Abstract
Neurological soft signs (NSS) comprise a broad range of subtle neurological deficits and are considered to represent external markers of sensorimotor dysfunction frequently found in mental disorders of presumed neurodevelopmental origin. Although NSS frequently occur in schizophrenia spectrum disorders (SSD), specific patterns of co‐altered brain structure and function underlying NSS in SSD have not been investigated so far. It is unclear whether gray matter volume (GMV) alterations or aberrant brain activity or a combination of both, are associated with NSS in SSD. Here, 37 right‐handed SSD patients and 37 matched healthy controls underwent motor assessment and magnetic resonance imaging (MRI) at 3 T. NSS were examined on the Heidelberg NSS scale. We used a multivariate data fusion technique for multimodal MRI data—multiset canonical correlation and joint independent component analysis (mCCA + jICA)—to investigate co‐altered patterns of GMV and intrinsic neural fluctuations (INF) in SSD patients exhibiting NSS. The mCCA + jICA model indicated two joint group‐discriminating components (temporoparietal/cortical sensorimotor and frontocerebellar/frontoparietal networks) and one modality‐specific group‐discriminating component (p < .05, FDR corrected). NSS motor score was associated with joint frontocerebellar/frontoparietal networks in SSD patients. This study highlights complex neural pathomechanisms underlying NSS in SSD suggesting aberrant structure and function, predominantly in cortical and cerebellar systems that critically subserve sensorimotor dynamics and psychomotor organization.
Keywords: gray matter volume, mCCA + jICA, motor abnormalities, neurological soft signs, resting‐state functional MRI, schizophrenia spectrum disorders
1. INTRODUCTION
In addition to severe sensorimotor symptoms such as catatonia, akathisia, and parkinsonism, patients with schizophrenia spectrum disorders (SSD) also exhibit subtle and clinically inconspicuous sensorimotor abnormalities (Hirjak et al., 2019; Hirjak, Kubera, Thomann, & Wolf, 2018; Hirjak, Meyer‐Lindenberg, Kubera, Thomann, & Wolf, 2018). These phenomena are referred to as neurological soft signs (NSS), which are subtle neurological abnormalities in the execution of motor or sensory tasks (Schroder et al., 1991; Schroder et al., 1998). Recent studies have shown that NSS are present in about 60–70% of SSD patients and show familial accumulation (Hirjak, Kubera, et al., 2018; Walther & Strik, 2012). NSS of nonaffected twins of monozygotic twin pairs discordant for SSD were significantly higher than those of healthy twins whereas the affected discordant twins showed higher NSS than their unaffected co‐twins (Niethammer et al., 2000). Both structural and functional magnetic resonance imaging (MRI) have been used to investigate the neural underpinnings of NSS in SSD patients. NSS‐related alterations in cortical structures such as the primary motor cortex (M1) and postcentral gyrus, premotor area, temporal and lingual gyri, middle and inferior frontal gyri, inferior parietal lobule, insula, precuneus, and occipital gyrus have been identified (Hirjak et al., 2013a; Zhao et al., 2014). Other studies showed that NSS are as well related to alterations within subcortical structures such as caudate nucleus (Dazzan et al., 2004; Hirjak et al., 2012; Janssen et al., 2009; Thomann et al., 2009), putamen (Dazzan et al., 2004; Kasparek et al., 2009; Venkatasubramanian, Jayakumar, Gangadhar, & Keshavan, 2008), globus pallidus (Dazzan et al., 2004; Hirjak et al., 2012), thalamus (Dazzan et al., 2004; Janssen et al., 2009; Thomann, Wustenberg, et al., 2009), cerebellum (Bottmer et al., 2005; Hirjak et al., 2015; Mouchet‐Mages et al., 2007; Thomann et al., 2009; Venkatasubramanian et al., 2008), and brainstem (Hirjak et al., 2013b; Fritze et al., 2019). Yet, although both abnormal brain structure and function can contribute to NSS symptom expression, it is unclear whether NSS are related to co‐altered patterns of brain structure and function, or whether gray matter volume (GMV) and intrinsic neural fluctuations (INF) are unique and different aspects associated with NSS. With the aim to examine the interaction between structural and functional abnormalities previously postulated in the pathogenesis of NSS in SSD, we applied multivariate data fusion techniques to structural and functional MRI data, using multiset canonical correlation analysis and joint independent component analysis (mCCA + jICA; He et al., 2017; Sugranyes et al., 2012; Sui et al., 2012; Sui et al., 2013). This blind source separation technique incorporates neuroimaging data from multiple modalities for each patient to extract maximally independent components (ICs) spanning across modalities in order to identify patterns of structural changes and local metabolic demands across brain regions that significantly covary across patients (He et al., 2017; Sui et al., 2012; Sui, He, Pearlson, et al., 2013).
We chose to investigate neural correlates of NSS with resting‐state fMRI because functional brain alterations underlying NSS in SSD are intrinsic and therefore they may be better examined without the confounders of a non‐ecological setting (e.g., scanner and paradigm) and the patient's motivational bias. Previous MRI studies have confirmed this notion and successfully used resting‐state fMRI (e.g., regional homogeneity (ReHo) to study NSS in healthy individuals (Hirjak, Thomann, Kubera, Stieltjes, & Wolf, 2016; Thomann, Hirjak, Kubera, Stieltjes, & Wolf, 2015). ReHo is a voxel‐based measure of focal connectivity that evaluates the synchronicity of the time series between a given voxel and each of its 26 neighboring voxels (Zang, Jiang, Lu, He, & Tian, 2004). ReHo is widely used as a proxy for ongoing activity, including spontaneous fluctuations neural signals in the so‐called default mode network (DMN), where ReHo increases at rest and decreases during task completion (Long et al., 2008; Y. Zang et al., 2004). However, previous studies were not able to reveal whether structural and functional alterations are unique or different aspects associated with NSS. Therefore, we sought to analyze multimodal data jointly and provide an insight into abnormal local function within selective networks underlying NSS in SSD. Furthermore, we chose to investigate intrinsic neural activity (INA), because INA explores the intrinsically (functionally) segregation or specialization of brain regions/networks (Logothetis, 2008; Zhang et al., 2016). For this particular reason, we used fractional amplitude of low‐frequency fluctuations (fALFF), because fALFF captures the relative magnitude of blood oxygen level‐dependent (BOLD) signal changes on INA and might help to identify brain regions/networks with aberrant local functioning (Egorova, Veldsman, Cumming, & Brodtmann, 2017; Hirjak et al., 2019; Kubera et al., 2019). Whereas ReHo measures connectivity between a given voxel and its neighbors in the time domain (Long et al., 2008; Zang et al., 2004), ALFF measures signal variability of a single voxel in the frequency domain (Xu, Zhuo, Qin, Zhu, & Yu, 2015). Furthermore, fALFF is designed to reduce the sensitivity to physiological noise. Nonetheless, both measures are highly correlated (Yuan et al., 2013). Still, one of the major benefits of using fALFF and combining both modalities is getting the information about both GMV and INA as well as increasing the power of data fusion analysis. There are also several recent fMRI studies that have successfully used fusion ICA methods with fALFF in healthy and SSD samples (Di et al., 2013; Hirjak, Rashidi, et al., 2019; Kubera et al., 2019; Lottman et al., 2018; Sui et al., 2013; Xu et al., 2015). Finally, GMV volume changes can cause spatiotemporal changes in INA and these joint network alterations might result in aberrant sensorimotor functioning and the development of NSS in SSD patients (Mittal, Bernard, & Northoff, 2017; Northoff & Duncan, 2016).
In the present study, we conducted a fusion analysis on structural MRI (sMRI) and resting‐state functional (rs‐fMRI) data by applying the mCCA + jICA framework in order to expand the knowledge on abnormalities spanning across multiple dimensions of neural integrity, that is, GMV and INA. This study had two main hypotheses: First, we predicted that there will be a difference in transmodal (i.e., structure and function) ICs comprising frontoparietal and frontostriatal networks between SSD patients and healthy controls (HC). Second, we predicted that both transmodal and modality‐specific ICs in distinct cortico‐subcortical networks (as involved in sensorimotor processing and integration of spatiotemporal information) will show significant relationships with distinct NSS dimensions in SSD patients.
2. METHODS
2.1. Study participants
For the present investigation, we approached and examined a total of 37 (17 females) right‐handed patients satisfying DSM‐IV‐TR (Sass, Wittchen, & Zaudig, 2003) for schizophrenia (n = 34; paranoid type) or schizoaffective disorder (n = 3) and 37 (17 females) HC matched for age, gender, education, and handedness. Patients in this sample have been also included elsewhere (Hirjak, Kubera, et al., 2019). Patients were consecutively recruited from the Department of Psychiatry and Psychotherapy at the Central Institute of Mental Health in Mannheim, Germany. Diagnoses were made by staff psychiatrists and confirmed using the German versions of the Structured Clinical Interview for DSM‐IV‐TR axis I and II disorders (SCID) and examination of the case notes by two experienced psychiatrists (D.H. and S.F.). Clinical evaluation included ascertainment of personal and family history and detailed physical and neurological examination. Patients were excluded if (a) they were aged <18 or >65 years; (b) they had a history of brain trauma or neurological disease (especially movement disorders); or (c) they had shown alcohol/substance use disorder within 12 months prior to participation. HCs were recruited through advertisements and screened for major psychiatric disorders before being included. Clinical evaluation included ascertainment of personal and family history and detailed physical and neurological examination. None of the HC had a lifetime history of neurological or medical illness, head injury, or substance abuse. All included study participants were right‐handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). The Ethics Committee of Medical Faculty at Heidelberg University, Germany approved the study. Written informed consent was obtained from all SSD patients and HC after all aims and procedures of the study had been fully explained.
2.2. Clinical assessments
Patients were recruited and examined within 1 week after reaching partial remission defined as >25% reduction of symptoms according to Positive and Negative Syndrome Scale (PANSS) total score (Kay, Fiszbein, & Opler, 1987). We considered partial remission as necessary for the motor assessment, since acute psychotic symptoms, agitation and severe formal thought disorder may considerably affect patients' cooperation as well as their capability to understand the instructions. At the time of the psychometric testing, motor assessment and MRI examination, none of the SSD patients had taken benzodiazepines and all patients were on stable antipsychotic medication for at least 2 weeks. Daily doses of antipsychotic medication were converted to olanzapine equivalents (OLZe) according to the classical mean dose method (Leucht et al., 2015). The duration between enrollment/consenting to the study, motor assessment, and MRI scanning was less than 3 days. NSS were examined with the Heidelberg Scale (Schroder et al., 1991), which consists of five items assessing motor coordination (MOCO; Ozeretski's test, diadochokinesia, pronation/supination, finger‐to‐thumb opposition, speech articulation), three items assessing integrative functions (IF; station and gait, tandem walking, two‐point discrimination), two items assessing complex motor tasks (CMT; finger‐to‐nose test, fist‐edge‐palm test), four items assessing right/left and spatial orientation (RLSPO; right/left orientation, graphesthesia, face‐hand test, stereognosis), and two items assessing hard signs (HS; arm holding test, mirror movements). Ratings are given on a 0‐ to 3‐point scale (no vs. marked prevalence). A sufficient internal reliability and test–retest reliability have been established previously (Bachmann, Bottmer, & Schroder, 2005; Schroder et al., 1991). The Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962) and the PANSS were used to assess the severity of clinical symptoms. Potential extrapyramidal motor symptoms (EPMS) and Parkinsonism according to Abnormal Involuntary Movement Scale (AIMS; Guy, 1976), Simpson and Angus scale (SAS; Simpson & Angus, 1970), and Barnes Akathisia Rating Scale (BARS; Barnes, 1989, 2003) were assessed during the study. All motor rating scales were performed by two experienced raters (S.F. and D.H.), who reached an intraclass correlation coefficient >0.85.
2.3. Image data acquisition
MRI scans were acquired at the Central Institute of Mental Health, Mannheim, Germany on a 3 Tesla MR scanner (MAGNETOM Tim Trio Siemens Medical Systems, Erlangen, Germany). The scanner protocol included three measurements: a resting‐state scan and two structural scans (diffusion‐tensor‐imaging data and 3‐D‐MPRAGE images). For rs‐fMRI (duration = 6 min), 167 whole‐brain echo planar imaging (EPI) volumes were recorded in an axial orientation with the following imaging parameters: repetition time = 1,790 ms, echo time = 28 ms, field of view = 192 mm, flip angle = 76°, voxel size = 3 × 3 × 3 mm, 34 slices, slice thickness = 3 mm. For acquisition of rs‐fMRI data, participants were instructed to remain as still as possible, to keep their eyes closed, to not think about anything in particular, and not to fall asleep. Adherence to these instructions was verified by verbal contact immediately after the resting‐state scan and as part of a post‐scanning exit interview. T1‐weighted three‐dimensional magnetization‐prepared rapid gradient‐echo (3D‐MPRAGE) data with following parameters were obtained: 176 sagittal slices, image matrix = 256 × 256, voxel size = 1 × 1 × 1 mm, TR = 2,530 ms, TE = 3.8 ms, TI = 1,100 ms, flip angle = 7°. All MRI brain scans were reviewed by two experienced physicians D.H. and S.F.
2.4. MRI data analysis
2.4.1. Modality‐specific data processing
We calculated voxel‐wise GMV estimates from T1‐weighted structural images with voxel‐based morphometry (VBM) using the CAT12 toolbox (http://dbm.neuro.uni-jena.de/cat/) implemented in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/). Data processing included (a) segmentation of images into gray matter, white matter, and cerebrospinal fluid, (b) normalization using the DARTEL approach (Ashburner, 2007), and (c) smoothing the GMV segments using an 8‐mm full‐width half‐maximum (FWHM) isotropic Gaussian kernel.
Processing of rs‐fMRI images included the application of the fractional amplitude of low‐frequency fluctuations (fALFF) method using the Data Processing Assistant for Resting‐State fMRI (DPARSF; C. Yan & Zang, 2010). The pipeline consisted of (a) slice timing with the middle slice as the reference frame, (b) head motion correction, (c) spatial normalization in Montreal Neurological Institute (MNI) space, (d) spatial smoothing with an 8‐mm FWHM isotropic Gaussian kernel, and (e) regressing of nuisance covariates including mean signals from white matter and cerebrospinal fluid as well as the Friston 24‐parameter model (6 head motion parameters, 6 head motion parameters one time point before, and the 12 corresponding squared items; Friston, Williams, Howard, Frackowiak, & Turner, 1996).
In the present study, we did not regress out the global mean signal. Global signal regression (GSR) is a disputable but not yet resolved topic in fMRI (Murphy & Fox, 2017; C. G. Yan et al., 2013). Although GSR is a relevant topic for fMRI in general, recent study in more than 1,400 subjects confirmed that GSR does not affect ALFF comparisons across psychiatric groups (major depression; Xia et al., 2019).
2.4.2. Multimodal data fusion
MCCA as a dimensionality reduction method and jICA (Li, Adali, Wang, & Calhoun, 2009; Sui, He, Pearlson, et al., 2013) were performed on both GMV and fALFF data using the Fusion ICA Toolbox (FIT; http://mialab.mrn.org/software/fit) in MATLAB 9.0.0 (R2016a). First, structural and functional 3D‐data were reshaped in a one‐dimensional vector per each subject and stacked, thus resulting in a 74 × [number of voxels] matrix for each modality. Then, each matrix was normalized to have the same average sum‐of‐squares (computed across subjects and voxels modalities). Relative scaling within the data was preserved for each modality. Second, data were reduced using mCCA and decomposed using a jICA approach with the infomax algorithm. The number of components for each modality was estimated using both the minimum description length (MDL) and the Akaike information criterion (AIC), as described in Calhoun, Adali, Pearlson, and Pekar (2001). Both approaches converged on five independent components (ICs) that were estimated for each modality. ICASSO selects the most central run, thus ensuring results replicability. In addition, the quality index (Iq) from the 100 random iterations of ICASSO (Himberg, Hyvärinen, & Esposito, 2004) software was used to check the reliability and stability of IC decomposition. The Iq ranges from zero to one with values close to one corresponding to components with high reliability, and close to zero with low reliability. ICs with the Iq value below 0.80 from 100 ICASSO iterations were defined to be excluded and all of the ICs had an above threshold Iq. This procedure resulted in a source matrix (i.e., voxel‐wise loadings) and a mixing matrix (i.e., loading coefficients for each component and each subject) for each modality. Here, joint ICs have a shared variance across modalities. For each subject, a loading coefficient represents individual contribution to the whole group data for each modality. No IC was excluded as all ICs had an Iq above 0.80. For component visualization, each source matrix was reshaped back to a 3D‐image, scaled to unit standard deviation (z), and thresholded at z > 1.5. Spatial maps from ICs described in the results section were overlaid onto a Montreal Neurological Institute (MNI) template. Anatomical labels and stereotaxic coordinates were derived from clusters above a threshold of z > 3.5 by linking the ICA output images (i.e., the chosen components of interest) to the Talairach Daemon database (http://www.talairach.org/daemon.html)).
2.5. Statistical analysis
The procedure was similar to that described by Sui, He, Yu, et al. (2013) and Lottman et al. (2018). In a first step, two‐tailed independent‐samples t‐tests (p < .05; FDR corrected for multiple comparisons) were used to compare the mean of loading coefficients of the five identified ICs with the same indices (e.g., the first IC from the sMRI modality with the first IC from the rs‐fMRI modality) in SSD patients and HC. Joint group‐differentiating ICs are those with the same index components that are able to separate patients and HCs on both modalities, whereas a modality‐unique group‐differentiating IC is a component that is able to distinguish groups along with a single modality. In a second step, we chose to establish relationships between both modality‐common and modality‐specific group‐discriminating ICs and NSS scores in SSD patients. To establish such associations, two‐tailed partial correlations (p < .05; FDR corrected for multiple comparisons) adjusted for OLZe and PANSS total score were used to investigate the relationship between the loading coefficients of ICs showing significant differences across groups and NSS subscale scores in SSD patients. All reported p values were corrected for multiple comparisons using the false discovery rate (FDR) method (Benjamini & Hochberg, 1995).
In the present study, we have strictly matched the two groups according to age, because previous studies on age‐specific NSS level differences in schizophrenia showed contradictory results (Bombin, Arango, & Buchanan, 2005): While the majority of earlier studies could not identify any effects of age on NSS scores in SZ (Bartko, Zador, Horvath, & Herczeg, 1988; Buchanan & Heinrichs, 1989; Lane et al., 1996; Mohr et al., 1996), a number of studies showed that NSS levels might increase with age suggesting a progressive worsening of sensorimotor functioning (Cuesta, Peralta, & de Leon, 1996; Griffiths, Sigmundsson, Takei, Rowe, & Murray, 1998; Herold et al., 2018). Further, our study did not include duration of illness (DOI) as a covariate in the statistical analyses, because the question whether NSS are early markers of motor dysfunction preceding the illness onset or consequences of progressive worsening are not conclusively clarified yet. The majority of NSS studies in SSD found no significant association between NSS levels and DOI (Ismail, Cantor‐Graae, Cardenal, & McNeil, 1998; Lane et al., 1996; Mohr et al., 1996; Smith, Hussain, Chowdhury, & Stearns, 1999). Additionally, a recent study of our own group found no significant difference in the NSS total score between first‐ and multiple‐episode SSD patients (Fritze et al., 2019).
3. RESULTS
No significant differences in age, gender, and years of education were identified between SSD patients and HC (Table 1). Results of the two‐tailed independent‐samples t tests indicated two joint (i.e., showing significant differences between SSD patients and HC in both GMV and fALFF modalities) and one modal‐specific (fALFF) group‐discriminative ICs (Figure 1).
Table 1.
Demographics and clinical scores for healthy controls (HC) and patients with schizophrenia spectrum disorders (SSD)
| Variable | HC (n = 37) | SSD (n = 37) | t a | df | Significance |
|---|---|---|---|---|---|
| Mean ± SD (range) | Mean ± SD (range) | ||||
| Age | 34.08 ± 11.83 (19–59) | 34.41 ± 11.00 (19–63) | −0.12 | 72 | 0.90 |
| Gender (M/F)b | 20/17 | 20/17 | 1 | 1 | |
| Education (years) | 14.43 ± 1.81 | 13.54 ± 2.95 | 1.57 | 72 | 0.12 |
| Olanzapine equivalents | 15.81 ± 9.27 | ||||
| Duration of illness (years) | 8.76 ± 9.81 (0–30) | ||||
| PANSS positive score | 16.03 ± 8.51 | ||||
| PANSS negative score | 16.35 ± 8.02 | ||||
| PANSS global score | 35.62 ± 10.44 | ||||
| PANSS total score | 68.00 ± 22.49 | ||||
| NSS | |||||
| Motor coordination | 7.62 ± 3.73 | ||||
| Sensory integration | 2.43 ± 1.44 | ||||
| Complex motor tasks | 3.08 ± 2.37 | ||||
| Right/left and spatial orientation | 2.43 ± 2.18 | ||||
| Hard signs | 2.92 ± 1.72 |
Abbreviations: df, degree of freedom; NSS, neurological soft signs; PANSS, The Positive and Negative Syndrome Scale; SD, standard deviation.
The t values were obtained using a two‐tailed independent samples t‐test.
The p values for distribution of gender were obtained by chi‐square test.
Figure 1.
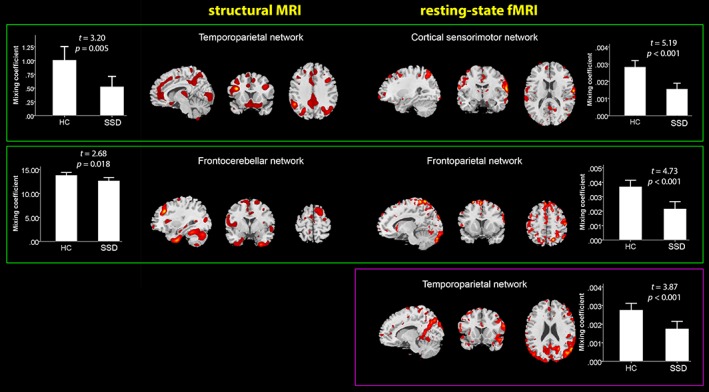
Spatial patterns of sMRI and rs‐fMRI networks thresholded at z > 1.5 which showed significant differences between HC (healthy controls) and patients with SSD (schizophrenia spectrum disorders). The green frame indicates an IC pair (i.e., showing significant differences between SSD patients and HC in both sMRI and rs‐fMRI modalities—component maps reflect jointly covarying patterns of GMV and INF), whereas a violet frame depicts a modal‐specific IC, showing significant differences only in the rs‐fMRI modality [Color figure can be viewed at http://wileyonlinelibrary.com]
The first identified joint sMRI IC (temporoparietal network) predominantly comprised the superior temporal gyrus, precentral gyrus (M1), posterior cingulate cortex (PCC), inferior parietal lobule (IPL), superior parietal lobule (SPL), supramarginal gyrus, and precuneus, t = 3.20, p = .005. The rs‐fMRI component for this IC pair (cortical sensorimotor network) had predominance in the superior frontal gyrus, postcentral gyrus, M1, SPL, and superior temporal gyrus, t = 5.19, p < .001. The second identified joint sMRI IC (Frontocerebellar network) comprised the middle frontal gyrus, PCC, and cerebellar subregions, t = 2.68, p = .018. The rs‐fMRI component for this IC pair (frontoparietal network) mainly included the precuneus, SPL, and superior frontal gyrus, t = 4.73, p < .001.
Furthermore, the modality‐specific IC showed significant difference in rs‐fMRI modality across groups. The modal‐specific rs‐fMRI IC (temporoparietal network) had predominance in the precuneus, PCC, IPL, angular gyrus, superior temporal gyrus, and superior occipital gyrus, t = 3.87, p < .001. For stereotaxic coordinates of sMRI and rsfMRI modality ICs, see Tables 2 and 3, respectively.
Table 2.
Spatial characteristics of resting‐state MRI ICs presented in Figure 1
| Area | Brodmann area | Volume (cc) | z‐max value (L/R; x, y, z) |
|---|---|---|---|
| Cortical sensorimotor network | |||
| Postcentral gyrus | 43 | 0.1/0.2 | 3.6 (−62, −8, 20)/5.0 (65, −5, 14) |
| Superior parietal lobule | 7 | 0.0/0.3 | −/4.5 (33, −61, 56) |
| Cuneus | 18, 30 | 0.1/0.3 | 3.6 (−21, −69, 12)/4.5 (18, −102, 0) |
| Superior temporal gyrus | 22 | 0.1/0.1 | 3.8 (−30, 10, −28)/4.2 (65, −20, 4) |
| Precentral gyrus | 0.0/0.1 | −/4.1 (65, −5, 20) | |
| Middle frontal gyrus | 6, 8, 10 | 0.2/0.1 | 4.0 (−33, 34, 43)/3.6 (48, 52, −3) |
| Transverse temporal gyrus | 0.0/0.1 | −/3.9 (65, −8, 11) | |
| Inferior frontal gyrus | 46 | 0.0/0.1 | −/3.8 (50, 47, 0) |
| Fusiform gyrus | 37 | 0.1/0.0 | 3.8 (−50, −56, −15)/− |
| Precuneus | 7 | 0.1/0.1 | 3.7 (−24, −70, 50)/3.6 (15, −70, 48) |
| Middle temporal gyrus | 21 | 0.0/0.1 | −/3.7 (65, −52, 8) |
| Superior frontal gyrus | 10 | 0.1/0.0 | 3.7 (−30, 62, 16)/− |
| Paracentral lobule | 31 | 0.1/0.0 | 3.6 (−3, −18, 45)/− |
| Anterior cingulate | 0.1/0.0 | 3.6 (0, 30, 21)/− | |
| Frontoparietal network | |||
| Precuneus | 7 | 0.0/0.1 | −/3.8 (18, −56, 53) |
| Superior parietal lobule | 0.0/0.1 | −/3.6 (30, −50, 58) | |
| Superior frontal gyrus | 6 | 0.0/0.1 | −/3.6 (12, 29, 57) |
| Temporoparietal network | |||
| Precuneus | 7, 19, 39 | 0.2/0.7 | 3.9 (−3, −74, 34)/5.4 (21, −57, 30) |
| Angular gyrus | 39 | 0.1/0.6 | 3.5 (−36, −71, 31)/5.1 (53, −65, 31) |
| Superior temporal gyrus | 39 | 0.1/0.3 | 3.7 (−56, −63, 25)/5.0 (56, −63, 28) |
| Posterior cingulate | 0.3/0.1 | 4.8 (−15, −55, 14)/3.6 (12, −49, 11) | |
| Inferior parietal lobule | 40 | 0.1/0.3 | 3.6 (−65, −39, 30)/4.4 (56, −56, 42) |
| Sub‐Gyral | 0.1/0.4 | 3.9 (−15, −57, 22)/4.3 (21, −60, 28) | |
| Middle temporal gyrus | 39 | 0.1/0.6 | 3.8 (−42, −75, 23)/4.2 (45, −72, 26) |
| Supramarginal gyrus | 40 | 0.0/0.1 | −/3.8 (59, −56, 36) |
| Superior parietal lobule | 7 | 0.1/0.0 | 3.8 (−30, −73, 45)/− |
| Superior occipital gyrus | 19 | 0.0/0.1 | −/3.6 (42, −80, 29) |
For the resting‐state fMRI networks shown in Figure 1, voxels with z > 3.5 were converted from MNI to Talairach coordinates and coupled with the Talairach Daemon database to provide anatomical labels. For each hemisphere (L: left; R: right), the maximum z‐value, stereotaxic coordinates (x, y, z), and the volume of voxels in cubic centimeters (cc) are provided.
Table 3.
Spatial characteristics of structural MRI ICs presented in Figure 1
| Area | Brodmann area | Volume (cc) | z‐max value (L/R; x, y, z) |
|---|---|---|---|
| Temporoparietal network | |||
| Superior temporal gyrus | 22, 39 | 0.1/1.3 | 3.6 (−43, −58, 26)/8.0 (43, −54, 21) |
| Posterior cingulate | 30 | 0.7/0.0 | 7.0 (−21, −65, 10)/− |
| Supramarginal gyrus | 40 | 1.1/0.2 | 6.5 (−49, −48, 36)/4.7 (40, −53, 27) |
| Precuneus | 7, 31, 39 | 0.8/0.8 | 5.0 (−24, −64, 36)/6.4 (25, −58, 42) |
| Middle frontal gyrus | 6, 8, 9, 46 | 1.4/1.0 | 6.0 (−36, −2, 44)/6.3 (37, 13, 28) |
| Cuneus | 17, 30 | 0.6/0.0 | 6.0 (−19, −69, 10)/− |
| Middle temporal gyrus | 21, 39 | 0.8/0.8 | 5.8 (−43, −64, 27)/5.9 (39, −54, 22) |
| Inferior parietal lobule | 39, 40 | 0.7/0.1 | 5.4 (−49, −51, 38)/3.5 (43, −67, 38) |
| Superior parietal lobule | 7 | 0.1/0.3 | 4.1 (−24, −63, 45)/5.4 (25, −60, 44) |
| Thalamus | 0.0/0.6 | −/5.2 (13, −23, 11) | |
| Angular gyrus | 0.1/0.5 | 4.8 (−40, −66, 30)/4.3 (45, −66, 30) | |
| Inferior frontal gyrus | 0.0/0.2 | −/4.7 (40, 10, 29) | |
| Lingual gyrus | 19 | 0.4/0.0 | 4.4 (−25, −61, −3)/− |
| Parahippocampal gyrus | 0.1/0.0 | 4.3 (−27, −57, −3)/− | |
| Precentral gyrus | 6 | 0.2/0.0 | 3.8 (−36, −5, 42)/− |
| Inferior occipital gyrus | 19 | 0.1/0.1 | 3.7 (−18, −89, −8)/3.6 (39, −74, −4) |
| Inferior temporal gyrus | 0.0/0.1 | −/3.7 (43, −71, 1) | |
| Frontocerebellar network | |||
| Middle frontal gyrus | 8, 9 | 0.0/0.8 | −/6.2 (34, 29, 30) |
| Culmen | 1.0/0.4 | 5.4 (−24, −55, −16)/4.0 (22, −55, −15) | |
| Declive | 1.3/1.1 | 5.2 (−21, −58, −15)/4.2 (19, −75, −21) | |
| Uncus | 20 | 0.0/0.2 | −/5.0 (30, −6, −38) |
| Uvula | 0.1/0.5 | 3.6 (−15, −78, −25)/5.0 (16, −77, −24) | |
| Posterior cingulate | 29, 30 | 0.0/0.5 | −/4.6 (12, −48, 8) |
| Middle temporal gyrus | 0.3/0.1 | 4.5 (−40, −70, 19)/3.6 (33, 1, −38) | |
| Inferior temporal gyrus | 20 | 0.1/0.3 | 4.2 (−43, −16, −28)/4.5 (34, −8, −36) |
| Cerebellar tonsil | 0.3/0.0 | 4.3 (−24, −53, −41)/− | |
| Fusiform gyrus | 20 | 0.0/0.2 | −/4.2 (36, −42, −18) |
| Parahippocampal gyrus | 0.0/0.2 | −/4.2 (22, −48, 5) | |
| Middle occipital gyrus | 0.1/0.0 | 4.1 (−28, −84, 10)/− | |
| Inferior semi‐lunar lobule | 0.0/0.4 | −/4.1 (15, −72, −39) | |
| Pyramis | 0.0/0.4 | −/4.1 (18, −77, −28) | |
| Tuber | 0.0/0.2 | −/4.0 (39, −68, −23) | |
| Superior temporal gyrus | 38, 39 | 0.1/0.1 | 3.8 (−49, −55, 11)/3.9 (42, 13, −31) |
| Precuneus | 0.1/0.1 | 3.8 (−12, −62, 35)/3.8 (21, −69, 50) | |
| Lingual gyrus | 0.0/0.1 | −/3.5 (15, −51, 5) |
For the gray matter volume networks shown in Figure 1, voxels with z > 3.5 were converted from MNI to Talairach coordinates and coupled with the Talairach Daemon database to provide anatomical labels. For each hemisphere (L: left; R: right), the maximum z‐value, stereotaxic coordinates (x, y, z), and the volume of voxels in cubic centimeters (cc) are provided.
To investigate the relationship between the mixing coefficients of the significant ICs (two IC pairs and one modal‐specific rsfMRI IC) and the NSS scores, partial correlation (adjusted for OLZe and PANSS total score) was performed. The mixing coefficients of the second IC pair both showed significant negative correlations with NSS motor score: frontocerebellar network, r = −.488, p = .037 (bootstrapped lower and upper 95% confidence intervals were −0.696 and −0.232, respectively), and frontoparietal network, r = −.524, p = .031 (bootstrapped lower and upper confidence intervals were −0.708 and −0.260, respectively). Scatter plots of these partial correlations are provided in Figure 2. No other significant correlation between the IC's mixing coefficients and NSS scores was identified (Table S1).
Figure 2.
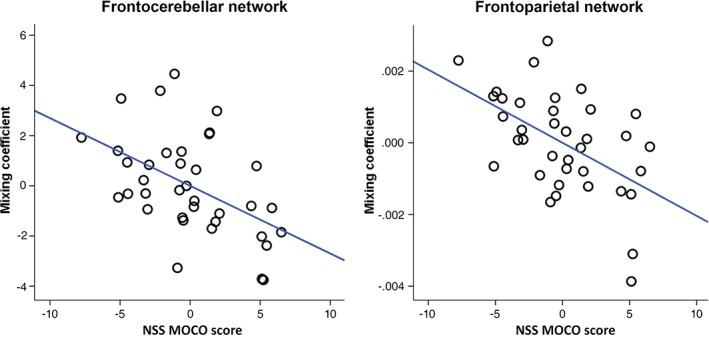
Partial correlation (adjusted for OLZe and PANSS total) between the mixing coefficients of the second group‐differentiating modality common IC pair [(frontocerebellar (r = −.488, p = .037) and frontoparietal (r = −.524, p = .031)] network and NSS motor coordination scores. p‐values are FDR corrected for multiple comparisons [Color figure can be viewed at http://wileyonlinelibrary.com]
To ensure the specificity of the significant networks for NSS, the partial correlation (adjusted for OLZe) between the mixing coefficients of these ICs and the PANSS positive and PANSS negative were tested separately. No significant correlation was observed, all ps ≥ .23 for the PANSS positive and all ps > .08 for the PANSS negative (Table S2). Additionally, the partial correlation (adjusted for OLZe and NSS scores) between the mixing coefficients and the PANSS positive and negative scores were tested. No significant correlation was observed (both p‐values > .11; Table S3).
4. DISCUSSION
The present study aimed at identifying joint and modality‐specific ICs underlying NSS in SSD patients. Two main findings emerged: First, results of the mCCA + jICA model indicated two joint group‐discriminating ICs comprising temporoparietal and frontocerebellar (GMV), as well as cortical sensorimotor and frontoparietal networks (INA). Second, the second joint IC comprising frontocerebellar and frontoparietal networks showed a negative correlation with MOCO scores in SSD patients.
4.1. Group differences
In accordance with our hypothesis, two ICs significantly differentiated SSD patients from HC in both modalities (GMV and INA/fALFF; Figure 1), suggesting that SSD‐specific pathology may lie in the co‐altered brain structure and function of temporoparietal and frontocerebellar as well as cortical sensorimotor and frontoparietal networks (Keshavan, Tandon, Boutros, & Nasrallah, 2008). The identified joint group‐discriminative ICs corroborate previous sMRI and fMRI studies that postulated either cortico‐cerebellar‐thalamo‐cortical circuit (CCTCC) dysfunction (Haijma et al., 2013; Horga et al., 2011; Job et al., 2002) or frontotemporal dysconnectivity hypothesis in the pathogenesis of SSD (Friston & Frith, 1995; Pettersson‐Yeo, Allen, Benetti, McGuire, & Mechelli, 2011). The temporoparietal and frontocerebellar networks significantly differentiated between SSD patients and HC suggesting significant decreases of GMV in the frontal gyrus, PCC, supramarginal gyrus, temporal gyrus, lingual gyrus, M1, precuneus, and cerebellar structures. GMV reduction in these regions, which are involved in important sensorimotor and cognitive functions, is consistent with previous MRI studies on SSD patients (Iwashiro et al., 2012; Kikinis et al., 2010; Koo et al., 2008; Shimizu et al., 2007; Tang et al., 2012; Torii et al., 2012; Zhou et al., 2005). Furthermore, the present study is in line with the recent mCCA + jICA study by Lottman and colleagues (Lottman et al., 2018), once again highlighting the relationship between GMV alterations and aberrant INA in SSD. In particular, the aberrant interrelationships between GMV and INA might contribute to the explanation of very heterogeneous symptoms in SSD patients (Kubera et al., 2019; Sumner, Bell, & Rossell, 2018a, 2018b).
Interestingly, GMV reduction in temporoparietal and frontocerebellar networks is associated with the functional abnormalities in the cortical sensorimotor and frontoparietal networks consisted of the middle frontal gyrus, temporal gyrus, M1, postcentral gyrus, SPL, and precuneus. These regions are similar to those identified by previous MRI studies and are characteristic areas in the pathogenesis of SSD as well (Zhao et al., 2014). Overall, these results converge with established theories, support earlier MRI studies in SSD patients and emphasize the joint structural and functional changes in frontoparietal and cerebellar networks as important sites in the pathogenesis of SSD.
4.2. Correlation with NSS
One trans‐modal group‐discriminative IC pair comprising frontocerebellar and frontoparietal networks was associated with NSS MOCO scores in SZ patients. This finding is important for several reasons: First, the significant association between the frontocerebellar network and MOCO scores in SZ patients supports the previously reported results by pointing out the importance of structural changes in the frontal gyrus, PCC, and precuneus in the pathogenesis of NSS (Hirjak et al., 2015). In particular, failure of the modulatory effect of the PCC on other brain areas involved in motor behavior in SSD might lead to aberrant goal‐directed behavior including disorders of motor response inhibition (suppression) and as a consequence the aberrant execution of pronation/supination (Wenderoth, Debaere, Sunaert, & Swinnen, 2005a, 2005b). Furthermore, the precuneus is necessary for movement control when two limbs have to be coordinated in space (Christensen et al., 2000; Wenderoth et al., 2005a, 2005b). These findings fit very well to the requirements of MOCO because patients have to coordinate their arms in space when performing Ozeretski's test. The connection between precuneus and cingulate gyrus is responsible for limb position imagery (Yu et al., 2011). Notably, the relationship between cingulate gyrus and precuneus and NSS were identified through previous MRI studies in schizophrenia patients (Dazzan et al., 2004; Kong, Bachmann, Thomann, Essig, & Schroder, 2012) and healthy participants (Hirjak et al., 2017; Thomann et al., 2015).
Second, alterations of the cerebellar structures and precuneus have been implicated in abnormal somatosensory control, motor adaptation, and the acquisition of new motor skills (Koziol et al., 2014; Ramnani, 2014; Ramnani, Toni, Josephs, Ashburner, & Passingham, 2000; Takakusaki, 2017). The precuneus is located in the medial parietal cortex and has its major connections to the prefrontal and cingulate cortex (Hirjak et al., 2017). Together with the parietal lobule, these regions play a crucial role in integration of stimuli, interpreting of sensory input (Corlett, Taylor, Wang, Fletcher, & Krystal, 2010) and experience of agency (Binder, 1997; Binder et al., 1997; Dean et al., 2006). From a systems neuroscience perspective, GMV decrease in cerebellar structures might strongly be associated with aberrant sensorimotor dynamics and lead to the development of motor symptoms in SSD patients (Mittal et al., 2017).
Third, the frontoparietal rs‐fMRI IC—comprising the frontal gyrus, SPL, and precuneus—is in line with previous studies on NSS in SSD (Zhao et al., 2014) and corresponds well with the cortico‐motor circuits that are involved in psychomotor organization and speed (Mittal et al., 2017). In particular, the frontal gyrus is involved mainly in cognitive and motor inhibition (Chamberlain & Sahakian, 2007; Lieberman, 2007; Swann et al., 2009; Tabibnia et al., 2011; Whelan et al., 2012) and the SPL is specialized in sensorimotor integration and maintaining of internal representation of one's own body (Wolpert, Goodbody, & Husain, 1998). These findings are remarkable because the identified network corresponds well with the requirements when performing individual MOCO (Ozeretski's test, diadochokinesia, pronation/supination, finger‐to‐thumb opposition, speech articulation) tests. Diadochokinesia, pronation/supination, and finger‐to‐thumb opposition require motor inhibition and integration of sensory input and motor output. Further, if you want to perform the Ozeretski's test or pronation/supination, you need spatial and right/left orientation as well as visual correction of your own bodily movements. Abnormalities of this covarying fALFF network can lead to impaired control of movements in space and motor disinhibition as is often manifested in SSD patients. These assumptions correlate well with the extant literature on NSS in SSD (Hirjak et al., 2015; Thomann, Wustenberg, et al., 2009; Venkatasubramanian et al., 2008). Furthermore, the frontoparietal network also appears important in the pathophysiology of NSS because it comprises orbitofrontal and prefrontal cortices. Both regions are crucial for the “horizontal” (i.e., predominantly cortico‐cortical) modulation of cognitive control and regulation of emotional stimuli and motor behavior (Hirjak et al., 2015, 2019; Northoff, 2000). Correspondingly, aberrant INA might lead to abnormalities in motor functioning, which can manifest itself as NSS or catatonia (Hirjak, Kubera, et al., 2019).
4.3. Strengths and limitations
Some of the strengths of our study are the excellent matching of both study groups and the use of comprehensive set of advanced joint multimodal analyses in SSD patients exhibiting NSS. Using a combination of structural and functional MRI methods, we show for the first time that aberrant INA/fALFF of fronto‐temporo‐parietal, temporo‐parieto‐occipital, and frontoparietal networks contribute to NSS expression in individuals with SSD. Our study shows convergent results and supports the interrelationships between reduced GMV and aberrant INA underlying NSS in SSD. However, this study has also specific limitations. First, SSD patients were taking antipsychotic medication that might have influenced both brain structure and function. Still, we used OLZe as a covariate in our analyses. Second, NSS was not assessed in HC. While we are not able to compare NSS scores between patients and HC, previous MRI studies in HC showed considerably lower NSS scores than in the present SSD patient sample (Hirjak et al., 2017; Thomann et al., 2015).
5. CONCLUSION
To the best of our knowledge, this is the first multimodal joint independent component analysis examining GMV and INA/fALFF alterations underlying NSS in SSD patients. This study did confirm earlier findings from MRI studies on NSS that examined imaging data independently. More importantly, this study essentially provides new insights into modality‐dependent inter‐relationships underlying NSS in SSD. In particular, the present study suggests GMV reduction and aberrant INA in networks subserving sensorimotor dynamics and psychomotor organization and speed to be closely associated with motor NSS in SSD. Focusing on the identified networks by future longitudinal studies holds great promise to define new biomarkers of external motor dysfunction and treatment response in SSD.
Supporting information
Table S1 Partial correlations (adjusted for OLZe and PANSS total scores) between NSS subscales scores and IC mixing coefficients. p‐values are FDR corrected for multiple comparisons.
Table S2 Two‐tailed partial correlation (adjusted for OLZe) between the ICs' mixing coefficients and the PANSS positive and PANSS negative scores. p‐values are FDR corrected for multiple comparisons.
Table S3 Two‐tailed partial correlation (adjusted for OLZe and NSS scores) between the ICs' mixing coefficients and the PANSS positive and PANSS negative scores. p‐values are FDR corrected for multiple comparisons.
ACKNOWLEDGMENTS
The authors have no potential conflicts of interest. We are grateful to all the participants and their families for their time and interest in this study. This study was supported by the German Research Foundation (DFG; grant number DFG HI 1928/2‐1 to D.H. and WO 1883/6‐1 to R.C.W.) and German Federal Ministry of Education and Research (BMBF, grant 01GQ1102 to H.T.). The DFG and BMBF had no further role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Hirjak D, Rashidi M, Fritze S, et al. Patterns of co‐altered brain structure and function underlying neurological soft signs in schizophrenia spectrum disorders. Hum Brain Mapp. 2019;40:5029–5041. 10.1002/hbm.24755
Funding information Bundesministerium für Bildung und Forschung, Grant/Award Number: 01GQ1102; Deutsche Forschungsgemeinschaft, Grant/Award Numbers: HI 1928/2‐1, WO 1883/6‐1
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage, 38(1), 95–113. 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Bachmann, S. , Bottmer, C. , & Schroder, J. (2005). Neurological soft signs in first‐episode schizophrenia: A follow‐up study. The American Journal of Psychiatry, 162(12), 2337–2343. 10.1176/appi.ajp.162.12.2337 [DOI] [PubMed] [Google Scholar]
- Barnes, T. R. (1989). A rating scale for drug‐induced akathisia. The British Journal of Psychiatry, 154, 672–676. [DOI] [PubMed] [Google Scholar]
- Barnes, T. R. (2003). The Barnes akathisia rating scale—revisited. Journal of Psychopharmacology, 17(4), 365–370. 10.1177/0269881103174013 [DOI] [PubMed] [Google Scholar]
- Bartko, G. , Zador, G. , Horvath, S. , & Herczeg, I. (1988). Neurological soft signs in chronic schizophrenic patients: Clinical correlates. Biological Psychiatry, 24(4), 458–460. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. , & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B, 57(1), 289–300. [Google Scholar]
- Binder, J. R. , (1997). Functional magnetic resonance imaging—Language mapping. Neurosurgery Clinics of North America, 8(3), 383–392. [PubMed] [Google Scholar]
- Binder, J. R. , Frost, J. A. , Hammeke, T. A. , Cox, R. W. , Rao, S. M. , & Prieto, T. (1997). Human brain language areas identified by functional magnetic resonance imaging. The Journal of Neuroscience, 17(1), 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombin, I. , Arango, C. , & Buchanan, R. W. (2005). Significance and meaning of neurological signs in schizophrenia: Two decades later. Schizophrenia Bulletin, 31(4), 962–977. 10.1093/schbul/sbi028 [DOI] [PubMed] [Google Scholar]
- Bottmer, C. , Bachmann, S. , Pantel, J. , Essig, M. , Amann, M. , Schad, L. R. , … Schroder, J. (2005). Reduced cerebellar volume and neurological soft signs in first‐episode schizophrenia. Psychiatry Research, 140(3), 239–250. 10.1016/j.pscychresns.2005.02.011 [DOI] [PubMed] [Google Scholar]
- Buchanan, R. W. , & Heinrichs, D. W. (1989). The neurological evaluation scale (NES): A structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Research, 27(3), 335–350. [DOI] [PubMed] [Google Scholar]
- Calhoun, V. D. , Adali, T. , Pearlson, G. D. , & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14(3), 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain, S. R. , & Sahakian, B. J. (2007). The neuropsychiatry of impulsivity. Current Opinion in Psychiatry, 20(3), 255–261. 10.1097/YCO.0b013e3280ba4989 [DOI] [PubMed] [Google Scholar]
- Christensen, L. O. , Johannsen, P. , Sinkjaer, T. , Petersen, N. , Pyndt, H. S. , & Nielsen, J. B. (2000). Cerebral activation during bicycle movements in man. Experimental Brain Research, 135(1), 66–72. [DOI] [PubMed] [Google Scholar]
- Corlett, P. R. , Taylor, J. R. , Wang, X. J. , Fletcher, P. C. , & Krystal, J. H. (2010). Toward a neurobiology of delusions. Progress in Neurobiology, 92(3), 345–369. 10.1016/j.pneurobio.2010.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta, M. J. , Peralta, V. , & de Leon, J. (1996). Neurological frontal signs and neuropsychological deficits in schizophrenic patients. Schizophrenia Research, 20(1–2), 15–20. [DOI] [PubMed] [Google Scholar]
- Dazzan, P. , Morgan, K. D. , Orr, K. G. , Hutchinson, G. , Chitnis, X. , Suckling, J. , … Murray, R. M. (2004). The structural brain correlates of neurological soft signs in AESOP first‐episode psychoses study. Brain, 127(Pt 1), 143–153. 10.1093/brain/awh015 [DOI] [PubMed] [Google Scholar]
- Dean, K. , Fearon, P. , Morgan, K. , Hutchinson, G. , Orr, K. , Chitnis, X. , … Dazzan, P. (2006). Grey matter correlates of minor physical anomalies in the AeSOP first‐episode psychosis study. The British Journal of Psychiatry, 189, 221–228. 10.1192/bjp.bp.105.016337 [DOI] [PubMed] [Google Scholar]
- Di, X. , Kim, E. H. , Huang, C. C. , Tsai, S. J. , Lin, C. P. , & Biswal, B. B. (2013). The influence of the amplitude of low‐frequency fluctuations on resting‐state functional connectivity. Frontiers in Human Neuroscience, 7, 118 10.3389/fnhum.2013.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova, N. , Veldsman, M. , Cumming, T. , & Brodtmann, A. (2017). Fractional amplitude of low‐frequency fluctuations (fALFF) in post‐stroke depression. Neuroimage Clinical, 16, 116–124. 10.1016/j.nicl.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. , & Frith, C. D. (1995). Schizophrenia: A disconnection syndrome? Clinical Neuroscience, 3(2), 89–97. [PubMed] [Google Scholar]
- Friston, K. J. , Williams, S. , Howard, R. , Frackowiak, R. S. , & Turner, R. (1996). Movement‐related effects in fMRI time‐series. Magnetic Resonance in Medicine, 35(3), 346–355. [DOI] [PubMed] [Google Scholar]
- Fritze, S. , Bertolino, A. L. , Kubera, K. M. , Topor, C. E. , Schmitgen, M. M. , Wolf, R. C. , & Hirjak, D. (2019). Differential contributions of brainstem structures to neurological soft signs in first‐ and multiple‐episode schizophrenia spectrum disorders. Schizophrenia Research. 10.1016/j.schres.2019.05.041. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Griffiths, T. D. , Sigmundsson, T. , Takei, N. , Rowe, D. , & Murray, R. M. (1998). Neurological abnormalities in familial and sporadic schizophrenia. Brain, 121(Pt 2), 191–203. 10.1093/brain/121.2.191 [DOI] [PubMed] [Google Scholar]
- Guy, E. (1976). Abnormal involuntary movement scale. Rockville, MD: U.S. National Institute of Health. [Google Scholar]
- Haijma, S. V. , Van Haren, N. , Cahn, W. , Koolschijn, P. C. , Hulshoff Pol, H. E. , & Kahn, R. S. (2013). Brain volumes in schizophrenia: A meta‐analysis in over 18 000 subjects. Schizophrenia Bulletin, 39(5), 1129–1138. 10.1093/schbul/sbs118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, H. , Sui, J. , Du, Y. , Yu, Q. , Lin, D. , Drevets, W. C. , … Calhoun, V. D. (2017). Co‐altered functional networks and brain structure in unmedicated patients with bipolar and major depressive disorders. Brain Structure & Function, 222(9), 4051–4064. 10.1007/s00429-017-1451-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold, C. J. , Lasser, M. M. , Seidl, U. W. , Hirjak, D. , Thomann, P. A. , & Schroder, J. (2018). Neurological soft signs and psychopathology in chronic schizophrenia: A cross‐sectional study in three age groups. Frontiers in Psychiatry, 9, 98 10.3389/fpsyt.2018.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg, J. , Hyvärinen, A. , & Esposito, F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage, 22(3), 1214–1222. 10.1016/j.neuroimage.2004.03.027 [DOI] [PubMed] [Google Scholar]
- Hirjak, D. , Huber, M. , Kirchler, E. , Kubera, K. M. , Karner, M. , Sambataro, F. , … Wolf, R. C. (2017). Cortical features of distinct developmental trajectories in patients with delusional infestation. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 76, 72–79. 10.1016/j.pnpbp.2017.02.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hirjak, D. , Kubera, K. M. , Northoff, G. , Fritze, S. , Bertolino, A. L. , Topor, C. E. , … Wolf, R. C. (2019). Cortical contributions to distinct symptom dimensions of catatonia. Schizophrenia Bulletin. 10.1093/schbul/sby192. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirjak, D. , Kubera, K. M. , Thomann, P. A. , & Wolf, R. C. (2018). Motor dysfunction as an intermediate phenotype across schizophrenia and other psychotic disorders: Progress and perspectives. Schizophrenia Research, 200, 26–34. 10.1016/j.schres.2017.10.007 [DOI] [PubMed] [Google Scholar]
- Hirjak, D. , Kubera, K. M. , Wolf, R. C. , & Northoff, G. (2019). Going Back to Kahlbaum's Psychomotor (and GABAergic) Origins: Is Catatonia More Than Just a Motor and Dopaminergic Syndrome? Schizophrenia Bulletin. 10.1093/schbul/sbz074. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirjak, D. , Kubera, K. M. , Wolf, R. C. , Thomann, A. K. , Hell, S. K. , Seidl, U. , & Thomann, P. A. (2015). Local brain gyrification as a marker of neurological soft signs in schizophrenia. Behavioural Brain Research, 292, 19–25. 10.1016/j.bbr.2015.05.048 [DOI] [PubMed] [Google Scholar]
- Hirjak, D. , Meyer‐Lindenberg, A. , Kubera, K. M. , Thomann, P. A. , & Wolf, R. C. (2018). Motor dysfunction as research domain in the period preceding manifest schizophrenia: A systematic review. Neuroscience and Biobehavioral Reviews, 87, 87–105. 10.1016/j.neubiorev.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Hirjak, D. , Rashidi, M. , Kubera, K. M. , Northoff, G. , Fritze, S. , Schmitgen, M. M. , … Wolf, R. C. (2019). Multimodal magnetic resonance imaging data fusion reveals distinct patterns of abnormal brain structure and function in catatonia. Schizophrenia Bulletin. 10.1093/schbul/sbz042. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirjak, D. , Thomann, P. A. , Kubera, K. M. , Stieltjes, B. , & Wolf, R. C. (2016). Cerebellar contributions to neurological soft signs in healthy young adults. European Archives of Psychiatry and Clinical Neuroscience, 266(1), 35–41. 10.1007/s00406-015-0582-4 [DOI] [PubMed] [Google Scholar]
- Hirjak, D. , Thomann, P. A. , Kubera, K. M. , Wolf, N. D. , Sambataro, F. , & Wolf, R. C. (2015). Motor dysfunction within the schizophrenia‐spectrum: A dimensional step towards an underappreciated domain. Schizophrenia Research, 169(1–3), 217–233. 10.1016/j.schres.2015.10.022 [DOI] [PubMed] [Google Scholar]
- Hirjak, D. , Thomann, P. A. , Wolf, R. C. , Kubera, K. M. , Goch, C. , Hering, J. , & Maier‐Hein, K. H. (2017). White matter microstructure variations contribute to neurological soft signs in healthy adults. Human Brain Mapping, 38(7), 3552–3565. 10.1002/hbm.23609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirjak, D. , Wolf, R. C. , Kubera, K. M. , Stieltjes, B. , Maier‐Hein, K. H. , & Thomann, P. A. (2015). Neurological soft signs in recent‐onset schizophrenia: Focus on the cerebellum. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 60, 18–25. 10.1016/j.pnpbp.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Hirjak, D. , Wolf, R. C. , Stieltjes, B. , Hauser, T. , Seidl, U. , Schroder, J. , & Thomann, P. A. (2013a). Cortical signature of neurological soft signs in recent onset schizophrenia. Brain Topography, 27, 296–306. 10.1007/s10548-013-0292-z [DOI] [PubMed] [Google Scholar]
- Hirjak, D. , Wolf, R. C. , Stieltjes, B. , Hauser, T. , Seidl, U. , Thiemann, U. , … Thomann, P. A. (2013b). Neurological soft signs and brainstem morphology in first‐episode schizophrenia. Neuropsychobiology, 68(2), 91–99. 10.1159/000350999 [DOI] [PubMed] [Google Scholar]
- Hirjak, D. , Wolf, R. C. , Stieltjes, B. , Seidl, U. , Schroder, J. , & Thomann, P. A. (2012). Neurological soft signs and subcortical brain morphology in recent onset schizophrenia. Journal of Psychiatric Research, 46(4), 533–539. 10.1016/j.jpsychires.2012.01.015 [DOI] [PubMed] [Google Scholar]
- Horga, G. , Bernacer, J. , Dusi, N. , Entis, J. , Chu, K. , Hazlett, E. A. , … Buchsbaum, M. S. (2011). Correlations between ventricular enlargement and gray and white matter volumes of cortex, thalamus, striatum, and internal capsule in schizophrenia. European Archives of Psychiatry and Clinical Neuroscience, 261(7), 467–476. 10.1007/s00406-011-0202-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail, B. T. , Cantor‐Graae, E. , Cardenal, S. , & McNeil, T. F. (1998). Neurological abnormalities in schizophrenia: Clinical, etiological and demographic correlates. Schizophrenia Research, 30(3), 229–238. [DOI] [PubMed] [Google Scholar]
- Iwashiro, N. , Suga, M. , Takano, Y. , Inoue, H. , Natsubori, T. , Satomura, Y. , … Yamasue, H. (2012). Localized gray matter volume reductions in the pars triangularis of the inferior frontal gyrus in individuals at clinical high‐risk for psychosis and first episode for schizophrenia. Schizophrenia Research, 137(1–3), 124–131. 10.1016/j.schres.2012.02.024 [DOI] [PubMed] [Google Scholar]
- Janssen, J. , Diaz‐Caneja, A. , Reig, S. , Bombin, I. , Mayoral, M. , Parellada, M. , … Arango, C. (2009). Brain morphology and neurological soft signs in adolescents with first‐episode psychosis. The British Journal of Psychiatry, 195(3), 227–233. 10.1192/bjp.bp.108.052738 [DOI] [PubMed] [Google Scholar]
- Job, D. E. , Whalley, H. C. , McConnell, S. , Glabus, M. , Johnstone, E. C. , & Lawrie, S. M. (2002). Structural gray matter differences between first‐episode schizophrenics and normal controls using voxel‐based morphometry. NeuroImage, 17(2), 880–889. [PubMed] [Google Scholar]
- Kasparek, T. , Prikryl, R. , Schwarz, D. , Tronerova, S. , Ceskova, E. , Mikl, M. , & Vanicek, J. (2009). Movement sequencing abilities and basal ganglia morphology in first‐episode schizophrenia. The World Journal of Biological Psychiatry, 10(4 Pt 3), 752–762. 10.1080/15622970701882433 [DOI] [PubMed] [Google Scholar]
- Kay, S. R. , Fiszbein, A. , & Opler, L. A. (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin, 13(2), 261–276. [DOI] [PubMed] [Google Scholar]
- Keshavan, M. S. , Tandon, R. , Boutros, N. N. , & Nasrallah, H. A. (2008). Schizophrenia, "just the facts": What we know in 2008 part 3: Neurobiology. Schizophrenia Research, 106(2–3), 89–107. 10.1016/j.schres.2008.07.020 [DOI] [PubMed] [Google Scholar]
- Kikinis, Z. , Fallon, J. H. , Niznikiewicz, M. , Nestor, P. , Davidson, C. , Bobrow, L. , … Shenton, M. E. (2010). Gray matter volume reduction in rostral middle frontal gyrus in patients with chronic schizophrenia. Schizophrenia Research, 123(2–3), 153–159. 10.1016/j.schres.2010.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L. , Bachmann, S. , Thomann, P. A. , Essig, M. , & Schroder, J. (2012). Neurological soft signs and gray matter changes: A longitudinal analysis in first‐episode schizophrenia. Schizophrenia Research, 134(1), 27–32. 10.1016/j.schres.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Koo, M. S. , Levitt, J. J. , Salisbury, D. F. , Nakamura, M. , Shenton, M. E. , & McCarley, R. W. (2008). A cross‐sectional and longitudinal magnetic resonance imaging study of cingulate gyrus gray matter volume abnormalities in first‐episode schizophrenia and first‐episode affective psychosis. Archives of General Psychiatry, 65(7), 746–760. 10.1001/archpsyc.65.7.746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziol, L. F. , Budding, D. , Andreasen, N. , D'Arrigo, S. , Bulgheroni, S. , Imamizu, H. , … Yamazaki, T. (2014). Consensus paper: The cerebellum's role in movement and cognition. Cerebellum, 13(1), 151–177. 10.1007/s12311-013-0511-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera, K. M. , Rashidi, M. , Schmitgen, M. M. , Barth, A. , Hirjak, D. , Sambataro, F. , … Wolf, R. C. (2019). Structure/function interrelationships in patients with schizophrenia who have persistent auditory verbal hallucinations: A multimodal MRI study using parallel ICA. Progress in Neuro‐Psychopharmacology & Biological Psychiatry, 93, 114–121. 10.1016/j.pnpbp.2019.03.007 [DOI] [PubMed] [Google Scholar]
- Lane, A. , Colgan, K. , Moynihan, F. , Burke, T. , Waddington, J. L. , Larkin, C. , & O'Callaghan, E. (1996). Schizophrenia and neurological soft signs: Gender differences in clinical correlates and antecedent factors. Psychiatry Research, 64(2), 105–114. [DOI] [PubMed] [Google Scholar]
- Leucht, S. , Samara, M. , Heres, S. , Patel, M. X. , Furukawa, T. , Cipriani, A. , … Davis, J. M. (2015). Dose equivalents for second‐generation antipsychotic drugs: The classical mean dose method. Schizophrenia Bulletin, 41(6), 1397–1402. 10.1093/schbul/sbv037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Adali, T. , Wang, W. , & Calhoun, V. D. (2009). Joint blind source separation by multiset canonical correlation analysis. IEEE Transactions on Signal Processing, 57(10), 3918–3929. 10.1109/TSP.2009.2021636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, M. D. (2007). Social cognitive neuroscience: A review of core processes. Annual Review of Psychology, 58, 259–289. 10.1146/annurev.psych.58.110405.085654 [DOI] [PubMed] [Google Scholar]
- Logothetis, N. K. (2008). What we can do and what we cannot do with fMRI. Nature, 453, 869–878. [DOI] [PubMed] [Google Scholar]
- Long, X. Y. , Zuo, X. N. , Kiviniemi, V. , Yang, Y. , Zou, Q. H. , Zhu, C. Z. , … Zang, Y. F. (2008). Default mode network as revealed with multiple methods for resting‐state functional MRI analysis. Journal of Neuroscience Methods, 171(2), 349–355. [DOI] [PubMed] [Google Scholar]
- Lottman, K. K. , White, D. M. , Kraguljac, N. V. , Reid, M. A. , Calhoun, V. D. , Catao, F. , & Lahti, A. C. (2018). Four‐way multimodal fusion of 7 T imaging data using an mCCA+jICA model in first‐episode schizophrenia. Human Brain Mapping, 39(4), 1475–1488. 10.1002/hbm.23906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal, V. A. , Bernard, J. A. , & Northoff, G. (2017). What can different motor circuits tell us about psychosis? An RDoC perspective. Schizophrenia Bulletin, 43(5), 949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr, F. , Hubmann, W. , Cohen, R. , Bender, W. , Haslacher, C. , Honicke, S. , … Werther, P. (1996). Neurological soft signs in schizophrenia: Assessment and correlates. European Archives of Psychiatry and Clinical Neuroscience, 246(5), 240–248. [DOI] [PubMed] [Google Scholar]
- Mouchet‐Mages, S. , Canceil, O. , Willard, D. , Krebs, M. O. , Cachia, A. , Martinot, J. L. , … Meder, J. F. (2007). Sensory dysfunction is correlated to cerebellar volume reduction in early schizophrenia. Schizophrenia Research, 91(1–3), 266–269. 10.1016/j.schres.2006.11.031 [DOI] [PubMed] [Google Scholar]
- Murphy, K. , & Fox, M. D. (2017). Towards a consensus regarding global signal regression for resting state functional connectivity MRI. NeuroImage, 154, 169–173. 10.1016/j.neuroimage.2016.11.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niethammer, R. , Weisbrod, M. , Schiesser, S. , Grothe, J. , Maier, S. , Peter, U. , … Sauer, H. (2000). Genetic influence on laterality in schizophrenia? A twin study of neurological soft signs. The American Journal of Psychiatry, 157(2), 272–274. 10.1176/appi.ajp.157.2.272 [DOI] [PubMed] [Google Scholar]
- Northoff, G. (2000). Brain imaging in catatonia: Current findings and a pathophysiologic model. CNS Spectrums, 5(7), 34–46. [DOI] [PubMed] [Google Scholar]
- Northoff, G. , & Duncan, N. W. (2016). How do abnormalities in the brain's spontaneous activity translate into symptoms in schizophrenia? From an overview of resting state activity findings to a proposed spatiotemporal psychopathology. Progress in Neurobiology, 145–146, 26–45. 10.1016/j.pneurobio.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Overall, J. E. , & Gorham, D. R. (1962). The brief psychiatric rating scale. Psychological Reports, 10, 799–812. [Google Scholar]
- Pettersson‐Yeo, W. , Allen, P. , Benetti, S. , McGuire, P. , & Mechelli, A. (2011). Dysconnectivity in schizophrenia: Where are we now? Neuroscience and Biobehavioral Reviews, 35(5), 1110–1124. 10.1016/j.neubiorev.2010.11.004 [DOI] [PubMed] [Google Scholar]
- Ramnani, N. (2014). Automatic and controlled processing in the corticocerebellar system. Progress in Brain Research, 210, 255–285. 10.1016/B978-0-444-63356-9.00010-8 [DOI] [PubMed] [Google Scholar]
- Ramnani, N. , Toni, I. , Josephs, O. , Ashburner, J. , & Passingham, R. E. (2000). Learning‐ and expectation‐related changes in the human brain during motor learning. Journal of Neurophysiology, 84(6), 3026–3035. 10.1152/jn.2000.84.6.3026 [DOI] [PubMed] [Google Scholar]
- Sass, H. , Wittchen, H. U. , Zaudig, M. , & Houben, I. (2003). Diagnostisches und Statistisches Manual Psychischer Störungen DSM‐IV‐TR: Text revision. Göttingen: Hogrefe Verlag. [Google Scholar]
- Schroder, J. , Niethammer, R. , Geider, F. J. , Reitz, C. , Binkert, M. , Jauss, M. , & Sauer, H. (1991). Neurological soft signs in schizophrenia. Schizophrenia Research, 6(1), 25–30. [DOI] [PubMed] [Google Scholar]
- Schroder, J. , Silvestri, S. , Bubeck, B. , Karr, M. , Demisch, S. , Scherrer, S. , … Sauer, H. (1998). D2 dopamine receptor up‐regulation, treatment response, neurological soft signs, and extrapyramidal side effects in schizophrenia: A follow‐up study with 123I‐iodobenzamide single photon emission computed tomography in the drug‐naive state and after neuroleptic treatment. Biological Psychiatry, 43(9), 660–665. [DOI] [PubMed] [Google Scholar]
- Shimizu, E. , Hashimoto, K. , Ochi, S. , Fukami, G. , Fujisaki, M. , Koike, K. , … Iyo, M. (2007). Posterior cingulate gyrus metabolic changes in chronic schizophrenia with generalized cognitive deficits. Journal of Psychiatric Research, 41(1–2), 49–56. 10.1016/j.jpsychires.2005.04.015 [DOI] [PubMed] [Google Scholar]
- Simpson, G. M. , & Angus, J. W. (1970). A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica. Supplementum, 212, 11–19. [DOI] [PubMed] [Google Scholar]
- Smith, R. C. , Hussain, M. I. , Chowdhury, S. A. , & Stearns, A. (1999). Stability of neurological soft signs in chronically hospitalized schizophrenic patients. The Journal of Neuropsychiatry and Clinical Neurosciences, 11(1), 91–96. 10.1176/jnp.11.1.91 [DOI] [PubMed] [Google Scholar]
- Sugranyes, G. , Kyriakopoulos, M. , Dima, D. , O'Muircheartaigh, J. , Corrigall, R. , Pendelbury, G. , … Frangou, S. (2012). Multimodal analyses identify linked functional and white matter abnormalities within the working memory network in schizophrenia. Schizophrenia Research, 138(2–3), 136–142. 10.1016/j.schres.2012.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, J. , He, H. , Liu, J. , Yu, Q. , Adali, T. , Pearlson, G. D. , & Calhoun, V. D. (2012). Three‐way FMRI‐DTI‐methylation data fusion based on mCCA+jICA and its application to schizophrenia Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 2012, 2692–2695. 10.1109/EMBC.2012.6346519 [DOI] [PubMed] [Google Scholar]
- Sui, J. , He, H. , Pearlson, G. D. , Adali, T. , Kiehl, K. A. , Yu, Q. , … Calhoun, V. D. (2013). Three‐way (N‐way) fusion of brain imaging data based on mCCA+jICA and its application to discriminating schizophrenia. NeuroImage, 66, 119–132. 10.1016/j.neuroimage.2012.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, J. , He, H. , Yu, Q. , Chen, J. , Rogers, J. , Pearlson, G. , … Calhoun, V. (2013). Combination of resting state fMRI, DTI, and sMRI data to discriminate schizophrenia by N‐way MCCA + jICA. Frontiers in Human Neuroscience, 7, 235 10.3389/fnhum.2013.00235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner, P. J. , Bell, I. H. , & Rossell, S. L. (2018a). A systematic review of task‐based functional neuroimaging studies investigating language, semantic and executive processes in thought disorder. Neuroscience and Biobehavioral Reviews, 94, 59–75. 10.1016/j.neubiorev.2018.08.005 [DOI] [PubMed] [Google Scholar]
- Sumner, P. J. , Bell, I. H. , & Rossell, S. L. (2018b). A systematic review of the structural neuroimaging correlates of thought disorder. Neuroscience and Biobehavioral Reviews, 84, 299–315. 10.1016/j.neubiorev.2017.08.017 [DOI] [PubMed] [Google Scholar]
- Swann, N. , Tandon, N. , Canolty, R. , Ellmore, T. M. , McEvoy, L. K. , Dreyer, S. , … Aron, A. R. (2009). Intracranial EEG reveals a time‐ and frequency‐specific role for the right inferior frontal gyrus and primary motor cortex in stopping initiated responses. The Journal of Neuroscience, 29(40), 12675–12685. 10.1523/JNEUROSCI.3359-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabibnia, G. , Monterosso, J. R. , Baicy, K. , Aron, A. R. , Poldrack, R. A. , Chakrapani, S. , … London, E. D. (2011). Different forms of self‐control share a neurocognitive substrate. The Journal of Neuroscience, 31(13), 4805–4810. 10.1523/JNEUROSCI.2859-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki, K. (2017). Functional neuroanatomy for posture and gait control. Journal of Movement Disorders, 10(1), 1–17. 10.14802/jmd.16062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J. , Liao, Y. , Zhou, B. , Tan, C. , Liu, W. , Wang, D. , … Chen, X. (2012). Decrease in temporal gyrus gray matter volume in first‐episode, early onset schizophrenia: An MRI study. PLoS One, 7(7), e40247 10.1371/journal.pone.0040247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomann, P. A. , Hirjak, D. , Kubera, K. M. , Stieltjes, B. , & Wolf, R. C. (2015). Neural network activity and neurological soft signs in healthy adults. Behavioural Brain Research, 278, 514–519. 10.1016/j.bbr.2014.10.044 [DOI] [PubMed] [Google Scholar]
- Thomann, P. A. , Roebel, M. , Dos Santos, V. , Bachmann, S. , Essig, M. , & Schroder, J. (2009). Cerebellar substructures and neurological soft signs in first‐episode schizophrenia. Psychiatry Research, 173(2), 83–87. 10.1016/j.pscychresns.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Thomann, P. A. , Wustenberg, T. , Santos, V. D. , Bachmann, S. , Essig, M. , & Schroder, J. (2009). Neurological soft signs and brain morphology in first‐episode schizophrenia. Psychological Medicine, 39(3), 371–379. 10.1017/S0033291708003656 [DOI] [PubMed] [Google Scholar]
- Torii, Y. , Iritani, S. , Sekiguchi, H. , Habuchi, C. , Hagikura, M. , Arai, T. , … Ozaki, N. (2012). Effects of aging on the morphologies of Heschl's gyrus and the superior temporal gyrus in schizophrenia: A postmortem study. Schizophrenia Research, 134(2–3), 137–142. 10.1016/j.schres.2011.10.024 [DOI] [PubMed] [Google Scholar]
- Venkatasubramanian, G. , Jayakumar, P. N. , Gangadhar, B. N. , & Keshavan, M. S. (2008). Neuroanatomical correlates of neurological soft signs in antipsychotic‐naive schizophrenia. Psychiatry Research, 164(3), 215–222. 10.1016/j.pscychresns.2007.12.021 [DOI] [PubMed] [Google Scholar]
- Walther, S. , & Strik, W. (2012). Motor symptoms and schizophrenia. Neuropsychobiology, 66(2), 77–92. 10.1159/000339456 [DOI] [PubMed] [Google Scholar]
- Wenderoth, N. , Debaere, F. , Sunaert, S. , & Swinnen, S. P. (2005a). The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. The European Journal of Neuroscience, 22(1), 235–246. 10.1111/j.1460-9568.2005.04176.x [DOI] [PubMed] [Google Scholar]
- Wenderoth, N. , Debaere, F. , Sunaert, S. , & Swinnen, S. P. (2005b). Spatial interference during bimanual coordination: Differential brain networks associated with control of movement amplitude and direction. Human Brain Mapping, 26(4), 286–300. 10.1002/hbm.20151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan, R. , Conrod, P. J. , Poline, J. B. , Lourdusamy, A. , Banaschewski, T. , Barker, G. J. , … Consortium, I. (2012). Adolescent impulsivity phenotypes characterized by distinct brain networks. Nature Neuroscience, 15(6), 920–925. 10.1038/nn.3092 [DOI] [PubMed] [Google Scholar]
- Wolpert, D. M. , Goodbody, S. J. , & Husain, M. (1998). Maintaining internal representations: The role of the human superior parietal lobe. Nature Neuroscience, 1(6), 529–533. 10.1038/2245 [DOI] [PubMed] [Google Scholar]
- Xia, M. , Si, T. , Sun, X. , Ma, Q. , Liu, B. , Wang, L. , … DIDA‐Major Depressive Disorder Working Group . (2019). Reproducibility of functional brain alterations in major depressive disorder: Evidence from a multisite resting‐state functional MRI study with 1,434 individuals. NeuroImage, 189, 700–714. 10.1016/j.neuroimage.2019.01.074 [DOI] [PubMed] [Google Scholar]
- Xu, Y. , Zhuo, C. , Qin, W. , Zhu, J. , & Yu, C. (2015). Altered spontaneous brain activity in schizophrenia: A meta‐analysis and a large‐sample study. BioMed Research International, 2015, 204628–204631. 10.1155/2015/204628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. , & Zang, Y. (2010). DPARSF: A MATLAB toolbox for "pipeline" data analysis of resting‐state fMRI. Frontiers in Systems Neuroscience, 4, 13 10.3389/fnsys.2010.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. G. , Cheung, B. , Kelly, C. , Colcombe, S. , Craddock, R. C. , Di Martino, A. , … Milham, M. P. (2013). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage, 76, 183–201. 10.1016/j.neuroimage.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C. , Zhou, Y. , Liu, Y. , Jiang, T. , Dong, H. , Zhang, Y. , & Walter, M. (2011). Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. NeuroImage, 54(4), 2571–2581. 10.1016/j.neuroimage.2010.11.018 [DOI] [PubMed] [Google Scholar]
- Yuan, R. , Di, X. , Kim, E. H. , Barik, S. , Rypma, B. , & Biswal, B. B. (2013). Regional homogeneity of resting‐state fMRI contributes to both neurovascular and task activation variations. Magnetic Resonance Imaging, 31(9), 1492–1500. 10.1016/j.mri.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang, Y. , Jiang, T. , Lu, Y. , He, Y. , & Tian, L. (2004). Regional homogeneity approach to fMRI data analysis. Neuroimage, 22(1), 394–400. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Li, X. , Lv, J. , Jiang, X. , Guo, L. , & Liu, T. (2016). Characterizing and differentiating task‐based and resting state fMRI signals via two‐stage sparse representations. Brain Imaging and Behavior, 10(1), 21–32. 10.1007/s11682-015-9359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. , Li, Z. , Huang, J. , Yan, C. , Dazzan, P. , Pantelis, C. , … Chan, R. C. (2014). Neurological soft signs are not "soft" in brain structure and functional networks: Evidence from ALE meta‐analysis. Schizophrenia Bulletin, 40(3), 626–641. 10.1093/schbul/sbt063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, S. Y. , Suzuki, M. , Hagino, H. , Takahashi, T. , Kawasaki, Y. , Matsui, M. , … Kurachi, M. (2005). Volumetric analysis of sulci/gyri‐defined in vivo frontal lobe regions in schizophrenia: Precentral gyrus, cingulate gyrus, and prefrontal region. Psychiatry Research, 139(2), 127–139. 10.1016/j.pscychresns.2005.05.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Partial correlations (adjusted for OLZe and PANSS total scores) between NSS subscales scores and IC mixing coefficients. p‐values are FDR corrected for multiple comparisons.
Table S2 Two‐tailed partial correlation (adjusted for OLZe) between the ICs' mixing coefficients and the PANSS positive and PANSS negative scores. p‐values are FDR corrected for multiple comparisons.
Table S3 Two‐tailed partial correlation (adjusted for OLZe and NSS scores) between the ICs' mixing coefficients and the PANSS positive and PANSS negative scores. p‐values are FDR corrected for multiple comparisons.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


