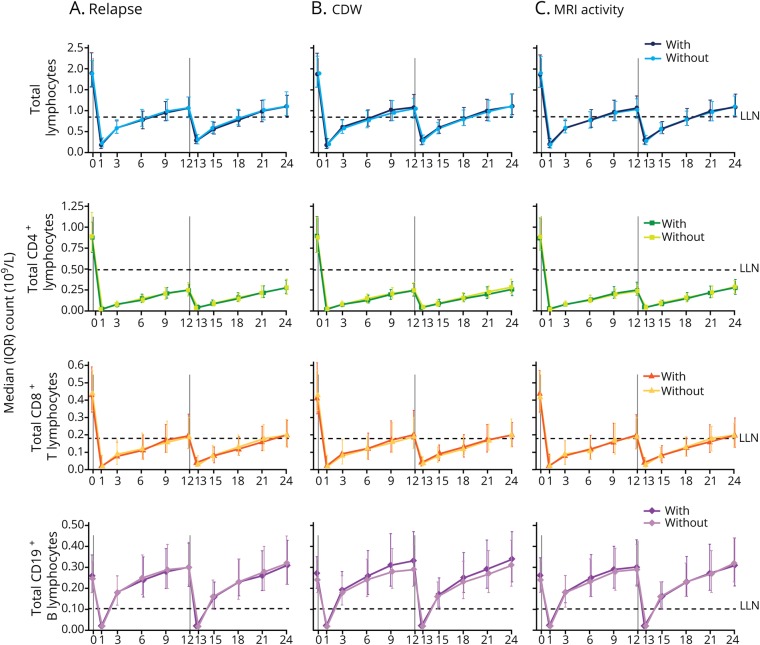
Figure 3. Median lymphocyte counts in alemtuzumab-treated patients with or without disease activity.
Median total lymphocyte, CD4+ and CD8+ T-lymphocyte, and CD19+ B-lymphocyte counts in Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis patients treated with alemtuzumab 12 mg, stratified by the presence or absence of (A) relapse, (B) 6-month CDW, and (C) MRI disease activity. N value range over 24 months: 356–388 patients without relapse; 395–414 patients with relapse; 567–606 patients without CDW; 184–196 patients with CDW; 263–280 patients without MRI activity; 485–516 patients with MRI activity. Vertical lines indicate administration of alemtuzumab. CDW = confirmed disability worsening; IQR = interquartile range; LLN = lower limit of normal.