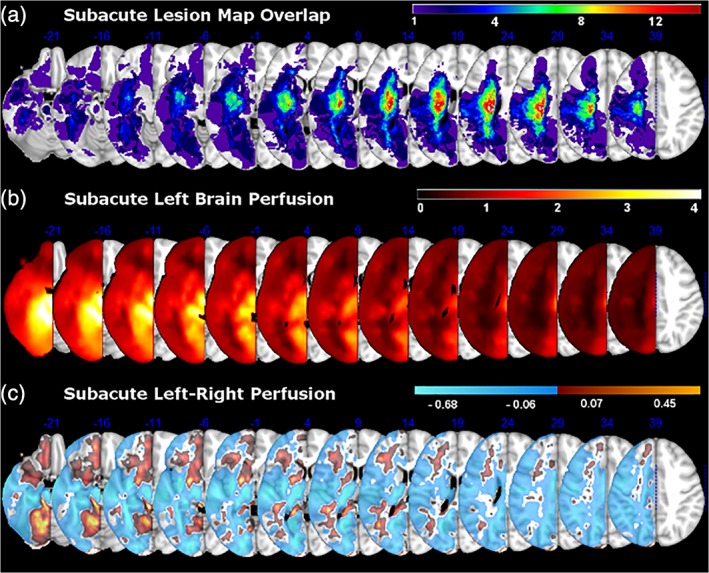
(a) The distribution of brain coverage by lesions within our patient sample. (b) Group‐averaged left‐brain perfusion. (c) Differences in left‐brain perfusion relative to right‐brain perfusion on initial assessment. For each patient, continuous perfusion values were used in a subtraction of left–right perfusion and vice versa. Individual maps were then averaged into a group subtraction map
2.4.3. ASL data
ASL data were brain extracted using BET and registered to the structural image using FLIRT with 6° of freedom, normalized mutual information cost function, and sinc interpolation (Jenkinson, Bannister, Brady, & Smith, 2002). Tag‐control ASL images were motion corrected using MCFLIRT (Jenkinson et al., 2002). For one participant, 9 out of 52 tag‐control pairs of images were removed due to excessive motion. To quantify perfusion in absolute units (ml/100 g/min), we applied kinetic model inversion within the oxford_asl software (Chappell, Groves, Whitcher, & Woolrich, 2009). A single delay pASL model was used for analysis. Calibration was performed using a single M0 pretag image value with CSF reference. We applied spatial smoothing to improve ASL signal and partial volume correction to account for perfusion in voxels partly occupied by both white and gray matter (Chappell et al., 2011). Partial volume estimates were created using white and gray matter masks derived from each patient's segmented structural brain image. The final ASL images for each participant were normalized to individual mean white matter perfusion. For group analyses, transformation matrices for each patient's structural brain to the MNI152_1mm template were applied to the normalized ASL images to bring them into a common atlas space. Binary left and right hemisphere masks derived from the MNI152_1mm template and transformed into structural brain space were applied to the data for the analysis of global lateralized perfusion differences.
A word reading meta‐analysis mask generated from the Neurosynth database mapping tool (Yarkoni, Poldrack, & Nichols, 2011) was applied to the data for regional analyses. We used topic‐based meta‐analysis results from Neurosynth with 100 topics extracted from 11,400 papers. We selected the most relevant one, topic 15, where the top loading terms were “reading,” “words,” “language,” “word,” and “phonological.” This topic summarized the results of 684 studies (Figure 3). We used the association test map (formerly, the reverse inference map), that is, the map that showed a statistically significant (FDR corrected at 0.01) association with the topic in question. In the left hemisphere of each participant, we additionally masked out their individual lesioned areas. The left and right reading network masks were obtained from the same image by masking either the right or the left hemisphere. The left reading network mask included areas in the left posterior inferior temporal, inferior parietal, posterior, mid, and anterior temporal; and inferior and superior frontal cortex; while the right reading network mask included several homologous areas in the right hemisphere (See Table S1 for cluster peaks, extent, and locations). The left reading network mask included areas thought to support semantic processing, for example, angular gyrus, anterior fusiform/inferior temporal, anterior superior temporal, and triangular inferior frontal cortex (Abel et al., 2009; Binder, Desai, Graves, & Conant, 2009; Lambon‐Ralph, Jefferies, Patterson, & Rogers, 2016; Price, 2012; Vigneau et al., 2006), and areas implicated in phonological processing, such as posterior superior temporal, supramarginal, and opercular inferior frontal cortex (Cattinelli, Borghese, Gallucci, & Paulesu, 2013; Graves et al., 2010; Taylor, Rastle, & Davis, 2012). Perilesional perfusion masks were created by first dilating each participant's lesion mask by 5 mm in all three dimensions (bounded by the outer brain edges and ventricular borders) and then calculating the difference between the original lesion mask and the dilated mask, leaving a perilesional ring (Figure 2c). Perfusion was previously shown to be significantly reduced in the radius of 15 mm from the lesion (Fridriksson et al., 2012). Perilesional area within a 5 mm radius of the lesion was chosen in order to better represent the entire perilesional ring for some cortical lesions, where lesion edge was close to the brain edges. The perilesional homolog masks were created by flipping the left perilesional masks in the x (left–right) dimension. Final perfusion values were extracted from each of the volumetric masks using the fslstats function and the AnalyzeFMRI package in the R statistical computing environment (Bordier, Dojat, & de Micheaux, 2011). Linear mixed‐effects statistical models were tested using the LME4 package in R (Bates, Mächler, Bolker, & Walker, 2015) and IBM SPSS Statistics version 21 (IBM Corporation, 2012). Hemispheric perfusion differences were queried with FSL Randomize analysis with 500 permutations (Winkler, Ridgway, Webster, Smith, & Nichols, 2014), a permutation based inference method for the general linear model conducted over brain images. This analysis results in a thresholded image with family‐wise error correction of p < .05.
Figure 3.
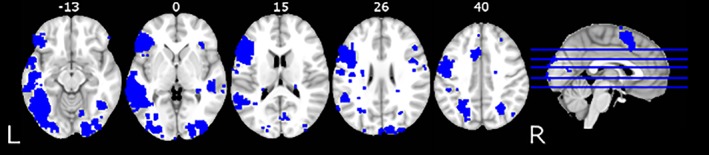
The reading network mask generated from the Neurosynth database (Yarkoni et al., 2011)
2.5. Statistical analysis
We examined perfusion in three different sets of regions: (a) entire left vs. right cerebral hemisphere (b) areas remote from the lesion but belonging to the left vs. right reading network, and (c) perilesional areas and their homologous right‐hemisphere regions. The comparisons were performed for the subacute period and the change from subacute to the chronic period. We also examined whether perfusion measures were associated with behavioral performance on the reading‐related tasks. The role of lesion extent and lesion load within the left reading network was considered in secondary analyses. False Discovery Rate (FDR) correction was performed for the group of paired‐samples tests performed on the perfusion measures with the corrected significance level of q = .04; q statistic is provided along with the p‐value. We did not apply FDR‐correction to the joint perfusion‐behavior tests because these tests were performed as part of planned comparisons to test our main hypotheses.
3. RESULTS
3.1. Subacute perfusion
We assessed perfusion differences between the left and right sides of the brain in three sets of comparisons: (a) the entire left hemisphere compared to the right hemisphere; (b) the left compared to right reading network; and (c) perilesional areas compared to their right brain homologs. This was done to quantify the degree to which left‐hemisphere strokes in our sample reduced cerebral blood flow to the left side of the brain.
3.1.1. Mean perfusion of the left compared to the right hemisphere
Regions of maximal lesion overlap (Figure 4a) coincided with areas of low perfusion in the left‐hemisphere (Figure 4b). Among all 31 patients, the overall mean normalized perfusion values were numerically greater for the right compared to the left side of the brain (M left = 0.93, SD = 0.14; M right = 1.02, SD = 0.16); however this difference did not reach statistical significance (p = .06; q = .044, ns). To better characterize this pattern, we plotted regional differences between mean normalized left and right brain perfusion (Figure 4c) and conducted a FSL Randomize analysis to identify clusters of significant regional differences in left and right‐brain perfusion (Table 3). Relative to the right brain, significant areas of low perfusion (shown in cool colors on Figure 4c) were noted throughout the brain, including superior parietal and lateral occipital cortex, cingulate gyrus, frontal and intracalcarine cortex, as well as thalamus and parts of the brainstem. None of the clusters of increased perfusion (shown in warm colors on Figure 4c) survived correction. The regional perfusion differences are likely due to various lesion locations among participants, which affected perfusion in and around the lesioned areas and the associated functional brain networks.
Table 3.
Significant clusters of perfusion differences between left and right brain areas among 31 patients on initial assessment
| Cluster | Size | X (max) | Y (max) | Z (max) | p |
|---|---|---|---|---|---|
| Right‐brain perfusion > left‐brain perfusion | |||||
| Precuneus, superior parietal lobule lateral occipital cortex, anterior and posterior cingulate gyrus, precentral and postcentral gyri, supplementary motor area, paracingulate cortex, superior and middle frontal gyri, intracalcarine cortex, thalamus, and brainstem | 174,874 | 103 | 71 | 80 | 0.002 |
| Inferior, middle, superior temporal, and occipitotemporal cortex | 2,098 | 142 | 130 | 30 | 0.02 |
Clusters are based on FSL randomize results with 500 permutations and threshold‐free cluster enhancement (FWE correction: p < .05).
3.1.2. Mean perfusion of the left compared to the right reading network
Based on previously published studies (Cloutman et al., 2011), we hypothesized that in addition to affecting the stroke core, left‐hemisphere infarctions, which may involve components of the functional network for reading, will affect perfusion of the entire functional network for reading when compared to the right‐hemisphere in the same individual. To conduct this analysis we identified the reading network areas using a topic‐based meta‐analysis from the Neurosynth database (Yarkoni et al., 2011). The reading network areas were used to create a binary mask following the procedure described in the Methods section (see also Figure 3). Normalized perfusion was lower in the left brain areas (M = 0.76, SD = 0.23) of the reading network, compared to the right brain areas (M = 1.10, SD = 0.34, t(30) = 5.04, p < .001, q = .003). Because the number of voxels within the meta‐analysis mask is smaller for the reading areas on the right side of the brain, we subsampled the left brain perfusion map to include the same number of voxels. Specifically, given all of the left mask voxels, we randomly sampled N voxels, where N was equal to the total number of right mask voxels. This produced similar results, again with greater mean perfusion within the right‐hemisphere reading areas compared to the left‐hemisphere reading areas (t[30] = 5.05, p < .001, q = .006). Furthermore, in order to compare homologous areas in both hemispheres, we created a conjunction mask of overlapping homologous regions between the left and right reading networks and applied it (as original and mirror‐reversed image) to the perfusion maps for the right and the left hemisphere. This produced congruent results, with greater mean perfusion within the right‐hemisphere reading areas compared to the left‐hemisphere reading areas (t(30) = 2.75, p < .05, q = .04).
3.1.3. Mean perilesional perfusion compared to homologous contralateral regions
It was expected that in the early stages of stroke recovery lower perfusion will be observed in the areas immediately surrounding the lesion. To determine the degree of perfusion in the perilesional area relative to homologous healthy tissue, we used a binary mask of the perilesional ring and its homolog in the right hemisphere. Perilesional perfusion (M = 0.72, SD = 0.36) was significantly lower than in the homologous tissue on the right (M = 0.88, SD = 0.40, t(30) = 2.91, p < .01, q = .027).
3.2. Do subacute perfusion and lesion load predict subacute reading ability?
To test if subacute perfusion was associated with subacute reading ability it was included as a predictor into a mixed‐linear effects model. The model also included participants as a random effect on the slopes for word imageability, frequency, and consistency. Each predictor was expected to account for independent variance. Because global perfusion values were not significantly different between the left and right side of the brain, and because these values correlated with regional perfusion values, global left and right brain perfusion were not considered in these and all subsequent analyses (See Table S2 for correlation among perfusion measures). Furthermore, because perfusion of the perilesional homologs in the right hemisphere was co‐linear with left perilesional perfusion, here and in subsequent analyses we studied it using separate linear models. Perfusion modulated subacute word reading accuracy, such that increased subacute perfusion of the right reading network was associated with worse subacute word reading accuracy (b 1 = −0.35, SE = 0.15, t(26.01) = −2.28, p < .05), whereas there was a nonsignificant trend for subacute perfusion of the left reading network to improve reading accuracy (b 1 = 0.37, SE = 0.25, t(26.01) = 1.46, p = .16). (Here we used Satterthwaite approximations of effective degrees of freedom, as required by the mixed effects modeling.) Similar results were obtained when we only considered homologous areas within the left and right reading networks, where right reading network perfusion was negatively associated with reading accuracy, (b 1 = −0.35, SE = 0.15, t(26.01) = −2.28, p < .05). Perilesional perfusion on the left (b 1 = 0.25, SE = 0.21, t(27.02) = 1.20, p = .24) or perfusion of its right homolog (b 1 = −0.25, SE = 0.19, t(27.02) = −1.30, p = .21) was not associated with word reading accuracy subacutely. None of the other linear models, testing for the effects of perfusion on accuracy in the orthography, phonology, and semantics touch screen tasks and the overall word and nonword reading aloud accuracy reached significance (Table S3). We also considered overall lesion volume and the overlap of lesion with the left reading network as predictors of subacute reading ability. We used the log transform of the lesion volume to ensure a normal distribution. Log lesion volume negatively correlated with subacute phonology task accuracy (r = −.43, p < .05), subacute semantic task (words) accuracy (r = −.36, p < .05), and subacute overall word reading accuracy (r = −.36, p < .05), however, these correlations do not survive multiple comparisons correction. The proportion of overlap between the lesion and the reading network negatively correlated with subacute phonology task accuracy (r = −.44, p < .05); subacute word reading accuracy (r = −.38, p < .05); and subacute nonword reading accuracy (r = −.47, p < .01). Only the latter result survived multiple comparisons correction. When the proportion of the reading network lesioned was included in the regression model for subacute nonword reading, together with subacute perfusion in the left and right reading network and subacute perfusion of the left perilesional area, it improved the model fit (R 2 change = .19, F(4, 29) = 3.68, p < .05, R 2 = .37), making the overall model significant. In fact, the overlap of the lesion with the reading network alone was a strong predictor of subacute nonword reading accuracy, F(1, 29) = 8.03, p < .01, R 2 = .22.
3.3. Longitudinal perfusion
We studied a potential brain marker of stroke recovery ‐ an improvement of cerebral blood flow to the initially hypoperfused areas. It was expected that an increase in perfusion would occur from the initial assessment to follow‐up. Because this analysis required longitudinal data, we included only the 15 participants who underwent two testing sessions (N = 15) and neuroimaging scans (N = 13) in the subacute and chronic stages of stroke recovery.
3.3.1. Mean perfusion changes of the left compared to the right hemisphere
Among the 13 participants who underwent both subacute and chronic scans, normalized perfusion values were numerically greater in the chronic compared to the subacute post‐stroke period. However, subacute‐to‐chronic perfusion changes for the left (M 1 = 0.94, SD = 0.15; M 2 = 0.96, SD = 0.15) or the right cerebral hemisphere (M 1 = 0.99, SD = 0.13; M 2 = 1.00, SD = 0.13) were not statistically significant. The differences between the overall left and right brain perfusion in the chronic period were also not significant (t(12) = 0.77, n.s.).
3.3.2. Mean perfusion changes of the left compared to the right reading network
We expected that an increase of cerebral perfusion within the left‐lateralized reading network would occur from the subacute to chronic period. An alternative hypothesis was that the right reading network perfusion would increase, similarly to the previously published observations in aphasia and motor recovery, where contra‐lateral functional homologs were activated during intermediate stages of recovery (Cramer, 2008; Saur et al., 2006). To test the effects of recovery time and laterality in a single statistical framework, we performed a repeated‐measures general linear model test with normalized perfusion as the dependent variable. The three within‐subject explanatory variables in this model were post‐stroke period, side of the brain, and their interaction. Neither the main effect of post‐stroke period, nor the interaction of post‐stroke period with side of the brain, was significant. That is, while perfusion numerically increased from the subacute to chronic period, primarily in the right reading network (M subacute = 1.08, SD = 0.32; M chronic = 1.11, SD = 0.24), this increase was not significant. The analysis did reveal a main effect of side, with higher perfusion on the right (M = 1.10, SE = 0.06) than the left (M = 0.78, SE = 0.06), F(1, 12) = 19.05, p < .005, q = .015.
Consistent with the previous result, normalized perfusion was greater within the right reading network (M = 1.11, SD = 0.24) compared to the left (M = 0.78, SD = 0.24), t(12) = 3.57, p < .005, q = .018, in the chronic period (Figure S1). As in the acute perfusion analysis, we masked out lesioned areas in the left brain to avoid biasing the results with low perfusion values in lesioned areas. When we subsampled the left‐brain reading network mask to include the same number of voxels as on the right, this effect remained significant, p < .005, q = .021. However, when considering only homologous regions in both hemispheres, perfusion was not significantly higher in the right hemisphere (M = 1.03, SD = 0.19), than in the left hemisphere (M = 0.94, SD = 0.30), p = .314, q = .05.
3.3.3. Mean perilesional perfusion changes compared to homologous contralateral regions
While a numerical difference in perilesional perfusion between the left (M = 0.54, SD = 0.33) and right side (M = 0.64, SD = 0.29) of the brain was still observed at follow up, this difference was only marginally significant, t(12) = 2.08, p = .06, q = .045, ns. There were no significant differences between acute and chronic perfusion either in the perilesional ring, or in the right brain homolog (Figure S1). To test if perfusion of the areas surrounding the lesion was below the participant's overall baseline in the chronic period, we compared perilesional perfusion to global left and right brain perfusion. Perilesional perfusion was significantly lower than perfusion of the entire left (t(12) = 5.13, p < .001, q = .009) or right brain (t(12) = 4.51, p < .005, q = .024).
3.4. Do subacute perfusion and lesion load predict chronic reading ability?
To test if subacute perfusion was associated with chronic reading performance, it was added as a predictor into three mixed‐linear effects models. The models included participants as a random effect on the slopes of word imageability, frequency, and consistency. In the first model, we tested the effects of subacute perfusion of the left and right reading network, and in two separate models, we examined the effects of subacute perfusion of perilesional areas and of the right perilesional homolog. Perfusion modulated chronic word reading accuracy, such that increased subacute perfusion of the right reading network was associated with worse subacute word reading accuracy (b 1 = −0.49, SE = 0.18, t[12.00] = −2.72, p < .05), whereas there was a nonsignificant trend for subacute perfusion of the left reading network to improve reading accuracy (b 1 = 0.54, SE = 0.28, t(12.00) = 1.93, p = .08). When we considered only the homologous regions within the right and left reading network, right reading network perfusion was negatively associated with reading accuracy, (b 1 = −0.64, SE = 0.14, t(12.01) = 4.45, p < .001), while left reading network perfusion was positively associated with reading accuracy, (b 1 = 0.43, SE = 0.16, t(12.01) = 2.66, p < .05). Perilesional perfusion on the left was not associated with chronic word reading accuracy (b 1 = −0.25, SE = 0.22, t(13.00) = 1.17, p = .27), but perfusion of its right homolog was negatively associated with chronic reading accuracy (b 1 = −0.49, SE = 0.16, t(13.00) = −3.05, p < .01).
We next examined the ability of the left and right regional perfusion in the subacute period to predict other reading‐related behavioral outcomes in the chronic period. To examine the independent contribution of each factor (subacute perfusion of the left and right reading network, and subacute perfusion of perilesional area and its homolog), we used a multiple regression model (See Table S4 for correlation among the variables used in our regression models). First, a multivariate linear regression model with three predictors (subacute perfusion of the left and right reading network, and subacute perfusion of perilesional area) was applied to each of our four touch‐screen behavioral measures of orthography, phonology, and semantics (Figure 1a). Among the participants with longitudinal data (N = 15), there was a significant relationship with subacute perfusion for phonology task performance. Higher subacute perfusion of the left reading network (b 1 = 0.38, SE = 0.16; t = 2.42, p < .05) was associated with better phonology task performance in the chronic period (right reading network: b 1 = −.10, SE = 0.10; t = −0.98, p = .35; left perilesional area: b 1 = −.13, SE = 0.10; t = −1.25, p = .24). Similar effects were obtained when using only homologous areas for the right and left reading network, with left reading network perfusion predicting higher phonology task accuracy (b 1 = 0.28, SE = 0.11; t = 2.37, p < .05; right reading network: b 1 = −0.17, SE = 0.10; t = −1.68, p = .12; left perilesional area:: b 1 = −0.09, SE = 0.10; t = −0.89, p = .40). A separate linear regression model was conducted for right hemisphere perilesional homolog and performance in the four touch‐screen behavioral measures. This analysis showed that higher subacute perfusion of the right homologous perilesional areas was associated with decreased phonology task accuracy during the chronic period (b 2 = −0.53, t = −2.28, p < .05). Figure 5 illustrates these effects (See also Table S5 for other nonsignificant regression models' statistics). We did not observe any associations between chronic perfusion and chronic reading ability. We also considered overall lesion volume as a predictor of chronic reading ability. Log lesion volume was not significantly associated with any of the chronic behavioral measures (all p's > .05; Table S6). Similarly, the proportion of overlap between the lesion and the reading network did not predict any of the chronic behavioral measures (all p's > .05; Table S7).
Figure 5.
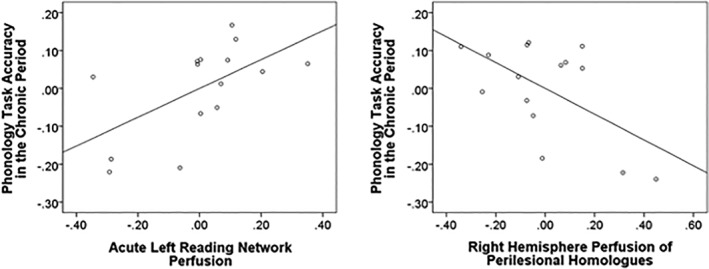
Partial regression plots for the significant (p < .05) associations between acute perfusion within the left reading network (left plot), the right reading network (right plot), and chronic ability to perform rhyming comparisons on pronounceable nonwords
4. DISCUSSION
In this study, we observed that regional perfusion of the left hemisphere, measured using ASL, was disrupted by left‐hemisphere stroke and some of this disruption persisted into the chronic period. In the subacute post‐stroke period, perfusion was lower in parts of the left hemisphere, including the perilesional areas and components of the neural network for reading as compared to their right‐hemisphere counterparts. While left–right differences in perfusion resolved during the chronic period, perfusion was still lower in perilesional areas compared to the rest of the brain. Importantly, higher subacute perfusion of the right reading network predicted worse reading ability during the subacute and chronic periods. In contrast, higher subacute perfusion of the left reading network predicted better reading performance in the chronic period. While lesion volume and overlap of lesion with the left‐hemisphere reading network predicted subacute performance, perfusion, rather than lesion load, was a better predictor of chronic performance. Thus, information about cerebral perfusion explained unique variance in chronic reading outcomes not accounted for by lesion load alone.
We tested two hypotheses about post‐stroke brain perfusion and reading. The first was that perilesional re‐perfusion supports functional recovery. Our results did not support this hypothesis. While we observed lower subacute perilesional perfusion compared to the nonlesioned contralateral homolog, perilesional perfusion did not increase in the chronic period. Therefore, it seems that subacute perilesional perfusion does not contribute to improved function. Increased perilesional perfusion in the subacute (as compared to chronic) period is pathological and may signal blood flow lingering because of extremely limited perfusion downstream near the infarct core (Detre et al., 1998, 2012; Zaharchuk, 2014). This idea is consistent with our observation that chronic perfusion decreased slightly (but nonsignificantly) in perilesional tissue, perhaps suggesting limited normalization of blood flow. It is also possible that local perfusion failed to improve from the subacute to chronic period, and even decreased slightly because irreversible cellular damage took place in the tissue immediately surrounding the lesion (Carmichael, 2016; Ten & Starkov, 2012). This is sometimes referred to as infarct growth (Zaharchuk, 2014). A nonsignificant decrease was also observed in perfusion of the right brain homolog from subacute to chronic period. This may be explained by initial upregulation of that region followed by a return to the baseline. However, because these effects were not found to be reliable, any conclusions about the longitudinal changes in perilesional perfusion are tentative and await further study.
The second hypothesis we tested was that re‐organization of the reading circuit supports recovery. Here, we defined re‐organization as re‐assignment of structure–function relationships within the existing functional network. Our results are consistent with this hypothesis. Because ASL enables measuring perfusion in absolute units, we could compare perfusion signal across participants and examine its relationship to behavioral measures. We found that higher subacute perfusion in the left reading network was associated both with greater chronic reading accuracy and with significantly higher chronic phonological competence. We did not observe a subacute‐to‐chronic perfusion increase in the reading network for our longitudinal sample, possibly because some perfusion changes occurred earlier in stroke recovery. Other studies have found an association between acute (1–2 days post‐stroke) perfusion and re‐perfusion and reading recovery (Fridriksson et al., 2002; Marsh & Hillis, 2005). Transient increases in perfusion using pharmacological agents to increase blood pressure have also been linked with improved aphasia symptoms (Hillis, 2007a), suggesting that perfusion operates on a fine time‐scale and that it is a promising target for early stroke interventions.
Previous longitudinal studies of cognitive recovery following stroke have characterized three types of stroke effects in the functional neuroimaging of language: decreased activity in areas remote from the lesion, but belonging to the same network (diaschisis), increased recruitment of the right homologs of the affected areas (explained by compensation or lack of inter‐hemispheric inhibition), and increasing ipsilesional activation with recovery (Geranmayeh, Brownsett, & Wise, 2014; Nair et al., 2015; Price & Crinion, 2005; Stockert et al., 2016; Thompson & den Ouden, 2008). We have demonstrated and further characterized two of these effects. First, we showed that areas in the left hemisphere, remote from the lesion, show decreased perfusion during the subacute period. When we consider functional areas in the left and right hemispheres found to be activated during reading in healthy individuals, left areas show reduced perfusion compared to the right, which persists into the chronic post‐stroke period. Perfusion is also higher in the right counterpart of the left perilesional area. However, higher contralesional perfusion predicted worse reading accuracy and phonological competency in the chronic period. It is possible that in patients with more severe disruption of blood flow around the stroke‐associated lesion, there may be an initial increase in perfusion of the contralateral hemisphere homologs, which may be related to higher neural activity in these areas. However, this increase is associated with poorer prognosis for recovery. Higher subacute perfusion of the right reading network was also associated with worse subacute and chronic reading ability. Overall, it appears that the right brain perfusion may be upregulated during stroke recovery. This could be due to the lack of transcallosal inhibition (Heiss, 2009), or compensatory activation, which serves to augment function that was impaired due to the loss of lesioned tissue. The latter compensatory mechanism seems more likely than inhibition, because we did not observe significant negative correlations between regional perfusion in the left and right hemisphere. Such a negative correlation would have been expected in the case of transcallosal inhibition. Ultimately, a compensatory activation of the right‐hemisphere appears to lead to suboptimal recovery compared to the ability to activate the left hemisphere.
How do our findings reconcile with others showing improved reading performance supported by the right hemisphere? The ability of the right hemisphere to augment functional recovery was supported by a case study of a patient with a left posterior fusiform stroke (the putative visual word form area; Cohen et al., 2002, 2003, Dehaene et al., 2010; Gaillard et al., 2006) and a hypoperfused splenium (Marsh & Hillis, 2005). The patient initially presented with a reading deficit. When his splenium was re‐perfused through spontaneous recovery, the patient's reading improved. Hillis (2007b) suggested that this finding indicated that the right hemisphere was able to communicate with the intact regions on the left and transfer location‐independent spatial representations via the splenium. Our finding that increased perfusion of the right hemisphere predicts poor reading in the subacute and chronic period underscores the importance of investigating neuroimaging trajectories across stages of stroke recovery. Stroke effects appear to evolve over time with a pattern of alternating dominant and nondominant hemisphere participation in recovery.
This study provides additional evidence that resting blood perfusion is an important biomarker in stroke. While lesion load predicted impairments during subacute post‐stroke period, resting perfusion within the reading network also predicted chronic recovery from reading deficits (see also Fridriksson et al., 2002 for a similar finding). We observed that for optimal reading function, preserved perfusion of the left reading network in the subacute period is required. Subacute right brain activation is sometimes seen as a necessary transition step in stroke recovery (Cramer, 2008; Stockert et al., 2016). Our findings challenge this view. We found that subacute right brain recruitment is associated with worse reading‐related outcomes. These data suggest that early activation and re‐perfusion of the left reading network is essential for better chronic reading performance. This finding could ultimately have prognostic value for stroke recovery. Our study also has implications for longitudinal functional neuroimaging studies, investigating the BOLD response in stroke survivors, because the magnitude of the BOLD response may change with changes in resting perfusion.
4.1. Limitations and future directions
Our study has several limitations. We did not have a nonstroke control group, and, therefore, we are not able to show that, in healthy individuals, global perfusion or perfusion of the left and right reading network is associated with performance on the reading tasks studied here. While our sample size is similar to other noteworthy studies in the field (Altamura et al., 2009; Bonakdarpour, Parrish, & Thompson, 2007; Detre et al., 1998; Roc et al., 2006; Saur et al., 2006), replication with a larger group of patients will be an important next step. In particular, our longitudinal data may have limited generalizability due to a relatively small sample size. Taking measurements at additional time‐points will also allow future studies to better characterize recovery trajectories and to account for the breadth of inter‐individual differences. The current study offers a roadmap for such expansion. Furthermore, the functional significance of the perilesional perfusion may differ for patients with lesions that primarily affect subcortical areas and white matter and patients whose lesions include the cortex. In future large‐scale studies, it would be important to group patients based on lesion location to fully characterize perilesional perfusion effects. In addition, we chose to use a hypothesis‐driven approach and studied perfusion within areas previously identified in functional neuroimaging studies of reading. This approach assumes that published literature is unbiased in representing all brain areas involved in reading. This may not be the case for areas with low signal on functional MRI, such as, for example, parts of the anterior temporal lobe. Likewise, although we compared global perfusion differences between the right and the left hemispheres, we did not consider the contribution of specific brain areas outside of the reading network to behavioral recovery. Future studies may be able to shed light on the role of such areas in recovery using voxelwise (Zhao, Lambon Ralph, & Halai, 2018), or region of interest analyses. Although we followed the current recommendations for pulsed ASL implementation in clinical patients (Alsop et al., 2015), we may not have captured hypoperfusion present at long delay intervals due to the limited window provided by the pulsed ASL technique. One of the future directions of the study is to use the pseudocontinuous ASL labeling technique, which has a higher signal‐to‐noise ratio with reduced signal loss and, thus, may be able to provide a more nuanced understanding of post‐stroke perfusion and re‐perfusion. Finally, ASL neuroimaging is susceptible to head motion, which may be increased in clinical populations. In this study, we were careful to apply motion correction and censor motion outliers, as described in the Methods section. In addition, spatial smoothing, averaging of multiple tag‐control volumes, and summary statistics were used in the majority of the analyses reported in this study. Thus, although motion could still be a contributing factor, it seems unlikely to explain the effects observed in this study.
5. CONCLUSIONS
Most improvement in post‐stroke language deficits occurs in the first 3 months after a stroke (Berthier, 2005; Robey, 1998). The goal of rehabilitation is to effectively use this time to administer the necessary therapy and help a stroke survivor return to work, school, or daily activity. For some, reading deficits may be the main obstacle to regaining their expected quality of life. In this study, we examined the neurobehavioral course of recovery from reading deficits. In 31 left‐brain stroke survivors, we found that rather than showing large‐scale (hemispheric) differences in subacute perfusion and longitudinal increases across the entire hemisphere, perfusion variations may operate on a finer scale. Consistent with this idea, we found that higher subacute perfusion of the left reading circuit is beneficial for chronic phonological competence. Thus, re‐perfusion of the left hemisphere‐reading network was integral to better reading ability.
Supporting information
Figure S1 Perfusion change from initial assessment to follow‐up (N = 13). Error bars represent standard error, * p < .005
Table S1 Cluster peaks for areas within the left and right reading network masks
Table S2. Pearson first‐order correlation values among subacute perfusion measures
Table S3. Summary of nonsignificant linear regression models carried out for the relationship of subacute reading ability and perfusion
Table S4. Pearson first‐order correlation values for the predictor and outcome variables utilized in the multiple regression analysis of chronic phonology task accuracy
Table S5. Summary of nonsignificant linear regression models carried out for the relationship of chronic reading ability and subacute perfusion
Table S6. Pearson first‐order correlation values for lesion volume and behavioral measures during the chronic period
Table S7. Pearson first‐order correlation values for proportion of lesion overlap with the reading network and behavioral measures during the chronic period
ACKNOWLEDGMENTS
This work was generously supported by a Mable H. Flory Foundation grant (AMB, OB, & WWG), NICHD NCMRR grant R21‐HD095488 (OB, AMB, & WWG) and NIH NICHD grant R00‐HD065839 (WWG). The authors would also like to thank Dr. Jeffrey Binder for providing materials for the touch‐screen task and for valuable feedback on an earlier version of this manuscript.
Boukrina O, Barrett AM, Graves WW. Cerebral perfusion of the left reading network predicts recovery of reading in subacute to chronic stroke. Hum Brain Mapp. 2019;40:5301–5314. 10.1002/hbm.24773
Funding information Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: R00‐HD065839; Kessler Foundation, Grant/Award Number: Pilot Grant; Mabel H. Flory Foundation; National Center for Medical Rehabilitation Research, Grant/Award Number: R21‐HD095488
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Abel, S. , Dressel, K. , Bitzer, R. , Kümmerer, D. , Mader, I. , Weiller, C. , & Huber, W. (2009). The separation of processing stages in a lexical interference fMRI‐paradigm. NeuroImage, 44, 1113–1124. 10.1016/j.neuroimage.2008.10.018 [DOI] [PubMed] [Google Scholar]
- Alsop, D. C. , Detre, J. A. , Golay, X. , Günther, M. , Hendrikse, J. , Hernandez‐Garcia, L. , … Zaharchuk, G. (2015). Recommended implementation of arterial spin‐labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magnetic Resonance in Medicine, 73, 102–116. 10.1002/mrm.25197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamura, C. , Reinhard, M. , Vry, M.‐S. , Kaller, C. P. , Hamzei, F. , Vernieri, F. , … Saur, D. (2009). The longitudinal changes of BOLD response and cerebral hemodynamics from acute to subacute stroke. A fMRI and TCD study. BMC Neuroscience, 10, 151 10.1186/1471-2202-10-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, J.‐C. , & Marchal, G. (1999). Ischemic core and penumbra in human stroke. Stroke, 30, 1150–1151. 10.1161/01.STR.29.9.1821 [DOI] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2015). Fitting linear mixed‐effects models using lme4. Journal of Statistical Software, 67, 51 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Berthier, M. L. (2005). Poststroke aphasia : Epidemiology, pathophysiology and treatment. Drugs & Aging, 22, 163–182. [DOI] [PubMed] [Google Scholar]
- Binder, J. R. , Desai, R. H. , Graves, W. W. , & Conant, L. L. (2009). Where is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cerebral Cortex, 19, 2767–2796. 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, J. R. , Medler, D. A. , Desai, R. , Conant, L. L. , & Liebenthal, E. (2005). Some neurophysiological constraints on models of word naming. NeuroImage, 27, 677–693. 10.1016/j.neuroimage.2005.04.029 [DOI] [PubMed] [Google Scholar]
- Binder, J. R. , Pillay, S. B. , Humphries, C. J. , Gross, W. L. , Graves, W. W. , & Book, D. S. (2016). Surface errors without semantic impairment in acquired dyslexia: A voxel‐based lesion‐symptom mapping study. Brain, 139, 1517–1526. 10.1093/brain/aww029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonakdarpour, B. , Parrish, T. B. , & Thompson, C. K. (2007). Hemodynamic response function in patients with stroke‐induced aphasia: Implications for fMRI data analysis. NeuroImage, 36, 322–331. 10.1016/j.surg.2006.10.010.Use [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier, C. , Dojat, M. , & de Micheaux, P. L. (2011). Temporal and spatial independent component analysis for fMRI data sets embedded in the AnalyzeFMRI R package. Journal of Statistical Software, 44, 1–24. [Google Scholar]
- Boukrina, O. , Barrett, A. M. , Alexander, E. J. , Yao, B. , & Graves, W. W. (2015). Neurally dissociable cognitive components of reading deficits in subacute stroke. Frontiers in Human Neuroscience, 9, 298 10.3389/fnhum.2015.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt, J. (1991). The Hopkins verbal learning test: Development of a new memory test with six equivalent forms. The Clinical Neuropsychologist, 5, 125–142. 10.1080/13854049108403297 [DOI] [Google Scholar]
- Brookshire, C. E. , Wilson, J. P. , Nadeau, S. E. , Gonzalez Rothi, L. J. , & Kendall, D. L. (2014). Frequency, nature, and predictors of alexia in a convenience sample of individuals with chronic aphasia. Aphasiology, 28, 1–17. 10.1080/02687038.2014.945389 [DOI] [Google Scholar]
- Brumm, K. P. , Perthen, J. E. , Liu, T. T. , Haist, F. , Ayalon, L. , & Love, T. (2010). An arterial spin labeling investigation of cerebral blood flow deficits in chronic stroke survivors. NeuroImage, 51, 995–1005. 10.1016/j.neuroimage.2010.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael, S. T. (2016). The 3 Rs of stroke biology: Radial, relayed, and regenerative. Neurotherapeutics, 13, 348–359. 10.1007/s13311-015-0408-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassar, M. , & Treiman, R. (1997). The beginnings of orthographic knowledge: Children's knowledge of double letters in words. Journal of Education & Psychology, 89, 631–644. 10.1037//0022-0663.89.4.631 [DOI] [Google Scholar]
- Cattinelli, I. , Borghese, N. A. , Gallucci, M. , & Paulesu, E. (2013). Reading the reading brain: A new meta‐analysis of functional imaging data on reading. Journal of Neurolinguistics, 26, 214–238. 10.1016/j.jneuroling.2012.08.001 [DOI] [Google Scholar]
- Chappell, M. A. , Groves, A. R. , MacIntosh, B. J. , Donahue, M. J. , Jezzard, P. , & Woolrich, M. W. (2011). Partial volume correction of multiple inversion time arterial spin labeling MRI data. Magnetic Resonance in Medicine, 65, 1173–1183. 10.1002/mrm.22641 [DOI] [PubMed] [Google Scholar]
- Chappell, M. A. , Groves, A. R. , Whitcher, B. , & Woolrich, M. (2009). Variational Bayesian inference for a non‐linear forward model. IEEE Transactions on Signal Processing, 57, 223–236. [Google Scholar]
- Chopp, M. , Zhang, Z. G. , & Jiang, Q. (2007). Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke, 38, 827–831. 10.1161/01.STR.0000250235.80253.e9 [DOI] [PubMed] [Google Scholar]
- Cloutman, L. L. , Newhart, M. , Davis, C. L. , Heidler‐Gary, J. , & Hillis, A. E. (2011). Neuroanatomical correlates of oral reading in actue left hemisphere stroke. Brain and Language, 116, 14–21. 10.1523/JNEUROSCI.3593-07.2007.Omega-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman, L. L. , Newhart, M. , Davis, C. L. , Kannan, V. C. , & Hillis, A. E. (2010). Patterns of reading performance in acute stroke: A descriptive analysis. Behavioural Neurology, 22, 35–44. 10.3233/BEN-2009-0258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, L. , Lehéricy, S. , Chochon, F. , Lemer, C. , Rivaud, S. , & Dehaene, S. (2002). Language‐specific tuning of visual cortex? Functional properties of the visual word form area. Brain, 125, 1054–1069. [DOI] [PubMed] [Google Scholar]
- Cohen, L. , Martinaud, O. , Lemer, C. , Lehéricy, S. , Samson, Y. , Obadia, M. , … Dehaene, S. (2003). Visual word recognition in the left and right hemispheres: Anatomical and functional correlates of peripheral Alexias. Cerebral Cortex, 13, 1313–1333. 10.1093/cercor/bhg079 [DOI] [PubMed] [Google Scholar]
- Cramer, S. C. (2008). Repairing the human brain after stroke: I. Mechanisms of Spontaneous Recovery. Annals of Neurology, 63, 272–287. 10.1002/ana.21393 [DOI] [PubMed] [Google Scholar]
- Crinion, J. , Holland, A. L. , Copland, D. a. , Thompson, C. K. , & Hillis, A. E. (2013). Neuroimaging in aphasia treatment research: Quantifying brain lesions after stroke. NeuroImage, 73, 208–214. 10.1016/j.neuroimage.2012.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosson, B. , Hampstead, B. M. , Krishnamurthy, L. C. , Krishnamurthy, V. , McGregor, K. M. , Nocera, J. R. , … Tran, S. M. (2017). Advances in neurocognitive rehabilitation research from 1992 to 2017: The Ascension of neural plasticity. Neuropsychology, 31, 900–920. 10.1037/neu0000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene, S. , Pegado, F. , Braga, L. W. , Ventura, P. , Nunes Filho, G. , Jobert, A. , … Cohen, L. (2010). How learning to read changes the cortical networks for vision and language. Science, 330, 1359–1364. 10.1126/science.1194140 [DOI] [PubMed] [Google Scholar]
- Detre, J. A. , Alsop, D. C. , Vives, L. R. , Maccotta, L. , Teener, J. W. W. , & Raps, E. C. C. (1998). Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology, 50, 633–641. 10.1212/WNL.50.3.633 [DOI] [PubMed] [Google Scholar]
- Detre, J. A. , Rao, H. , Wang, D. J. , Chen, Y. F. , & Wang, Z. (2012). Applications of arterial spin labeled MRI in the brain. Journal of Magnetic Resonance Imaging, 35, 1026–1037. 10.1002/jmri.23581.Applications [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty, L. C. , Bowers, D. , & Heilman, K. M. (1990). Florida mental status exam for progressive dementia screening. Gerontologist, 30 (20A (Special Issue)). [Google Scholar]
- Fiez, J. A. , Balota, D. A. , Raichle, M. E. , & Petersen, S. E. (1999). Effects of lexicality, frequency, and spelling‐to‐sound consistency on the functional anatomy of reading. Neuron, 24, 205–218. [DOI] [PubMed] [Google Scholar]
- Fridriksson, J. (2011). Measuring and inducing brain plasticity in chronic aphasia. Journal of Communication Disorders, 44, 557–563. 10.1016/j.surg.2006.10.010.Use [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson, J. , Holland, A. L. , Coull, B. M. , Plante, E. , Trouard, T. P. , & Beeson, P. (2002). Aphasia severity: Association with cerebral perfusion and diffusion. Aphasiology, 16, 859–871. 10.1080/02687030244000347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson, J. , Richardson, J. D. , Fillmore, P. , & Cai, B. (2012). Left hemisphere plasticity and aphasia recovery. NeuroImage, 60, 854–863. 10.1016/j.neuroimage.2011.12.057.Left [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost, S. J. , Mencl, C. A. W. E. , Sandak, R. , Moore, D. L. , Rueckl, J. G. , Katz, L. , … Pugh, K. R. (2005). A functional magnetic resonance imaging study of the tradeoff between semantics and phonology in reading aloud. NeuroReport, 16, 621–624. [DOI] [PubMed] [Google Scholar]
- Gaillard, R. , Naccache, L. , Pinel, P. , Clémenceau, S. , Volle, E. , Hasboun, D. , … Cohen, L. (2006). Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron, 50, 191–204. 10.1016/j.neuron.2006.03.031 [DOI] [PubMed] [Google Scholar]
- Geranmayeh, F. , Brownsett, S. L. E. , & Wise, R. J. S. (2014). Task‐induced brain activity in aphasic stroke patients: What is driving recovery? Brain, 137, 2632–2648. 10.1093/brain/awu163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves, W. W. , Desai, R. , Humphries, C. , Seidenberg, M. S. , & Binder, J. R. (2010). Neural systems for reading aloud: A multiparametric approach. Cerebral Cortex, 20, 1799–1815. 10.1093/cercor/bhp245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss, W. D. (2009). WSO leadership in stroke medicine award lecture Vienna, September 26, 2008: Functional imaging correlates to disturbance and recovery of language function. International Journal of Stroke, 4, 129–136. 10.1111/j.1747-4949.2009.00268.x [DOI] [PubMed] [Google Scholar]
- Hillis, A. E. (2007a). Pharmacological, surgical, and neurovascular interventions to augment acute aphasia recovery. American Journal of Physical Medicine & Rehabilitation, 86, 426–434. 10.1097/PHM.0b013e31805ba094 [DOI] [PubMed] [Google Scholar]
- Hillis, A. E. (2007b). Magnetic resonance perfusion imaging in the study of language. Brain and Language, 102, 165–175. 10.1016/j.bandl.2006.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis, A. E. , & Heidler, J. (2002). Mechanisms of early aphasia recovery. Aphasiology, 16, 885–895. 10.1080/0268703 [DOI] [Google Scholar]
- Hillis, A. E. , Vimal, S. , Newhart, M. , Aldrich, E. , Heidler, J. , & Ken, L. (2005). Reperfusion of selective areas is association with improved naming in acute stroke. Brain and Language, 95, 100–101. [Google Scholar]
- IBM Corporation . (2012). IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM. [Google Scholar]
- Jared, D. (2002). Spelling‐sound consistency and regularity effects in word naming. Journal of Memory and Language, 46(4), 723–750. 10.1006/jmla.2001.2827 [DOI] [Google Scholar]
- Jenkinson, M. , Bannister, P. , Brady, M. , & Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. J. , Woolrich, M. W. , & Smith, S. M. (2012). FSL. NeuroImage, 62, 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Kaplan, E. , Goodglass, H. , & Weintraub, S. (1983). Boston naming test. Philadelphia, PA: Lea and Febiger. [Google Scholar]
- Krupinski, J. , Kaluza, J. , Kumar, P. , Kumar, S. , & Wang, J. (1994). Role of angiogenesis in patients with cerebral ischemic stroke. Stroke, 25, 1794–1798. 10.1161/01.STR.25.9.1794 [DOI] [PubMed] [Google Scholar]
- Lambon‐Ralph, M. A. , Jefferies, E. , Patterson, K. , & Rogers, T. T. (2016). The neural and computational bases of semantic cognition. Nature Reviews. Neuroscience, 18, 42–55. 10.1038/nrn.2016.150 [DOI] [PubMed] [Google Scholar]
- Lichtenwalner, R. J. , & Parent, J. M. (2006). Adult neurogenesis and the ischemic forebrain. Journal of Cerebral Blood Flow and Metabolism, 26, 1–20. 10.1038/sj.jcbfm.9600170 [DOI] [PubMed] [Google Scholar]
- Macas, J. , Nern, C. , Plate, K. H. , & Momma, S. (2006). Increased generation of neuronal progenitors after ischemic injury in the aged adult human forebrain. The Journal of Neuroscience, 26, 13114–13119. 10.1523/JNEUROSCI.4667-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, E. B. , & Hillis, A. E. (2005). Cognitive and neural mechanisms underlying reading and naming: Evidence from letter‐by‐letter reading and optic aphasia. Neurocase, 11, 325–337. 10.1080/13554790591006320 [DOI] [PubMed] [Google Scholar]
- Monsell, S. (1991). The nature of word frequency effects in reading. In D. Besner & G. Humphreys (Eds.), Basic processes in reading: visual word recognition (pp. 148–197). Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Nair, V. A. , Young, B. M. , La, C. , Reiter, P. , Nadkarni, T. N. , Song, J. , … Prabhakaran, V. (2015). Functional connectivity changes in the language network during stroke recovery. Annals of Clinical Translational Neurology, 2, 185–195. 10.1002/acn3.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohab, J. J. , & Carmichael, S. T. (2008). Poststroke neurogenesis: Emerging principles of migration and localization of immature neurons. Neuroscience, 14, 369–380. [DOI] [PubMed] [Google Scholar]
- Ohab, J. J. , Fleming, S. , Blesch, A. , & Carmichael, S. T. (2006). A neurovascular niche for neurogenesis after stroke. The Journal of Neuroscience, 26, 13007–13016. 10.1523/JNEUROSCI.4323-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay, S. B. , Gross, W. L. , Graves, W. W. , Humphries, C. , Book, D. S. , & Binder, J. R. (2018). The neural basis of successful word Reading in aphasia. Journal of Cognitive Neuroscience, 30, 514–525. 10.1162/jocn_a_01214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay, S. B. , Stengel, B. C. , Humphries, C. , Book, D. S. , & Binder, J. R. (2014). Cerebral localization of impaired phonological retrieval during rhyme judgment. Annals of Neurology, 76, 738–746. 10.1002/ana.24266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut, D. C. , McClelland, J. L. , Seidenberg, M. S. , & Patterson, K. (1996). Understanding normal and impaired word reading: Computational principles in quasi‐regular domains. Psychological Review, 103, 56–115. [DOI] [PubMed] [Google Scholar]
- Price, C. J. (2012). A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62, 816–847. 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price, C. J. , & Crinion, J. (2005). The latest on functional imaging studies of aphasic stroke. Current Opinion in Neurology, 18, 429–434. 10.1097/01.wco.0000168081.76859.c1 [DOI] [PubMed] [Google Scholar]
- Rapcsak, S. Z. , Beeson, P. M. , Henry, M. L. , Kim, E. , Rising, K. , Andersen, S. , & Cho, H. (2009). Phonological dyslexia and dysgraphia: Cognitive mechanisms and neural substrates. Cortex, 45, 575–591. 10.1016/j.cortex.2008.04.006.Phonological [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey, R. R. (1998). A meta‐analysis of clinical outcomes in the treatment of aphasia. Journal of Speech, Language, and Hearing Research, 41, 172–187. [DOI] [PubMed] [Google Scholar]
- Roc, A. C. , Wang, J. , Ances, B. M. , Liebeskind, D. S. , Kasner, S. E. , & Detre, J. A. (2006). Altered hemodynamics and regional cerebral blood flow in patients with hemodynamically significant stenoses. Stroke, 37, 382–387. 10.1161/01.STR.0000198807.31299.43 [DOI] [PubMed] [Google Scholar]
- Saur, D. , Lange, R. , Baumgaertner, A. , Schraknepper, V. , Willmes, K. , Rijntjes, M. , & Weiller, C. (2006). Dynamics of language reorganization after stroke. Brain, 129, 1371–1384. 10.1093/brain/awl090 [DOI] [PubMed] [Google Scholar]
- Seidenberg, M. S. , & McClelland, J. L. (1989). A distributed, developmental model of word recognition and naming. Psychological Review, 96, 523–568. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert, A. , Kummerer, D. , & Saur, D. (2016). Insights into early language recovery: From basic principles to practical applications. Aphasiology, 39, 517–541. [Google Scholar]
- Strain, E. , Patterson, K. , & Seidenberg, M. S. (1995). Semantic effects in single‐word naming. Journal of Experimental Psychology. Learning, Memory, and Cognition, 21(5), 1140–1154. [DOI] [PubMed] [Google Scholar]
- Taraban, R. , & McClelland, J. L. (1987). Conspiracy effects in word pronunciation. Journal of Memory and Language, 26, 608–631. [Google Scholar]
- Taylor, J. S. H. , Rastle, K. , & Davis, M. H. (2012). Can cognitive models explain brain activation during word and pseudoword reading? A meta‐analysis of 36 neuroimaging studies. Psychological Bulletin, 139, 766–791. 10.1037/a0030266 [DOI] [PubMed] [Google Scholar]
- Ten, V. S. , & Starkov, A. (2012). Hypoxic‐ischemic injury in the developing brain: The role of reactive oxygen species originating in mitochondria. Neurology Research International, 2012, 1–10. 10.1155/2012/542976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, C. K. , & den Ouden, D.‐B. (2008). Neuroimaging and recovery of language in aphasia. Current Neurology and Neuroscience Reports, 8, 1–14. 10.1097/MPG.0b013e3181a15ae8.Screening [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, C. K. , den Ouden, D.‐B. , Bonakdarpour, B. , Garibaldi, K. , & Parrish, T. B. (2010). Neural plasticity and treatment‐induced recovery of sentence processing in agrammatism. Neuropsychologia, 48, 3211–3227. 10.1126/scisignal.2001449.Engineering [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau, M. , Beaucousin, V. , Hervé, P. Y. , Duffau, H. , Crivello, F. , Houdé, O. , … Tzourio‐Mazoyer, N. (2006). Meta‐analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. NeuroImage, 30, 1414–1432. 10.1016/j.neuroimage.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Winkler, A. M. , Ridgway, G. R. , Webster, M. A. , Smith, S. M. , & Nichols, T. E. (2014). Permutation inference for the general linear model. NeuroImage, 92, 381–397. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollams, A. M. , Ralph, M. A. , Plaut, D. C. , & Patterson, K. (2007). SD‐squared: On the association between semantic dementia and surface dyslexia. Psychological Review, 114, 316–339. 10.1037/0033-295X.114.2.316 [DOI] [PubMed] [Google Scholar]
- Yarkoni, T. , Poldrack, R. , & Nichols, T. (2011). Large‐scale automated synthesis of human functional neuroimaging data. Nature Methods, 8, 665–670. 10.1038/nmeth.1635.Large-scale [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage, J. A. , Brink, T. L. , Rose, T. L. , Lum, O. , Huang, V. , Adey, M. , & Leirer, V. O. (1982). Development of validation of a geriatric screening scale: A preliminary report. Journal of Psychiatric Research, 17, 37–49. [DOI] [PubMed] [Google Scholar]
- Zaharchuk, G. (2014). Arterial spin‐labeled perfusion imaging in acute ischemic stroke. Stroke, 45, 1202–1207. 10.1161/STROKEAHA.113.003612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Lambon Ralph, M. A. , & Halai, A. D. (2018). Relating resting‐state hemodynamic changes to the variable language profiles in post‐stroke aphasia. NeuroImage Clinical, 20, 611–619. 10.1016/j.nicl.2018.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Perfusion change from initial assessment to follow‐up (N = 13). Error bars represent standard error, * p < .005
Table S1 Cluster peaks for areas within the left and right reading network masks
Table S2. Pearson first‐order correlation values among subacute perfusion measures
Table S3. Summary of nonsignificant linear regression models carried out for the relationship of subacute reading ability and perfusion
Table S4. Pearson first‐order correlation values for the predictor and outcome variables utilized in the multiple regression analysis of chronic phonology task accuracy
Table S5. Summary of nonsignificant linear regression models carried out for the relationship of chronic reading ability and subacute perfusion
Table S6. Pearson first‐order correlation values for lesion volume and behavioral measures during the chronic period
Table S7. Pearson first‐order correlation values for proportion of lesion overlap with the reading network and behavioral measures during the chronic period
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


