Abstract
Differentially methylated genes (DMGs) serve a crucial role in the pathogenesis of glioma via the regulation of the cell cycle, proliferation, apoptosis, migration, infiltration, DNA repair and signaling pathways. This study aimed to identify aberrant DMGs and pathways by comprehensive bioinformatics analysis. The gene expression profile of GSE28094 was downloaded from the Gene Expression Omnibus (GEO) database, and the GEO2R online tool was used to find DMGs. Gene Ontology (GO) functional analysis and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis of the DMGs were performed by using the Database for Annotation Visualization and Integrated Discovery. A protein-protein interaction (PPI) network was constructed with Search Tool for the Retrieval of Interacting Genes. Analysis of modules in the PPI networks was performed by Molecular Complex Detection in Cytoscape software, and four modules were performed. The hub genes with a high degree of connectivity were verified by The Cancer Genome Atlas database. A total of 349 DMGs, including 167 hypermethylation genes, were enriched in biological processes of negative and positive regulation of cell proliferation and positive regulation of transcription from RNA polymerase II promoter. Pathway analysis enrichment revealed that cancer regulated the pluripotency of stem cells and the PI3K-AKT signaling pathway, whereas 182 hypomethylated genes were enriched in biological processes of immune response, cellular response to lipopolysaccharide and peptidyl-tyrosine phosphorylation. Pathway enrichment analysis revealed cytokine-cytokine receptor interaction, type I diabetes mellitus and TNF signaling pathway. A total of 20 hub genes were identified, of which eight genes were associated with survival, including notch receptor 1 (NOTCH1), SRC proto-oncogene (also known as non-receptor tyrosine kinase, SRC), interleukin 6 (IL6), matrix metallopeptidase 9 (MMP9), interleukin 10 (IL10), caspase 3 (CASP3), erb-b2 receptor tyrosine kinase 2 (ERBB2) and epidermal growth factor (EGF). Therefore, bioinformatics analysis identified a series of core DMGs and pathways in glioma. The results of the present study may facilitate the assessment of the tumorigenicity and progression of glioma. Furthermore, the significant DMGs may provide potential methylation-based biomarkers for the precise diagnosis and targeted treatment of glioma.
Keywords: glioma, bioinformatics, methylation, biomarker
Introduction
Glioma is a type of primary central nervous system (CNS) solid tumor that arises from glial cells and is aggressive and lethal (1). It comprises ~30% of all CNS tumors and 80% of all malignant brain tumors (2). According to the 2016 World Health Organization (WHO) guidelines, gliomas can be classified into grades I–IV depending on ‘integrated diagnosis’, combining histopathological and molecular features (3). The most malignant type is grade IV glioblastoma (GBM), the overall survival (OS) time of which is only ~14 months (4). Lower grade glioma (LGG; grades II and III) exhibits benign tendencies and leads to a favorable prognosis for patients (5). However, it has a high rate of recurrence and increases in grade over time (6). Currently, the common treatment strategies for the treatment of glioma involve surgical resection, after which adjuvant chemotherapy and/or radiotherapy are used; however, the clinical outcomes of glioma remain unsatisfactory. The pathogenesis of glioma involves multiple factors and steps, comprising genetic and epigenetic alterations. Despite a number of mechanisms of oncogenesis have been verified in glioma, including retinoblastoma, p53 and receptor tyrosine kinase signaling pathways, the genetic factors and precise mechanism of human glioma remain poorly understood (7). Therefore, vital molecular biomarkers and oncogenic pathways associated with accurate diagnosis and patient survival in glioma require further identification.
Aberrant CpG island methylation is a primary epigenetic modified form of DNA sequence in malignancy (8). The functions of key genes can be altered through methylation to modify their expression, including hypermethylation of tumor suppressor genes (TSGs) and hypomethylation of oncogenes (9,10). The methylation status of multiple TSGs may serve as a biomarker for the early diagnosis and prediction of prognosis (11). For example, promoter methylation-induced silencing of the O6-methylguanine-DNA methyltransferase DNA-repair gene has been demonstrated to be a powerful predictor of the sensitivity of alkylating chemotherapy in patients with glioma with long survival rates (12). The methylation level of death associated protein kinase 1 is associated with clinical features and outcomes of glioma; hypermethylation at site-1,527 or together with site-1,543 indicates good sensitivity to postoperative therapies, particularly radiotherapy (13). A number of TSGs associated with cell cycle, proliferation and DNA repair have been identified in glioma, including cyclin dependent kinase inhibitor 2A (CDKN2A, also known as P16INK4a, P14ARF), TNF receptor superfamily member 11a (TNFRSF11A) and nuclear receptor binding SET domain protein 1 (NSD1) (14–17). Therefore, identifying differentially methylated genes (DMGs) by comparing the differences in DNA methylation status of glioma with that of normal brain tissue is crucial for elucidating the development, progression and metastasis of glioma and may be used as a guide for targeting drugs for glioma.
At present, microarray analysis based on high-throughput platforms is an efficient tool that has been applied to screen virtual genetic or epigenetic alterations in carcinogenesis, and to identify biomarkers for the diagnosis and prognosis of cancer (18). In the present study, DMGs were examined in glioma by online bioinformatics resources. The expression profile of GSE28094 from the Gene Expression Omnibus (GEO) database was downloaded to identify the DMGs between glioma and normal brain tissue. Subsequently, hierarchical clustering and functional enrichment analyses of the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways by Database for Annotation Visualization and Integrated Discovery (DAVID) for the DMGs were performed. A protein-protein interaction (PPI) network of DMGs was constructed and the main hub genes with high degree of connectivity were examined by Search Tool for the Retrieval of Interacting Genes (STRING), and the results were further validated by The Cancer Genome Atlas (TCGA) data to identify DMGs in the expression of glioma. This study provided comprehensive biological information for DMGs and novel targets for the diagnosis and accurate treatment of glioma.
Materials and methods
Microarray data
To identify DMGs between glioma and normal brain tissue, the GSE28094 gene expression microarray, contributed by Fernandez et al (19), was downloaded from the GEO database (https://www.ncbi.nlm.nih.gov/gds/) of the National Center for Biotechnology Information, a public functional genomics data repository. GSE28094 was based on the GPL9183 platform (Illumina GoldenGate Methylation Cancer Panel I). For the present study, 90 gliomas and 6 normal brain tissues were collected from GSE28094.
Data processing of DMGs
GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r/) online software was used to analyze GSE28094 and detect DMGs between gliomas and normal brain tissues. GEO2R is an interactive online tool in which ≥2 groups of samples can be compared in a GEO series to display differentially expressed genes under specific experimental conditions (20). The adjusted P-values were used to decrease the false positive rate using the Benjamini and Hochberg false discovery rate method. Adjusted P<0.05 and |t|>2 were used as the cut-off values for detecting DMGs. Finally, 349 DMGs were obtained, including 167 upregulated and 182 downregulated genes.
GO and KEGG pathway analyses of DMGs
GO analysis served as a crucial tool to annotate genes and gene products and to identify characteristic biological functions using high-throughput genome or transcriptome data (21), including biological process (BP), cellular component (CC) and molecular function (MF). KEGG is a collection of databases that can help to annotate genomes, biological pathways, diseases, chemical substances and drugs (22). DAVID (version 6.8; http://david.ncifcrf.gov/) was used to perform KEGG pathway enrichment analysis for the selected DMGs. P<0.05 was considered to indicate a statistically significant difference.
PPI network and module analysis
PPI analysis was performed to illustrate the interactions and functions of the selected DMGs via STRING (version 11.0; http://string-db.org). Cytoscape is a vital workflow component for executing network visualization, analysis and publishing tasks (23). To illustrate the potential interactions among those DMGs, STRING in Cytoscape (version 3.6.0) was applied and the DMGs were mapped into STRING. A confidence score ≥0.4 and a maximum number of interactors=0 were set as the cut-off criterion. Molecular complex detection (MCODE, version 1.5.1) is a method to analyze densely connected regions in PPI networks (24). This method was used to screen modules of the PPI network in Cytoscape with a degree cut-off=2, node score cut-off=0.2, k-core=2 and maximum depth=100. The pathway analysis of genes in these modules was also performed by DAVID. In addition, the top 20 hub genes with a high degree of connectivity were inserted into STRING with a confidence score of ≥0.4 and a maximum number of interactors=0. GO and KEGG pathway analyses were also used to examine the potential interactions.
TCGA data validation of the hub DMGs
In view of the small sample size of this study, further validation analysis was performed to verify the results of TCGA data. GEPIA (http://gepia.cancer-pku.cn/index.html) is a publicly available interactive web server that can analyze the RNA sequencing expression data of 9,736 tumors and 8,587 normal samples from TCGA and GTEx projects by a standard processing pipeline (25). It supports customizable functions, including tumor and normal tissue differential expression analyses, and information can be obtained on the expression level of hub DMGs in glioma and normal brain tissue. Boxplots were displayed to visualize the association. By acquiring pathologically confirmed immunohistochemical data of glioma and normal brain tissue based on the Human Protein Atlas (HPA; http://www.proteinatlas.org/), the expression of these hub DMGs was further validated. Hub DMGs in glioma were identified using HPA. From the extensive sample collection of antibodies against the DMGs, the sample with the strongest intensity and quantity was selected. The normal brain tissue stained for caspase 3 (CASP3) expression was from a female patient (age, 45 years; patient ID, 1539; staining, not detected; intensity, negative; quantity, negative; location, none), and the glioma tissue was from a male patient (age, 56 years; patient ID, 131; staining, medium; intensity, moderate; quantity, 75–25%; location, nuclear). The normal brain tissue stained for erb-b2 receptor tyrosine kinase 2 (ERBB2) expression was from a female patient (age, 54 years; patient ID, 2523; staining, not detected; intensity, negative; quantity, negative; location, none), and the glioma tissue was from a male patient (age, 66 years; patient ID, 206; staining, high; intensity, strong; quantity, >75%; location, cytoplasmic/membranous).
Survival analysis of hub genes
GEPIA was used to further investigate the relapse-free survival and OS time data of the DMGs by Kaplan-Meier survival curves. The hazard ratio (HR) with 95% confidence intervals and log-rank P-value were calculated and indicated in the plot. Log-rank P<0.05 was considered to indicate a statistically significant difference in the survival curve.
Results
Identification of DMGs in glioma
After the analysis of GSE28094, a total of 349 DMGs were obtained between glioma and normal brain tissue, including 167 upregulated (hypermethylated) and 182 downregulated (hypomethylated) genes (Table SI).
GO functional enrichment analysis
In order to further understand the underlying mechanism of these DMGs in glioma, all DMGs were imported to the DAVID software. GO function results showed that upregulated DMGs were particularly enriched in BP, including positive and negative regulation of ‘cell proliferation’, ‘positive regulation of transcription from RNA polymerase II promoter’, ‘protein autophosphorylation’ and ‘regulation of apoptosis’ (Table I). As for CC, the DMGs were enriched in ‘cytoplasm’, ‘plasma membrane’, ‘nucleoplasm’, ‘perinuclear region of cytoplasm’ and ‘extracellular region’ (Table I). In addition, MF was enriched predominantly in ‘protein binding’, ‘transcription factor binding’, ‘protein heterodimerization activity’, ‘receptor binding’ and ‘ATP binding’ (Table I).
Table I.
GO analysis of differentially methylated genes associated with glioma.
| A, Hypermethylation | ||||
|---|---|---|---|---|
| GO analysis | Term | Count | % | P-value |
| GOTERM_BP_DIRECT | GO:0008285~negative regulation of cell proliferation | 23 | 14.7436 | 1.43×10−11 |
| GOTERM_BP_DIRECT | GO:0008284~positive regulation of cell proliferation | 24 | 15.3846 | 5.14×10−11 |
| GOTERM_BP_DIRECT | GO:0045944~positive regulation of transcription from RNA polymerase II promoter | 34 | 21.7949 | 6.75×10−11 |
| GOTERM_BP_DIRECT | GO:0046777~protein autophosphorylation | 15 | 9.6154 | 6.80×10−10 |
| GOTERM_BP_DIRECT | GO:0042981~regulation of apoptotic process | 16 | 10.2564 | 1.23×10−9 |
| GOTERM_CC_DIRECT | GO:0005737~cytoplasm | 79 | 50.6410 | 6.58×10−9 |
| GOTERM_CC_DIRECT | GO:0005886~plasma membrane | 61 | 39.1026 | 3.46×10−6 |
| GOTERM_CC_DIRECT | GO:0005654~nucleoplasm | 45 | 28.8462 | 1.77×10−5 |
| GOTERM_CC_DIRECT | GO:0048471~perinuclear region of cytoplasm | 18 | 11.5385 | 2.05×10−5 |
| GOTERM_CC_DIRECT | GO:0005576~extracellular region | 30 | 19.2308 | 7.41×10−5 |
| GOTERM_MF_DIRECT | GO:0005515~protein binding | 121 | 77.5641 | 4.77×10−12 |
| GOTERM_MF_DIRECT | GO:0008134~transcription factor binding | 16 | 10.2564 | 4.30×10−8 |
| GOTERM_MF_DIRECT | GO:0046982~protein heterodimerization activity | 18 | 11.5385 | 1.01×10−6 |
| GOTERM_MF_DIRECT | GO:0005102~receptor binding | 15 | 9.6154 | 3.80×10−6 |
| GOTERM_MF_DIRECT | GO:0005524~ATP binding | 32 | 20.5128 | 8.54×10−6 |
| B, Hypomethylation | ||||
| GO analysis | Term | Count | % | P-value |
| GOTERM_BP_DIRECT | GO:0006955~immune response | 32 | 18.4971 | 1.89×10−18 |
| GOTERM_BP_DIRECT | GO:0071222~cellular response to lipopolysaccharide | 15 | 8.6705 | 6.78×10−12 |
| GOTERM_BP_DIRECT | GO:0018108~peptidyl-tyrosine phosphorylation | 16 | 9.2486 | 3.60×10−11 |
| GOTERM_BP_DIRECT | GO:0006954~inflammatory response | 22 | 12.7168 | 2.12×10−10 |
| GOTERM_BP_DIRECT | GO:0022617~extracellular matrix disassembly | 10 | 5.7804 | 6.50×10−8 |
| GOTERM_CC_DIRECT | GO:0005615~extracellular space | 56 | 32.3699 | 3.22×10−22 |
| GOTERM_CC_DIRECT | GO:0005576~extracellular region | 55 | 31.7919 | 6.24×10−18 |
| GOTERM_CC_DIRECT | GO:0009986~cell surface | 22 | 12.7168 | 2.62×10−8 |
| GOTERM_CC_DIRECT | GO:0005887~integral component of plasma membrane | 35 | 20.2312 | 1.66×10−7 |
| GOTERM_CC_DIRECT | GO:0005886~plasma membrane | 67 | 38.7283 | 6.33×10−7 |
| GOTERM_MF_DIRECT | GO:0005125~cytokine activity | 20 | 11.5607 | 1.17×10−14 |
| GOTERM_MF_DIRECT | GO:0004713~protein tyrosine kinase activity | 17 | 9.8266 | 2.94×10−13 |
| GOTERM_MF_DIRECT | GO:0008083~growth factor activity | 15 | 8.6705 | 8.36×10−10 |
| GOTERM_MF_DIRECT | GO:0046934~phosphatidylinositol-4,5-bisphosphate 3-kinase activity | 9 | 5.2023 | 1.74×10−7 |
| GOTERM_MF_DIRECT | GO:0004715~non-membrane spanning protein tyrosine kinase activity | 8 | 4.6243 | 3.29×10−7 |
GO, Gene Ontology; BP, biological process; CC, cell component; MF, molecular function.
For downregulated DMGs, enriched BP included ‘immune response’, ‘cellular response to lipopolysaccharide’, ‘peptidyl-tyrosine phosphorylation’, ‘inflammatory response’ and ‘extracellular matrix disassembly’ (Table I). CC analysis revealed enrichment in ‘extracellular space and region’, ‘cell surface’, ‘integral component of plasma membrane’ and ‘plasma membrane’ (Table I). In addition, MF showed enrichment in ‘cytokine activity’, ‘protein tyrosine kinase activity’, ‘growth factor activity’, ‘phosphatidylinositol-4,5-bisphosphate 3-kinase activity’ and ‘non-membrane spanning protein tyrosine kinase activity’ (Table I). This information suggested that DMGs may serve a crucial function in the tumor immune microenvironment in glioma.
Pathway functional enrichment analysis
KEGG pathway enrichment analysis identified that upregulated DMGs were significantly enriched in pathways in ‘cancer’, ‘signaling pathways regulating pluripotency of stem cells’, ‘PI3K-AKT signaling pathway’, ‘focal adhesion’ and ‘melanoma’. However, downregulated DMGs exhibited enrichment in the pathways of ‘cytokine-cytokine receptor interaction’, ‘type I diabetes mellitus’, ‘graft-vs.-host disease’, ‘rheumatoid arthritis’ and ‘TNF signaling pathway’. These screened pathways suggested that DMGs may have a vital function in the etiology and pathogenesis of glioma (Table II).
Table II.
KEGG pathway analysis of differentially methylated genes associated with glioma.
| A, Hypermethylation | ||||
|---|---|---|---|---|
| KEGG analysis | Term | Count | % | P-value |
| KEGG_PATHWAY | hsa05200:Pathways in cancer | 35 | 22.4 | 1.73×10−17 |
| KEGG_PATHWAY | hsa04550:Signaling pathways regulating pluripotency of stem cells | 15 | 9.6 | 2.32×10−8 |
| KEGG_PATHWAY | hsa04151:PI3K-AKT signaling pathway | 21 | 13.5 | 2.17×10−7 |
| KEGG_PATHWAY | hsa04510:Focal adhesion | 16 | 10.3 | 4.91×10−7 |
| KEGG_PATHWAY | hsa05218:Melanoma | 10 | 6.4 | 1.17×10−6 |
| B, Hypomethylation | ||||
| KEGG analysis | Term | Count | % | P-value |
| KEGG_PATHWAY | hsa04060:Cytokine-cytokine receptor interaction | 25 | 14.5 | 1.68×10−12 |
| KEGG_PATHWAY | hsa04940:Type I diabetes mellitus | 12 | 6.9 | 6.46×10−11 |
| KEGG_PATHWAY | hsa05332:Graft-vs.-host disease | 11 | 6.4 | 9.97×10−11 |
| KEGG_PATHWAY | hsa05323:Rheumatoid arthritis | 15 | 8.7 | 1.94×10−10 |
| KEGG_PATHWAY | hsa04668:TNF signaling pathway | 15 | 8.7 | 2.79×10−9 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; hsa, Homo sapiens.
PPI network construction and module analysis
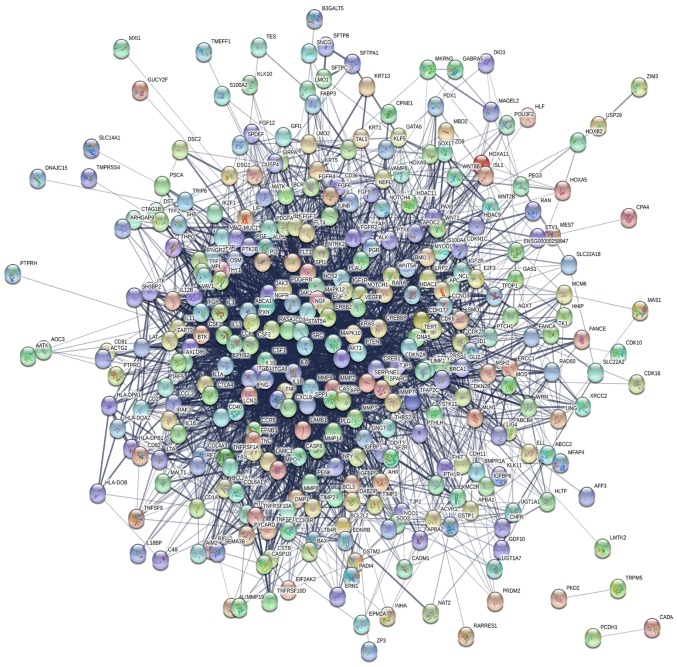
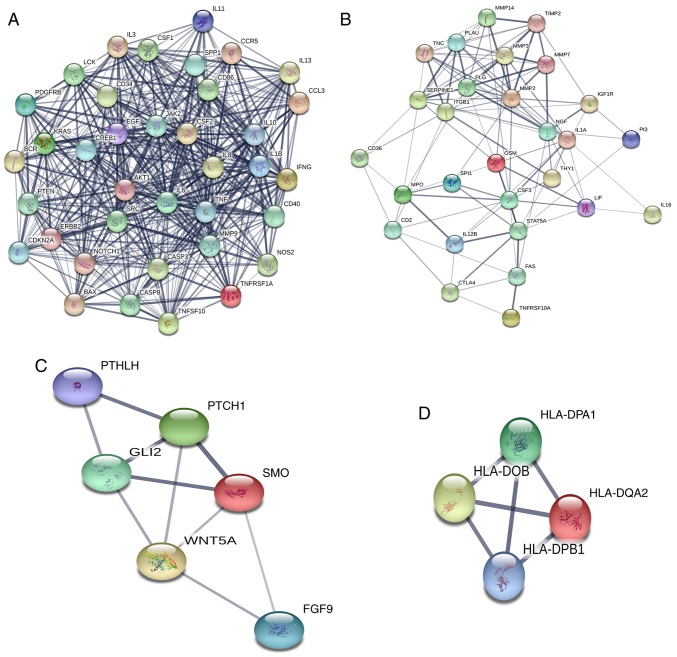
Based on the information in the STRING protein query and the data downloaded from public databases, a PPI DMG network was constructed with 349 nodes and 3,796 edges (Fig. 1). To examine the significant modules in this PPI network, MCODE in Cytoscape was performed. Interactions with a score of >0.9 were considered significant. The top four modules with the highest scores in the network were selected (Table III). The four modules were mapped into STRING (Fig. 2), and these four core modules were mainly associated with ‘cytokine-cytokine receptor interaction’, ‘proteoglycans in cancer’, ‘basal cell carcinoma’, ‘Jak-STAT signaling pathway’ and ‘cancer pathway’ by KEGG pathway enrichment analysis (Table IV).
Figure 1.
The PPI network of differentially methylated genes.
Table III.
Modules of the protein-protein interaction networks.
| Category | Score | Nodes | Edges | Genes |
|---|---|---|---|---|
| 1 | 27.056 | 37 | 487 | IL10, IL1B, IFNG, MMP9, CREB1, CASP8, TNFSF10, IL13, LCK, IL8, BAX, AKT1, NOS2, PTEN, SPP1, KRAS, JAK2, TNFRSF1A, TNF, CD34, CD86, PDGFRB, IL11, IL6, CD40, CASP3, EGF, NOTCH1, ERBB2, CCL3, CCR5, BCR, SRC, CSF1, CDKN2A, IL3, CSF2 |
| 2 | 8 | 28 | 108 | TNC, CSF3, ITGB1, IL1A, CD2, FAS, OSM, MPO, TNFRSF10A, PLG, MMP7, STAT5A, THY1, CD36, IGF1R, CTLA4, SERPINE1, SPI1, PLAU, LIF, MMP14, IL16, NGF, IL12B, MMP3, PI3, MMP2, TIMP2 |
| 3 | 4 | 6 | 10 | SMO, PTCH1, GLI2, WNT5A, PTHLH, FGF9 |
| 4 | 4 | 4 | 6 | HLA-DQA2, HLA-DPA1, HLA-DPB1, HLA-DOB |
Score defined as the product of the complex subgraph, density and the number of vertices in the complex subgraph (24).
Figure 2.
Top four modules for differentially methylated genes were selected. (A) Module 1. (B) Module 2. (C) Module 3. (D) Module 4.
Table IV.
The enriched pathways of modules.
| Module | Term | P-value | FDR | Genes |
|---|---|---|---|---|
| 1 | hsa05152:Tuberculosis | 2.06×10−12 | 2.45×10−9 | AKT1, TNFRSF1A, CASP3, IL6, TNF, BAX, CREB1, CASP8, IFNG, IL1B, JAK2, NOS2, IL10 |
| 1 | hsa05145:Toxoplasmosis | 1.12×10−11 | 1.33×10−8 | AKT1, TNFRSF1A, CASP3, TNF, CCR5, CASP8, IFNG, JAK2, CD40, NOS2, IL10 |
| 1 | hsa05142:Chagas disease (American trypanosomiasis) | 2.27×10−10 | 2.69×10−7 | AKT1, TNFRSF1A, IL6, CCL3, TNF, CASP8, IFNG, IL1B, NOS2, IL10 |
| 1 | hsa04060:Cytokine-cytokine receptor interaction | 1.68×10−9 | 2.00×10−6 | TNFRSF1A, IL6, TNFSF10, CCL3, TNF, CCR5, IFNG, IL1B, IL13, CD40, IL10, IL11 |
| 1 | hsa05161:Hepatitis B | 4.48×10−9 | 5.33×10−6 | AKT1, CASP3, IL6, TNF, KRAS, BAX, CREB1, MMP9, CASP8, PTEN |
| 2 | hsa04060:Cytokine-cytokine receptor interaction | 1.44×10−4 | 1.57×10−1 | OSM, LIF, TNFRSF10A, CSF3, FAS, IL12B, IL1A |
| 2 | hsa05205:Proteoglycans in cancer | 5.35×10−4 | 5.82×10−1 | IGF1R, FAS, IL12B, MMP2, ITGB1, PLAU |
| 2 | hsa05162:Measles | 1.05×10−3 | 1.14 | TNFRSF10A, STAT5A, FAS, IL12B, IL1A |
| 2 | hsa04630:Jak-STAT signaling pathway | 1.45×10−3 | 1.57 | OSM, LIF, CSF3, STAT5A, IL12B |
| 2 | hsa05202:Transcriptional misregulation in cancer | 2.44×10−3 | 2.62 | IGF1R, SPI1, MPO, MMP3, PLAU |
| 3 | hsa05217:Basal cell carcinoma | 1.82×10−6 | 1.29×10−3 | WNT5A, SMO, PTCH1, GLI2 |
| 3 | hsa05200:Pathways in cancer | 1.05×10−5 | 7.46×10−3 | WNT5A, SMO, FGF9, PTCH1, GLI2 |
| 3 | hsa04340:Hedgehog signaling pathway | 8.86×10−5 | 6.29×10−2 | SMO, PTCH1, GLI2 |
| 3 | hsa05205:Proteoglycans in cancer | 4.86×10−3 | 3.40 | WNT5A, SMO, PTCH1 |
| 3 | hsa04390:Hippo signaling pathway | 8.50×10−2 | 46.77 | WNT5A, GLI2 |
| 4 | hsa05310:Asthma | 7.49×10−8 | 5.78×10−5 | HLA-DPA1, HLA-DPB1, HLA-DQA2, HLA-DOB |
| 4 | hsa05332:Graft-vs.-host disease | 1.01×10−7 | 7.77×10−5 | HLA-DPA1, HLA-DPB1, HLA-DQA2, HLA-DOB |
| 4 | hsa05330:Allograft rejection | 1.43×10−7 | 1.11×10−4 | HLA-DPA1, HLA-DPB1, HLA-DQA2, HLA-DOB |
| 4 | hsa04940:Type I diabetes mellitus | 2.12×10−7 | 1.63×10−4 | HLA-DPA1, HLA-DPB1, HLA-DQA2, HLA-DOB |
| 4 | hsa04672:Intestinal immune network for IgA production | 2.99×10−7 | 2.31×10−4 | HLA-DPA1, HLA-DPB1, HLA-DQA2, HLA-DOB |
FDR, false discovery rate; hsa, Homo sapiens.
Validation of the hub genes in TCGA database
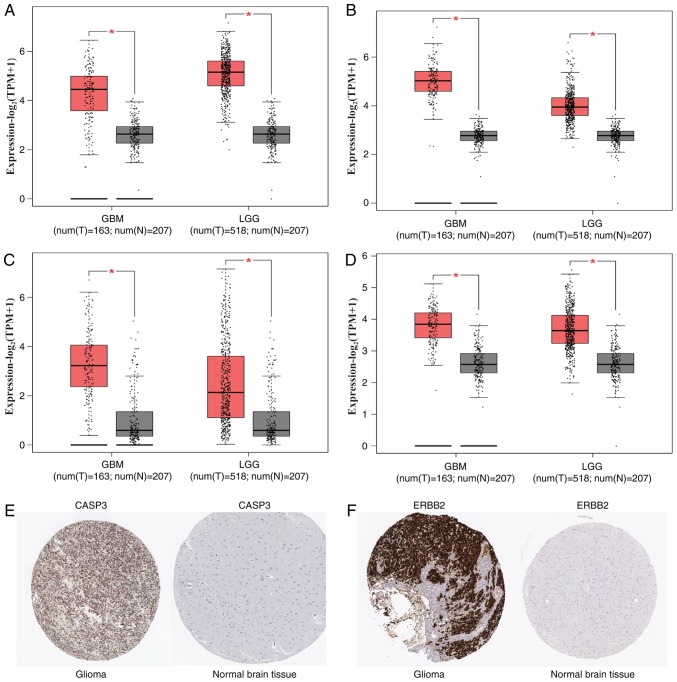
The top 20 DMGs with the highest degree of connectivity were selected as hub genes in the PPI network. The top 20 hub genes were annotated as AKT serine/threonine kinase 1 (AKT1), SRC proto-oncogene (also known as non-receptor tyrosine kinase; SRC), epidermal growth factor (EGF), tumor necrosis factor (TNF), IL6, NOTCH1, interleukin 8 (IL8), MMP9, IL10, CASP3, interleukin 1 β (IL1B), colony stimulating factor 2 (CSF2), phosphatase and tensin homolog (PTEN), ERBB2, CDKN2A, CD34 molecule (CD34), Janus kinase 2 (JAK2), interferon gamma (IFNG), KRAS proto-oncogene (KRAS) and cAMP responsive element binding protein 1 (CREB1). In addition, GEPIA was used to determine the expression levels of hub genes between glioma and normal brain tissue. Compared with that in normal brain tissue, the expression level of the four hub genes, including NOTCH1, CASP3, IL1B and CREB1, was significantly elevated both in LGG and GBM (Fig. 3). Boxplots were used to visualize the expression levels of these four hub genes. Furthermore, the immunohistochemical data in glioma and normal brain tissue based on HPA data showed that the expression of CASP3 and ERBB2 increased in glioma (Fig. 3). In addition, the expression level of hypomethylated genes increased in GBM compared with that in normal brain tissue, including IL6, notch receptor 1 (NOTCH1), matrix metallopeptidase 9 (MMP9), IL10, IL1B and CREB1 (Table V). For the aforementioned hub genes, the significant consistency of methylation and expression status partially confirmed the reliability and stability of the results.
Figure 3.
Expression level of the hub genes in glioma and normal brain tissues. (A-D) Expression level of (A) NOTCH1, (B) CASP3, (C) IL1B and (D) CREB1 in glioma and normal brain tissues. Red indicates glioma, gray indicates normal brain tissue. (E) CASP3 protein was strongly upregulated in glioma tissues compared with normal brain tissues based on the Human Protein Atlas database. (F) ERBB2 protein was strongly upregulated in glioma tissues compared with normal brain tissues based on the Human Protein Atlas database. *P<0.05. GBM, glioblastoma multiforme; LGG, brain lower grade glioma; TPM, transcripts per million. NOTCH1, notch receptor 1; CASP3, caspase 3; IL1B, interleukin 1 β; CREB1, cAMP responsive element binding protein 1; ERBB2, erb-b2 receptor tyrosine kinase 2.
Table V.
Top 20 hub genes with the highest degree of connectivity, and validation of the hub genes in The Cancer Genome Atlas database.
| Gene | Degree of connectivity | Methylation status | Adjusted P-value | P-value | Expression status |
|---|---|---|---|---|---|
| AKT1 | 121 | Hypermethylation | 1.09×10−5 | 4.71×10−7 | Upregulation |
| SRC | 112 | Hypomethylation | 2.08×10−3 | 2.27×10−4 | No change |
| EGF | 112 | Hypomethylation | 1.35×10−2 | 2.54×10−3 | No change |
| TNF | 110 | Hypomethylation | 4.62×10−2 | 1.43×10−2 | No change |
| IL6 | 108 | Hypomethylation | 4.76×10−2 | 1.47×10−2 | Upregulation |
| NOTCH1 | 88 | Hypomethylation | 5.76×10−13 | 4.59×10−15 | Upregulation |
| IL8 | 81 | Hypomethylation | 1.30 ×10−3 | 1.26×10−4 | No change |
| MMP9 | 77 | Hypomethylation | 2.64×10−2 | 6.41×10−3 | Upregulation |
| IL10 | 76 | Hypomethylation | 4.29×10−2 | 1.31×10−2 | Upregulation |
| CASP3 | 75 | Hypermethylation | 2.70×10−2 | 6.66×10−3 | Upregulation |
| IL1B | 74 | Hypomethylation | 1.43×10−2 | 2.78×10−3 | Upregulation |
| CSF2 | 73 | Hypomethylation | 3.50×10−2 | 9.68×10−3 | No change |
| PTEN | 72 | Hypermethylation | 1.90×10−2 | 4.13×10−3 | No change |
| ERBB2 | 70 | Hypermethylation | 1.18×10−2 | 2.10×10−3 | Upregulation |
| CDKN2A | 67 | Hypermethylation | 3.58×10−2 | 1.00×10−2 | Upregulation |
| CD34 | 66 | Hypermethylation | 8.65×10−4 | 7.93×10−5 | No change |
| JAK2 | 63 | Hypermethylation | 2.23×10−5 | 1.05×10−6 | No change |
| IFNG | 63 | Hypomethylation | 3.77×10−2 | 1.07×10−2 | No change |
| KRAS | 59 | Hypermethylation | 9.14×10−3 | 1.49×10−3 | No change |
| CREB1 | 58 | Hypomethylation | 1.53×10−5 | 7.03×10−7 | Upregulation |
Kaplan-Meier plots and expression level of hub DMGs
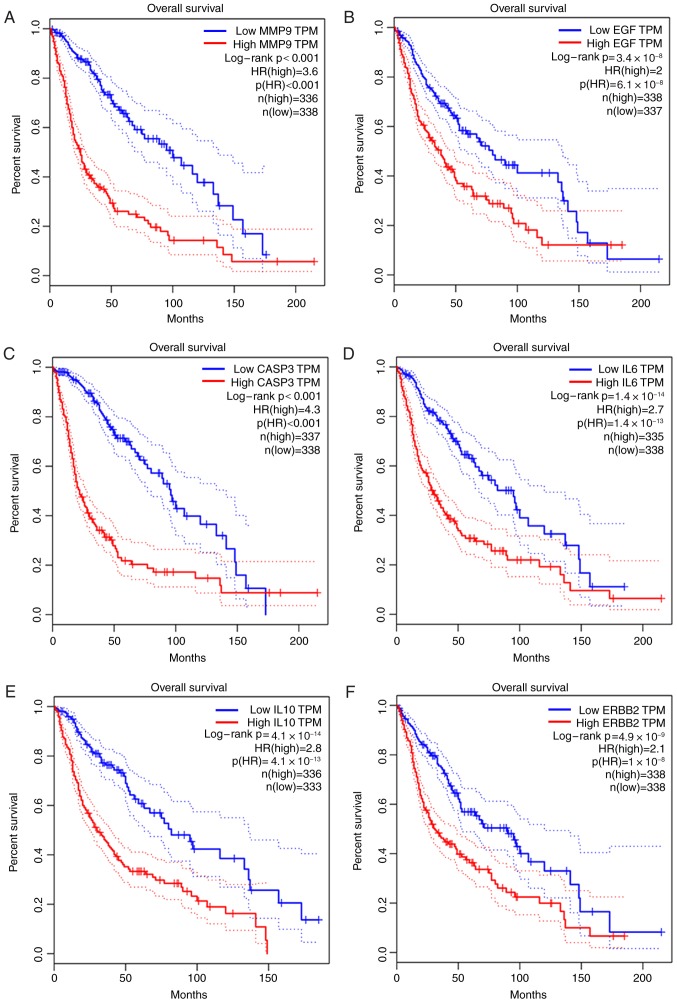
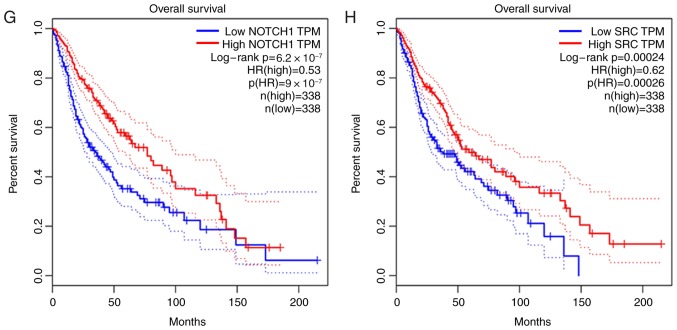
The prognostic data of the 20 hub DMGs were obtained from GEPIA. The results indicated that overexpression of MMP9 (HR, 3.6; P<0.001), EGF (HR, 2.0; P=6.1×10−8), CASP3 (HR, 4.3; P<0.001), IL6 (HR, 2.7; P=1.4×10−13), IL10 (HR, 2.8; P=4.1×10−13) and ERBB2 (HR, 2.1; P=1×10−8) was significantly associated with shorter OS time. By contrast, the high expression of NOTCH1 (HR, 0.53; P=9×10−7) and SRC (HR, 0.62; P=0.00026) was associated with longer OS time in glioma (Fig. 4).
Figure 4.
Prognostic value of eight differentially methylated genes in patients with glioma. (A-F) High expression of (A) MMP9, (B) EGF, (C) CASP3, (D) IL6, (E) IL10 and (F) ERBB2 was significantly associated with poor prognosis in glioma patients. (G and H) High expression of (G) NOTCH1 and (H) SRC was associated with improved prognosis in glioma patients. HR, hazard ratio; TPM, Transcripts per million; MMP9, matrix metallopeptidase 9; EGF, epidermal growth factor; CASP3, caspase 3; IL6, interleukin 6; IL10, interleukin 10; ERBB2, erb-b2 receptor tyrosine kinase 2; NOTCH1, notch receptor 1; SRC, SRC proto-oncogene.
Discussion
Glioma is the most common malignant tumor of the CNS (26). Despite drastic treatment, including neurosurgical resection after which radiotherapy in combination with temozolomide is used, a poor prognosis and high recurrence rate remains (27). Genetic and epigenetic alterations play a vital role in cellular transformation and therapy resistance (28). Therefore, potential specific molecular biomarkers and core therapeutic targets require further investigation. Global genomic hypomethylation and promoter hypermethylation of key genes is a hallmark of various types of cancer, such as lymphoma, breast and ovarian cancer (29). DNA methylation can alter the expression level of genes, which can further influence the proliferation and migration of tumor cells (30). Hypomethylation has been verified to result in chromosome instability by activating the transcription of the commonly silenced transposons, similar to repetitive sequences (31). Aberrant gene promoter region hypermethylation of certain tumor suppressor genes, such as MLH1, MGMT, and CDKN2A genes, can lead to epigenetic silencing of such gene expression, which is associated with vital biological processes, including DNA repair and cell cycle control (32). Therefore, in order to gain a further understanding of the underlying mechanisms that affect the incidence and development of glioma, epigenetic alterations require further investigation. Bioinformatics analysis provides objective data for this purpose. In the present study, the GSE28094 dataset, containing 90 gliomas and 6 normal brain tissues, was extracted from GEO as a discovery set. Numerous bioinformatics tools detected a total of 349 DMGs, including 167 hypermethylated genes and 182 hypomethylated genes, which may be associated with the tumorigenicity and progression of glioma.
As revealed by GO analysis, hypomethylated genes in glioma were enriched in BPs of ‘immune response’, ‘cellular response to lipopolysaccharide’, ‘peptidyl-tyrosine phosphorylation’, ‘inflammatory response’ and ‘extracellular matrix (ECM) disassembly’. MF indicated enrichment in ‘cytokine activity’, ‘protein tyrosine kinase activity’, ‘growth factor activity’, ‘phosphatidylinositol-4,5-bisphosphate 3-kinase activity’ and ‘non-membrane spanning protein tyrosine kinase activity’. In addition, for hypermethylated genes in glioma, GO analysis indicated that enriched BPs were positive and negative regulation of ‘cell proliferation’, ‘positive regulation of transcription from RNA polymerase II promoter’, ‘protein autophosphorylation’ and ‘regulation of apoptotic process’. MF enrichment revealed ‘protein binding’, ‘transcription factor binding’, ‘protein heterodimerization activity’, ‘receptor binding’ and ‘ATP binding’. These results suggested that dysregulation of cell proliferation and apoptosis may serve an important role in the occurrence and development of tumors, which was in agreement with previous studies (33,34). As a major component of tumor local microenvironment, the ECM affects tumor progression by promoting cell transformation and metastasis (35). Notably, ECM anomalies also lead to stromal cell deregulation and facilitate tumor-associated angiogenesis and inflammation, which comprise the tumorigenic microenvironment (35). It has been reported that active but dysfunctional immune responses are associated with cancer (36). Clinical data have indicated that immune stimulation and immune suppression simultaneously occur in patients with cancer, with increased concentrations of TNFα, interleukin (IL)6, IL8 and IL10 (37), which is in accordance with the results of the present study. Therefore, tumor-promoting immune disorder may serve a pivotal role in glioma oncogenesis, suggesting that cancer immunotherapy is likely to become a key part of the clinical management of glioma.
KEGG analysis revealed that DMGs were significantly enriched in pathways including ‘cancer’, ‘signaling pathways regulating pluripotency of stem cells’, ‘PI3K-AKT signaling pathway’, ‘focal adhesion’ and ‘melanoma’. Activated AKT modulates the function of numerous substrates, such as mTOR, glycogen synthase kinase 3 β or forkhead box protein O1, which are involved in the regulation of cell metabolism, proliferation, cell cycle progression and survival (38). In recent years, the PI3K/AKT signaling pathway has been associated with tumorigenesis and development of GBM (39). Pluripotent mouse embryonic stem cells can be expanded in large numbers in vitro by symmetrical self-renewal (40). Self-renewal entails proliferation with a concomitant suppression of differentiation, which is responsible for the occurrence and progression of cancer (40). Furthermore, differentially methylated regions in induced pluripotent stem cells, derived by epigenetic reprogramming, are significantly enriched in specific tissues and cancer, demonstrating that hypermethylation and hypomethylation of cytosine-phosphate-guanine islands are broadly involved in tissue differentiation, epigenetic reprogramming and cancer (41).
In addition, focal adhesion is associated with tumor cell migration and invasion (42). A previous study have shown that the expression and activity of focal adhesion kinase, an intracellular non-receptor tyrosine kinase, is frequently associated with cell adhesion, migration, survival, proliferation, differentiation and angiogenesis in cancer (43). These findings demonstrated the reliability of the present results, indicating the critical role of the PI3K/AKT signaling pathway, pluripotency of stem cells and focal adhesion kinase in the tumorigenicity of glioma, providing a promising therapeutic target for glioma.
The PPI network for DMGs uncovered the overview of their functional connections. The top 20 hub genes were also selected, in which five DMGs with a high degree of connectivity were identified: AKT1, SRC, EGF, TNF and IL6 (gene count >100). Furthermore, TCGA database analysis was used to validate the top 20 hub genes. The results revealed that the hypomethylation of IL6, NOTCH1, MMP9, IL10, IL1B and CREB1 was associated with high expression levels in glioma. Moreover, immunochemistry images of these hub genes were obtained from HPA. Compared with that of the other eight hub genes (AKT1, IL6, NOTCH1, MMP9, IL10, IL1B, CDKN2A and CREB1), the expression intensity and quantity of CASP3 and ERBB2 were higher in glioma. Therefore, the immunochemistry images of CASP3 and ERBB2 were selected to visualize their differential expression level in glioma vs. normal brain tissue. It was revealed that eight genes were associated with survival, which were either protective or risky, based on HR. NOTCH1 and SRC were defined as protective with HR<1, whereas IL6, MMP9, IL10, CASP3, ERBB2 and EGF were defined as risky with HR>1. The aforementioned genes identified in the present study may serve as valuable prognostic biomarkers in glioma.
In previous studies, a number of these hub genes have already been examined as promising predictors of glioma. For instance, ILs, including IL6 and IL10, two important Th2-associated cytokines, are often co-expressed in glioma and further participate in malignancy progression and lead to an unfavorable prognosis (44,45). MMP9 contributes to the degradation of ECM, angiogenesis and the invasiveness of glioma (46). AKT1 kinase is a crucial member of activated proliferation and survival pathways in cancer, which has the ability to suppress apoptosis, deregulate the cell cycle and alter the angiogenic potential (47). Increased levels of AKT in ~80% of all GBM cases suggested that activation of the AKT pathway was strongly implicated in the development of GBM (48). Epidermal growth factor receptor (EGFR) is activated by binding of its extracellular ligand, EGF, resulting in enhanced cell proliferation, growth and survival (49). A series of signaling pathway cascades activated by EGF/EGFR are associated with GBM progression, including activation of K-RAS and AKT signaling and mammalian target of rapamycin, together with PI3K pathways (50). Accumulating evidence shows that NOTCH1 is upregulated in classical and proneural subtypes of GBM, and is associated with tumor grade (51). NOTCH1 inhibition suppresses the biological behavior of metastasis, invasion and epithelial-mesenchymal transition (52). SRC is one of several oncogenic tyrosine kinases, which is activated in various types of cancer to influence survival and metastasis, such as lung cancer, breast cancer and melanoma (53). The SRC-linked STAT3/MMP pathway is responsible for glioma stem cell (GSC) maintenance and growth (54); therefore, the inhibition of the SRC/STAT3 pathway may be a novel therapeutic target in glioma. The CREB1 gene plays a crucial role in the development and progression in various types of cancer, such as colon cancer, ovarian cancer and bladder cancer (55). Overexpression of CREB1 is significantly associated with advanced WHO grade, Karnofsky Performance Scale and short survival time of patients with glioma (56). Furthermore, inhibition of CREB1 activation can prevent GSC self-renewal and suppress neoplastic growth (57). The ERBB2 proto-oncogene (also known as HER2) is one of the most useful molecules for classification and prognosis in cancer, and its activation and downstream signals are highly dependent on dimerization with EGFR (58). ERBB2, in combination with survivin and CD99 molecule, has the ability to form branched multipeptides, which stably bind with T2 cells to exhibit 40–60 and 60–80% cytotoxic activity against the U251 GBM and primary cells, respectively; therefore, they are promising candidates for immunotherapeutic GBM treatment (59). Caspases are well-renowned proteases that play a central role in initiating and executing cell apoptosis (60). A number of human cancer cell lines, such as MCF7, HCC1500, CRL1500, MDA-MB-231, A549, DU145, HepG2, HeLa and HGC27, upregulate ERBB2 expression, which promotes tumor survival via inhibiting the apoptotic activity of CASP3/8 (61). Therefore, apoptosis-associated CASP3 is an important gene in human carcinogenesis. According to previous studies, the underlying mechanism of IFNG in glioma remains unclear, and further research is necessary (62).
In summary, through bioinformatics analysis of glioma microarray gene expression data, a total of 349 DMGs were obtained between glioma and normal brain tissue, including 167 hypermethylated and 182 hypomethylated genes. The results showed that IL6, MMP9, IL10, CASP3, ERBB2 and EGF, which were associated with the tumor immune microenvironment, may act in the progression of malignant glioma. Molecular biological experiments are further required to elucidate the function and mechanism of these identified candidate DMGs in glioma, thereby offering new implications on glioma immune microenvironment and immune-related therapy.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the Resource Sharing Platform Construction of Science and Technology Department of the Autonomous Region (grant no. PT1802) and the Xinjiang Medical University Graduate Innovation Entrepreneurship Start-up Fund (grant no. CXCY2018018).
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Authors' contributions
All authors participated in the preparation of the manuscript. WZ conceived and designed the study. JX, HXG and WS conducted the research. YZ and QW standardized the procedure for sequence search. WLC, ML and MBW contributed to data analysis and revised the manuscript. JX wrote the first draft of the manuscript. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
References
- 1.Hu T, Xi J. Identification of COX5B as a novel biomarker in high-grade glioma patients. Onco Targets Ther. 2017;10:5463–5470. doi: 10.2147/OTT.S139243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song X, Zhang N, Han P, Moon BS, Lai RK, Wang K, Lu W. Circular RNA profile in gliomas revealed by identification tool UROBORUS. Nucleic Acids Res. 2016;44:e87. doi: 10.1093/nar/gkw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 4.Komotar RJ, Otten ML, Gaetan M, Connolly ES., Jr Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma-a critical review. Clin Med Oncol. 2008;2:421–422. doi: 10.4137/cmo.s390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sturm D, Pfister SM, Jones DTW. Pediatric gliomas: Current concepts on diagnosis, biology, and clinical management. J Clin Oncol. 2017;35:2370–2377. doi: 10.1200/JCO.2017.73.0242. [DOI] [PubMed] [Google Scholar]
- 6.Duffau H. Paradoxes of evidence-based medicine in lower-grade glioma: To treat the tumor or the patient? Neurology. 2018;91:657–662. doi: 10.1212/WNL.0000000000006288. [DOI] [PubMed] [Google Scholar]
- 7.Demuth T, Berens ME. Molecular mechanisms of glioma cell migration and invasion. J Neurooncol. 2004;70:217–228. doi: 10.1007/s11060-004-2751-6. [DOI] [PubMed] [Google Scholar]
- 8.Yang AS, Estecio MR, Garcia-Manero G, Kantarjian HM, Issa JP. Comment on ‘Chromosomal instability and tumors promoted by DNA hypomethylation’ and ‘Induction of tumors in nice by genomic hypomethylation’. Science. 2003;302:1153. doi: 10.1126/science.1089523. [DOI] [PubMed] [Google Scholar]
- 9.Manel E. CpG island hypermethylation and tumor suppressor genes: A booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- 10.Feinberg AP, Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun. 1983;111:47–54. doi: 10.1016/S0006-291X(83)80115-6. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Wang M, Wang W, Mo J. Incidence and prognostic value of multiple gene promoter methylations in gliomas. J Neurooncol. 2014;116:349–356. doi: 10.1007/s11060-013-1301-5. [DOI] [PubMed] [Google Scholar]
- 12.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Pu J, Liu J, Chen Y, Li Y, Hou P, Shi B, Yang Q. The prognostic value of DAPK1 hypermethylation in gliomas: A site-specific analysis. Pathol Res Pract. 2018;214:940–948. doi: 10.1016/j.prp.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Arifin MT, Hama S, Kajiwara Y, Sugiyama K, Saito T, Matsuura S, Yamasaki F, Arita K, Kurisu K. Cytoplasmic, but not nuclear, p16 expression may signal poor prognosis in high-grade astrocytomas. J Neurooncol. 2006;77:273–277. doi: 10.1007/s11060-005-9037-5. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T, Katayama Y, Yoshino A, Yachi K, Ohta T, Ogino A, Komine C, Fukushima T. Aberrant hypermethylation of p14ARF and O6-methylguanine-DNA methyltransferase genes in astrocytoma progression. Brain Pathol. 2007;17:5–10. doi: 10.1111/j.1750-3639.2006.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darnay BG, Besse A, Poblenz AT, Lamothe B, Jacoby JJ. TRAFs in RANK Signaling. Adv Exp Med Biol. 2007;597:152–159. doi: 10.1007/978-0-387-70630-6_12. [DOI] [PubMed] [Google Scholar]
- 17.Berdasco M, Ropero S, Setien F, Fraga MF, Lapunzina P, Losson R, Alaminos M, Cheung NK, Rahman N, Esteller M. Epigenetic inactivation of the Sotos overgrowth syndrome gene histone methyltransferase NSD1 in human neuroblastoma and glioma. Proc Natl Acad Sci USA. 2009;106:21830–21835. doi: 10.1073/pnas.0906831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Q, Shai O, Lee LJ, Frey BJ, Blencowe BJ. Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nat Genet. 2008;40:1413–1415. doi: 10.1038/ng.259. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez AF, Assenov Y, Martin-Subero JI, Balint B, Siebert R, Taniguchi H, Yamamoto H, Hidalgo M, Tan AC, Galm O, et al. A DNA methylation fingerprint of 1628 human samples. Genome Res. 2012;22:407–419. doi: 10.1101/gr.119867.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis S, Meltzer PS. GEOquery: A bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics. 2007;23:1846–1847. doi: 10.1093/bioinformatics/btm254. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanehisa M, Goto S. KEGG: Kyoto Encyclopaedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ono K, Muetze T, Kolishovski G, Shannon P, Demchak B. CyREST: Turbocharging cytoscape access for external tools via a RESTful API. F1000Res. 2015;4:478. doi: 10.12688/f1000research.6767.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bader GD, Hogue CW. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics. 2003;4:2. doi: 10.1186/1471-2105-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–ii56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander BM, Cloughesy TF. Adult Glioblastoma. J Clin Oncol. 2017;35:2402–2409. doi: 10.1200/JCO.2017.73.0119. [DOI] [PubMed] [Google Scholar]
- 28.Bralten LB, French PJ. Genetic alterations in glioma. Cancers (Basel) 2011;3:1129–1140. doi: 10.3390/cancers3011129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaudet F, Hodgson JG, Eden A, Jackson-Grusby L, Dausman J, Gray JW, Leonhardt H, Jaenisch R. Induction of tumors in mice by genomic hypomethylation. Science. 2003;300:489–492. doi: 10.1126/science.1083558. [DOI] [PubMed] [Google Scholar]
- 30.Győrffy B, Bottai G, Fleischer T, Munkácsy G, Budczies J, Paladini L, Børresen-Dale AL, Kristensen VN, Santarpia L. Aberrant DNA methylation impacts gene expression and prognosis in breast cancer subtypes. Int J Cancer. 2016;38:87–97. doi: 10.1002/ijc.29684. [DOI] [PubMed] [Google Scholar]
- 31.Dai D, Zhou B, Xu W, Jin H, Wang X. CHFR promoter hypermethylation is associated with gastric cancer and plays a protective role in gastric cancer process. J Cancer. 2019;10:949–956. doi: 10.7150/jca.27224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang ZL, Zhang CB, Cai JQ, Li QB, Wang Z, Jiang T. Integrated analysis of genome-wide DNA methylation, gene expression and protein expression profiles in molecular subtypes of WHO II–IV gliomas. J Exp Clin Cancer Res. 2015;34:127. doi: 10.1186/s13046-015-0249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: A link between cancer genetics and chemotherapy. Cell. 2002;108:153–164. doi: 10.1016/S0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 34.Schlabach MR, Luo J, Solimini NL, Hu G, Xu Q, Li MZ, Zhao Z, Smogorzewska A, Sowa ME, Ang XL, et al. Cancer proliferation gene discovery through functional genomics. Science. 2008;319:620–624. doi: 10.1126/science.1149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu P, Weaver VM, Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Lippitz BE. Cytokine patterns in patients with cancer: A systematic review. Lancet Oncol. 2013;14:e218–e228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 38.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Majewska E, Szeliga M. AKT/GSK3β signaling in glioblastoma. Neurochem Res. 2017;42:918–924. doi: 10.1007/s11064-016-2044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/S0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 41.Doi A, Park IH, Wen B, Murakami P, Aryee MJ, Irizarry R, Herb B, Ladd-Acosta C, Rho J, Loewer S, et al. Differential methylation of tissue- and cancer-specific CpG island shores distinguishes human induced pluripotent stem cells, embryonic stem cells and fibroblasts. Nat Genet. 2009;41:1350–1353. doi: 10.1038/ng.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sood AK, Coffin JE, Schneider GB, Fletcher MS, DeYoung BR, Gruman LM, Gershenson DM, Schaller MD, Hendrix MJ. Biological significance of focal adhesion kinase in ovarian cancer: Role in migration and invasion. Am J Pathol. 2004;165:1087–1095. doi: 10.1016/S0002-9440(10)63370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo M, Guan JL. Focal adhesion kinase: A prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett. 2010;289:127–139. doi: 10.1016/j.canlet.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao F, Zhang Q, Chen W, Han C, He Y, Ran Q, Yao S. IL-6 increases SDCBP expression, cell proliferation, and cell invasion by activating JAK2/STAT3 in human glioma cells. Am J Transl Res. 2017;9:4617–4626. [PMC free article] [PubMed] [Google Scholar]
- 45.Samaras V, Piperi C, Korkolopoulou P, Zisakis A, Levidou G, Themistocleous MS, Boviatsis EI, Sakas DE, Lea RW, Kalofoutis A, Patsouris E. Application of the ELISPOT method for comparative analysis of interleukin (IL)-6 and IL-10 secretion in peripheral blood of patients with astroglial tumors. Mol Cell Biochem. 2007;304:343–351. doi: 10.1007/s11010-007-9517-3. [DOI] [PubMed] [Google Scholar]
- 46.Xue Q, Cao L, Chen XY, Zhao J, Gao L, Li SZ, Fei Z. High expression of MMP9 in glioma affects cell proliferation and is associated with patient survival rates. Oncol Lett. 2017;13:1325–1330. doi: 10.3892/ol.2017.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 48.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 49.Kjær IM, Olsen DA, Alnor A, Brandslund I, Bechmann T, Madsen JS. EGFR and EGFR ligands in serum in healthy women; reference intervals and age dependency. Clin Chem Lab Med. 2019 Jul 16; doi: 10.1515/cclm-2019-0376. doi: 10.1515/cclm-2019-0376 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 50.Thorne AH, Zanca C, Furnari F. Epidermal growth factor receptor targeting and challenges in glioblastoma. Neuro Oncol. 2016;18:914–918. doi: 10.1093/neuonc/nov319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hai L, Zhang C, Li T, Zhou X, Liu B, Li S, Zhu M, Lin Y, Yu S, Zhang K, et al. Notch1 is a prognostic factor that is distinctly activated in the classical and proneural subtype of glioblastoma and that promotes glioma cell survival via the NF-κB(p65) pathway. Cell Death Dis. 2018;9:158. doi: 10.1038/s41419-017-0119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarkar S, Mirzaei R, Zemp FJ, Wei W, Senger DL, Robbins SM, Yong VW. Activation of NOTCH signaling by Tenascin-C promotes growth of human brain tumor-initiating cells. Cancer Res. 2017;77:3231–3243. doi: 10.1158/0008-5472.CAN-16-2171. [DOI] [PubMed] [Google Scholar]
- 53.Li MY, Peng WH, Wu CH, Chang YM, Lin YL, Chang GD, Wu HC, Chen GC. PTPN3 suppresses lung cancer cell invasiveness by counteracting Src-mediated DAAM1 activation and actin polymerization. Oncogene. 2019 Aug 12; doi: 10.1038/s41388-019-0948-6. doi: 10.1038/s41388-019-0948-6 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 54.Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, MacSwords J, Eyler CE, McLendon RE, Heddleston JM, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong L, Wang Y, Ao Y, Sun X. CREB1 induced lncRNA HAS2-AS1 promotes epithelial ovarian cancer proliferation and invasion via the miR-466/RUNX2 axis. Biomed Pharmacother. 2019;115:108891. doi: 10.1016/j.biopha.2019.108891. [DOI] [PubMed] [Google Scholar]
- 56.Dong H, Cao W, Xue J. Long noncoding FOXD2-AS1 is activated by CREB1 and promotes cell proliferation and metastasis in glioma by sponging miR-185 through targeting AKT1. Biochem Biophys Res Commun. 2019;508:1074–1081. doi: 10.1016/j.bbrc.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 57.Mukherjee S, Tucker-Burden C, Kaissi E, Newsam A, Duggireddy H, Chau M, Zhang C, Diwedi B, Rupji M, Seby S, et al. CDK5 Inhibition resolves PKA/cAMP-independent activation of CREB1 signaling in glioma stem cells. Cell Rep. 2018;23:1651–1664. doi: 10.1016/j.celrep.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brennan PJ, Kumagai T, Berezov A, Murali R, Greene MI. HER2/Neu: Mechanisms of dimerization/oligomerization. Oncogene. 2000;19:6093–6101. doi: 10.1038/sj.onc.1203967. [DOI] [PubMed] [Google Scholar]
- 59.Kim YH, Tran TA, Lee HJ, Jung SI, Lee JJ, Jang WY, Moon KS, Kim IY, Jung S, Jung TY. Branched multipeptide immunotherapy for glioblastoma using human leukocyte antigen-A*0201-restricted cytotoxic T-lymphocyte epitopes from ERBB2, BIRC5 and CD99. Oncotarget. 2016;7:50535–50547. doi: 10.18632/oncotarget.10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bagnjuk K, Kast VJ, Tiefenbacher A, Kaseder M, Yanase T, Burges A, Kunz L, Mayr D, Mayerhofer A. Inhibitor of apoptosis proteins are potential targets for treatment of granulosa cell tumors-implications from studies in KGN. J Ovarian Res. 2019;12:76. doi: 10.1186/s13048-019-0549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arman K, Ergün S, Temiz E, Öztuzcu S. The interrelationship between HER2 and CASP3/8 with apoptosis in different cancer cell lines. Mol Biol Rep. 2014;41:8031–8036. doi: 10.1007/s11033-014-3700-x. [DOI] [PubMed] [Google Scholar]
- 62.Michaelsen SR, Urup T, Olsen LR, Broholm H, Lassen U, Poulsen HS. Molecular profiling of short-term and long-term surviving patients identifies CD34 mRNA level as prognostic for glioblastoma survival. J Neurooncol. 2018;137:533–542. doi: 10.1007/s11060-017-2739-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.