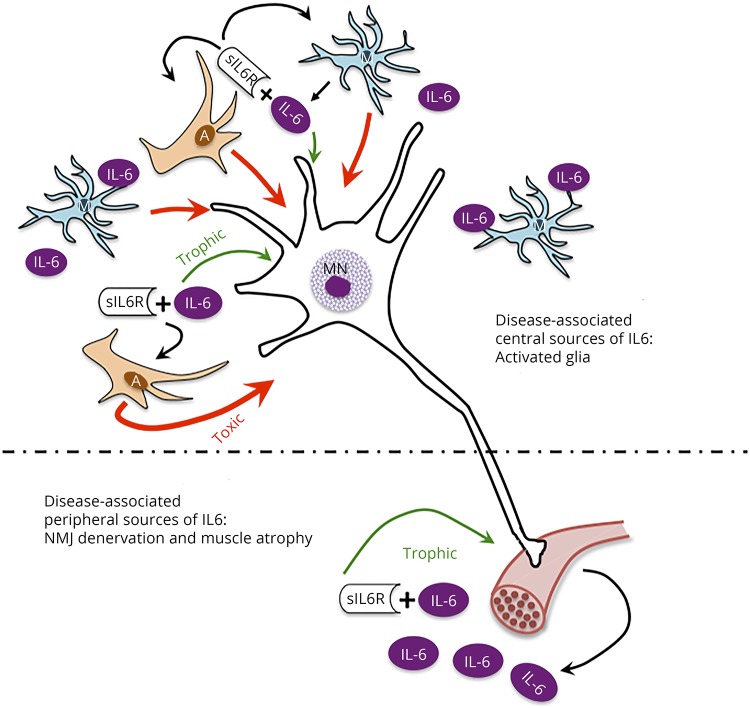
Figure 4. In amyotrophic lateral sclerosis (ALS), interleukin 6 (IL6) trans-signaling effects on motor neurons may shift the balance from trophic activity to promoting a toxic environment.
In conditions of axonal injury, muscle injury, muscle stress (e.g., exercise), and atrophy (e.g., sarcopenia), increases in muscle-specific expression of IL6 are correlated with increased levels of circulating IL6.9,38,39 Of relevance to MNs, IL6 is a member of the CNTF/CT/LIF gp130 family of trophic factors that are critical in MN development and survival following injury and disease.7 In the local environment, in the presence of sIL6R, the myokine-receptor complex can bind to gp130 on MNs acting as a trophic factor. Accordingly, IL6 signaling may be beneficial in maintaining NMJ innervation, promoting MN survival, and rebuilding muscle (lower panel). Despite the potential prosurvival effect of IL6 on neurons in the periphery, it may be that in *C patients, CNS IL6 trans-signaling effects on glial cells promote damaging effects in the CNS.9,38,39 In the CNS, microglia become activated in response to NMJ denervation, and both microglial and astrocytes become active in response to disease-associated MN pathology. These and other CNS cells have been reported to be sources of IL6 (upper panel). In this central environment, IL6 can bind sIL6R and, as in the periphery, bind gp130 on MNs promoting trophic activity. However, the IL6-sIL6R complex can also bind to gp130 on glial cells to further activate them. In ALS, activated astrocytes have been shown to create a toxic environment for MNs.38,39 We hypothesize that this toxic activity overpowers any potential trophic activity, shifting the balance for MNs toward dysfunction and degeneration.