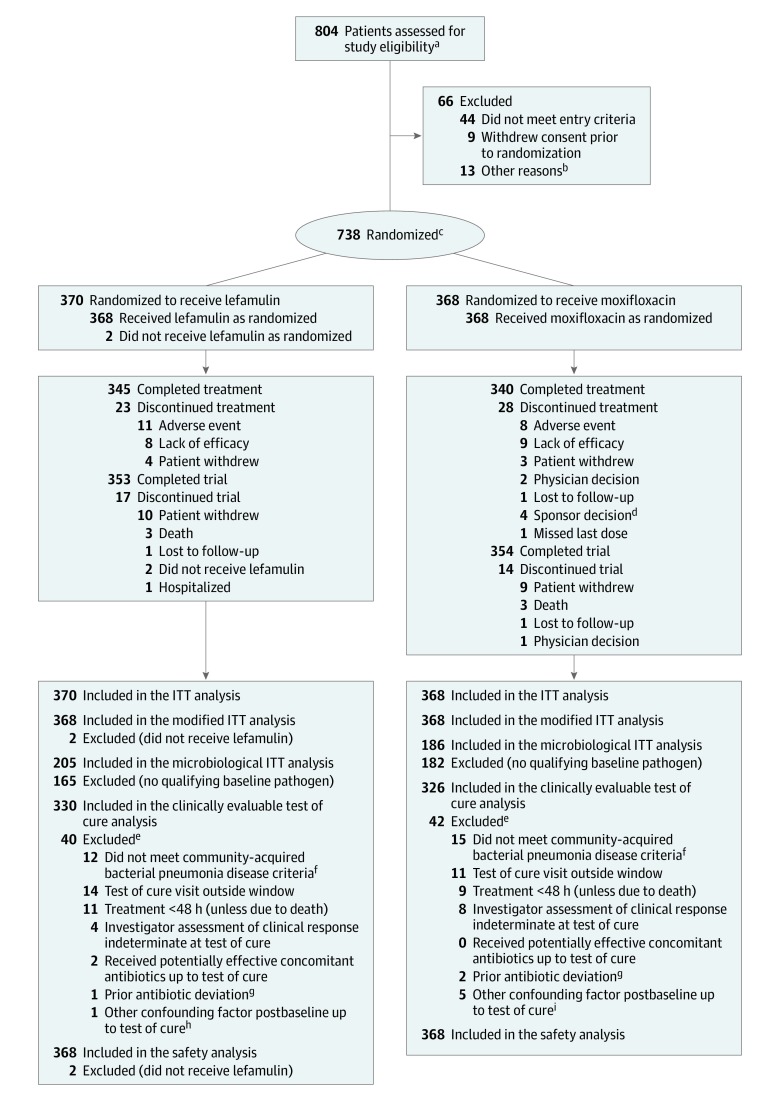
Figure. Patient Recruitment, Randomization, and Follow-up in the LEAP 2 Trial.
ITT indicates intent to treat; LEAP 2, Lefamulin Evaluation Against Pneumonia 2.
aThe number of patients with a Pneumonia Outcomes Research Team (PORT) risk class of II was capped at 50% of the total population; once this cap was reached, these patients were no longer assessed for study eligibility. All patients assessed for study eligibility provided informed consent.
bOne patient did not attend the randomization visit, 1 patient did not receive study drug due to insufficient drug stock at site, 1 patient with PORT risk class II was assessed after the per-protocol cap had been met, 1 patient had prolonged QTcF at the screening visit, 2 patients were excluded by the investigator, 6 patients were excluded due to randomization error, and 1 patient was excluded for an unknown reason.
cThe number of randomized patients who received a single dose of a short-acting antibiotic was capped at 25% of the total population; this cap was not reached and therefore this criterion had no effect on randomization.
dPatients were discontinued from treatment because they met per protocol exclusion or study withdrawal criteria: 1 patient due to baseline and postbaseline QTcF greater than 500 ms, 2 patients due to confirmed Staphylococcus aureus bacteremia, and 1 patient due to a complete left bundle branch block.
eMay have met more than 1 exclusion criterion.
fAs defined by inclusion criteria 3 through 7 and exclusion criteria 3, 5, and 6 in Supplement 1.
gAs defined by exclusion criterion 1 in Supplement 1.
hPatient had a lung abscess diagnosed by computed tomography within hours after randomization.
iOne patient was diagnosed with tuberculosis on study day 1; 1 patient was diagnosed with small cell lung cancer on study day 4; 1 patient was diagnosed with squamous cell carcinoma of the lung on study day 5; 1 patient was diagnosed with tuberculosis effusion on study day 24; and 1 patient was ultimately diagnosed with tuberculosis.