Abstract
Appropriate feeding practices are crucial for survival, growth, and development in childhood. This paper analyzes Pakistan's Demographic and Health Survey 2012–2013 to fill the knowledge gap in risk factors of poor complementary feeding practices in Pakistani children. Multilevel models were applied to fit the multistage cluster sample of 2,827 children aged 6–23 months from 489 communities. Introduction of solid, semi‐solid, or soft foods (intro) was achieved in 67% infants aged 6–8 months. Among children aged 6–23 months, the proportion of children meeting minimum meal frequency, dietary diversity (MDD), and acceptable diet criteria were 63%, 22% and 15%, respectively. Consumption of legumes and nuts, flesh foods, and vitamin A‐rich fruits and vegetables was low in all children (6–19%), even among children who met the MDD criteria (15–55%). Younger child age, especially between 6 and 11 months and delayed maternal postnatal checkup were significant individual‐level risk factors that consistently increased the odds of not meeting all four criteria examined. Fewer antenatal care visits predicted the odds of achieving intro and minimum meal frequency while younger maternal age and household poverty predicted the odds of achieving MDD and minimum acceptable diet. Community‐level factors included geographic region and general access to maternal and child health care services. The overall poor quality of children's complementary diets in Pakistani calls for stronger policy and program action to promote the consumption of key nutrient‐dense foods while prioritizing interventions for the most vulnerable children and populations.
Keywords: complementary foods and feeding, demographic and health survey, dietary diversity, multilevel model, Pakistan, risk factors
1. INTRODUCTION
Undernutrition, including suboptimal nutrition from conception through infancy and early childhood, is a cause of 3.1 million child deaths or 45% of all under five mortality worldwide (Black et al., 2013). Appropriate feeding practices, including optimal breastfeeding and complementary feeding in the critical window from birth to 2 years of age, are key to achieving growth and developmental potential throughout childhood and likely preferable cardiovascular profile and nutritional status in later adulthood (Black et al., 2013; Horta & Victora, 2013). Complementary foods must be diverse, of sufficient energy density, and introduced at the right time to meet a growing infant's needs. The timing of introduction of complementary foods is critical because both early and delayed introduction are associated with poor nutritional status and increased morbidity (Behar et al., 1986; Gross, Giay, Sastroamidjojo, Schultink, & Lang, 2000; Pearce, Taylor, & Langley‐Evans, 2013). Therefore, the World Health Organization (WHO) recommends to introduce other foods beyond human milk at 6 months of age not only to help infants to meet nutrients needs but also to avoid the risk of exposure to food‐borne pathogens to developmentally unready infants (Dewey, 2003). Sufficient energy intake supports child growth, development, and physical activity. Given limitations in gastric capacity, infants must be fed frequently and with sufficient energy density to meet those needs. Dietary diversity is a good predictor of micronutrient density in child diets (Moursi et al., 2008; Working Group on Infant and Young Child Feeding Indicators, 2006) and has shown its predictive value in child growth (Arimond & Ruel, 2004; Rah et al., 2010; Sawadogo et al., 2006) and development (Frongillo et al., 2017). Given the small amount of complementary foods consumed in infancy and early childhood, feeding children a high‐quality diet with a variety of foods is crucial to meet the need for high‐micronutrient density to support optimal growth and development.
Pakistan is home to the second largest number of stunted children in South Asia after India (UNICEF, 2013) and suffers from the highest prevalence of child undernutrition in the world, with 47% of children born small for gestational age (Black et al., 2013) and 45% of children younger than 5 years stunted (National Institute of Population Studies & ICF International, 2013). Poor quality diets in young children is a known immediate cause of child malnutrition (Black et al., 2010). Since the 1990s, national and regional efforts, such as the Maternal, Neonatal and Child Health Program (Ministry of Health Government of Pakistan, 2006), and more recently, the Enhanced Nutrition for Mothers and Children Project (World Bank, 2014), have prioritized to improve child health and nutrition knowledge and practice, given the known effectiveness of nutritional counseling and education in improving child weight and height (Dewey & Adu‐Afarwuah, 2008; Imdad, Yakoob, & Bhutta, 2011; Saleem, Mahmud, Baig‐Ansari, & Zaidi, 2014; Zaman, Ashraf, & Martines, 2008).
Yet until recently, there has been a lack of data on the core aspects of complementary feeding beyond timely introduction of complementary foods, including meal frequency and dietary diversity. In 2010, the World Health Organization released a set of standardized indicators with minimum threshold criteria for use in assessing the quality of complementary feeding diets (World Health Organization, 2010). These include the timely introduction of complementary foods, minimum meal frequency, minimum dietary diversity, and minimum acceptable diets. This has allowed for a greater focus on complementary feeding, standardized cross‐country comparisons, and evaluations of predictors of poor practices.
Pakistan's Demographic Health Survey (PDHS) conducted in 2012–2013 is the third survey of its kind to collect nationally representative information on maternal and child survival, health, and nutrition (National Institute of Population Studies & ICF International, 2013). For the first time, the latest round of PDHS included the WHO's Infant and Young Child Feeding module. Thus, it offers a new opportunity to explore the current state of complementary feeding practices. Prior analyses in Bangladesh (Kabir et al., 2012), India (Patel et al., 2012), Nepal (Joshi, Agho, Dibley, Senarath, & Tiwari, 2012), and Sri Lanka (Senarath, Godakandage, Jayawickrama, Siriwardena, & Dibley, 2012) have revealed a number of strong and consistent risk factors, including younger child age, poor maternal education, and low household wealth, yet data had not been previously available for Pakistan. In this exploratory study, we aim to confirm empirically if these and/or other risk factors are independently associated with poor feeding practices in this context. In addition to proximal risk factors at individual and household level, we wish to examine contextual factors, such as access to health care and education at community level, which may also serve as underlying determinants of feeding practices (Stewart, Iannotti, Dewey, Michaelsen, & Onyango, 2013).
The aims of our analysis are (a) to describe the distribution of complementary feeding practices in Pakistan using the WHO indicators of complementary feeding, (b) to examine dietary diversity by disaggregating the consumption of individual food groups and identifying the key food groups, and (c) to identify factors at individual, household, and community levels that predict positive complementary feeding practices. The overall objective of this work is to better inform future program design and policy development in Pakistan and South Asia for better targeting and intervening to support the needs of vulnerable children.
Key messages.
Poor child feeding practices are prevalent in Pakistan. Delayed introduction of complementary foods and insufficient feeding frequency each affect one in three children. Most children (78%) have poor dietary diversity and only 15% receive a minimally acceptable diet.
Several nutrient‐rich food groups are rarely consumed; fewer than one in five children consume legumes, meat or fish, or vitamin A‐rich fruits and vegetables.
Common risk factors for poor child feeding are poverty and poor access to health and nutrition services in the community. This underlines the need to improve the capacity of programs, health professionals, and community workers to support good complementary feeding.
2. PARTICIPANTS AND METHODS
2.1. Study sample
The study uses data from the 2012–2013 PDHS. Comprehensive methods have been described previously (National Institute of Population Studies & ICF International, 2013). In brief, PDHS 2012–2013 included a nationally representative sample of ~14,000 households, excluding Azad Jammu and Kashmir, Federally Administered Tribal Areas, and restricted military and protected areas. Households were selected in two stages: the first stage was to randomly select enumeration blocks, known as the primary sampling units (PSUs, total n = 500), in urban (divisions of cities and towns, n = 248) and rural (villages, mouzas, a local administrative district, or dehs, known as hamlets, n = 252) areas; and the second stage was to select households randomly within each PSUs (on average 28 households/PSU selected) using a systematic sampling technique. For the purposes of this analysis, we use data from the Household Questionnaire, which collected information on dwelling characteristics, and the Woman's Questionnaire, which collected information from eligible women about their characteristics and those of their children, including foods consumed by their children in the previous day or night. The response rates of interviews for eligible households and women were 96.1% and 93.1%, respectively. We include in the analytic sample the youngest living singletons aged 6 to 23 months whose mothers were between the ages of 15–49 years, and we exclude children who did not usually live with their mothers at the time of the survey.
2.2. Complementary feeding practices outcome data
The four indicators of appropriate complementary feeding practices recommended by WHO are defined as follows (World Health Organization, 2010): introduction of solid, semi‐solid, or soft foods (intro) is defined as the proportion of infants 6–8 months of age who received solid, semi‐solid, or soft foods in the previous day or night. Minimum meal frequency (MMF) is defined as the proportion of breastfed and nonbreastfed children 6–23 months of age who received solid, semi‐solid, or soft foods the minimum number of times or more in the previous day or night. The recommended MMF differs by child age and current feeding mode (breastfed or nonbreastfed) and is defined as 2 times for breastfed infants 6–8 months, 3 times for breastfed children 9–23 months, or 4 times (including milk feeds) for nonbreastfed children 6–23 months. Minimum dietary diversity (MDD) is defined as the proportion of children 6–23 months of age who received foods from four or more food groups in the previous day or night. We generated seven standard food groups: (a) grains, roots, and tubers; (b) legumes and nuts; (c) dairy products; (d) flesh foods; (e) eggs; (f) vitamin A‐rich fruits and vegetables; and (g) other fruits and vegetables. We also examined consumption of individual food groups. Minimum acceptable diet (MAD) is defined as the proportion of children 6–23 months of age who received at least the MDD and the MMF in the previous day or night. MAD is met if breastfed infants received at least four food groups and the MMF for their age. For nonbreastfed children, feeding practices MAD is met if the child received at least four food groups (excluding dairy products) and two milk feeds and was fed solid, semi‐solid, or soft foods and dairy products at least 4 times in the previous day or night. All four outcomes, intro, MMF, MDD, and MAD, as well as the consumption of individual food groups, were expressed as dichotomous variables.
2.3. Risk factors
On the basis of prior research in South Asia (Joshi et al., 2012; Kabir et al., 2012; Khanal, Sauer, & Zhao, 2013; Patel et al., 2012; Senarath et al., 2012) and an existing conceptual framework (Stewart et al., 2013), we selected the explanatory variables from three levels: individual, household, and community characteristics.
We considered individual‐level factors describing child, maternal, and paternal characteristics. Child characteristics included child sex, breastfeeding status, age, birth order, birth interval, use of micronutrient supplements (vitamin A and iron), vaccination status, and child morbidity in the previous 2 weeks (diarrhea, fever, and cough). Maternal characteristics included age, smoking status, reproductive health care, education, occupation, work place, marital status, exposure to media, ethnicity, and empowerment. An empowerment index, ranging from 0 to 5, was created by summing five dichotomized items querying women's involvement in decision making on (a) allocation or use of income earned by husband or partner, (b) large household purchases, (c) visiting family and friends, (d) own health care, and (e) attitude towards domestic violence (Na, Jennings, Talegawkar, & Ahmed, 2015). Paternal characteristics included age, education and occupation.
Household characteristics included general indicators (sex of household head, number of household members, number of children 0–59 months old, and types of cooking fuel), sanitation characteristics (indicators measuring access to improved drinking water and improved toilet facilities), and a composite PDHS‐derived wealth index from principal component analysis using socioeconomic data and categorized into quintiles (Rutstein & Johnson, 2004).
We generated community‐level variables using information from all surveyed households or respondents in each of the 489 PSUs to capture characteristics of the community in which women and children live. General characteristics include rural or urban location, geographic region, proportion of women who completed primary or higher education, mean level of women's empowerment, and access to sanitation facilities (percentage with improved toilets and percentage with shared toilets). We created a summary score reflecting general access to maternal and child health and nutrition services at the community level including 10 indicators describing coverage of child vaccination, percentage of reproductive health care usage (percentage of health facility delivery, caesarean delivery, professional assisted delivery, appropriate number of antenatal care visits, and receipt of a timely postnatal checkup for women and children), and coverage of nutritional supplementation in women and their youngest children aged 0–5 years (child vitamin A supplementation and iron supplementation for children and women). Communities were ranked on each indicator and the summed rank of all indicators was created as the composite index of overall access to health and nutrition services. Scores were categorized into quintiles for analysis.
2.4. Statistical analysis
We used STATA/SE 14.1 to analyze data. Demographic and socioeconomic characteristics at the individual, household, and community level and the distribution of complementary feeding practices were all described adjusting for the complex sampling design of the PDHS using the “svy” command. The Taylor series linearization method was used to estimate confidence intervals around prevalence estimates (Wolter, 2007). The Pearson's chi‐squared statistics corrected for complex survey design were computed to test independence between feeding practices by child age and feeding status. A score test for trend of odds was applied to test linear trend in proportions by child characteristics.
To investigate the association between potential risk factors and the four complementary feeding indicators in all eligible children under the hierarchical survey structure of PDHS, we applied multilevel models to generate robust standard errors around estimates of association on two levels of factors: individual or household level and community level factors. Because bias may be introduced if variance within the PSU sample is large (Kravdal, 2006), we have checked the intraclass correlation (ICC) in each community‐level attribute to understand potential bias.
First, an intercept only model (null model and no risk factors) was fitted to understand the source of variance at individual or household (level 1) and community (level 2) levels. Log likelihood ratio tests were used to compare null models with single‐level models. Second, bivariate multilevel logistic models (random intercept only) were fit for each outcome variable and risk factor pairs. For categorical variables, the referent group was chosen as the group with the largest sample size. Third, multivariable multilevel logistic models were constructed through backward stepwise selection at p = .1 level. Fourth, collinearity was checked by estimating variance inflation factors (VIFs) and variables with VIF > 5 were removed from multivariable models one at a time beginning with the variable with highest VIF until all were reduced below 5. Fifth, a final set of multivariable multilevel logistic models were performed on variables that satisfied both significance (step 3) and noncollinearity (step 4) criteria. The multilevel logistic models were developed without adjusting for the multistage sampling design and are considered as internally valid (Rutstein & Rojas, 2006). The external validity of results was examined by comparing the demographic and socioeconomic characteristics between included and excluded samples and by comparing final results to those in the published literature.
A set of sensitivity analyses were performed to check the validity and consistency of the multilevel models, including (a) selecting only variables with p values <.05 in the full model for stepwise backward elimination; (b) fixing covariates including child sex and maternal and paternal age in the final models; and (c) comparing results from current models with results from traditional multivariate logistic regression and three‐level multilevel models (level 1: individual or household, level 2: community, and level 3: region).
3. RESULTS
3.1. Sample characteristics
The sample was drawn from 489 communities and the total unweighted and weighted sample sizes were 2,827 and 2,806, respectively. Individual, household, and community characteristics are presented in Table 1. About half of the children were girls (49%), second to fourth born (50%), born with an interpregnancy interval of 24 months or longer (51%) and had completed age‐appropriate vaccinations (50%). The majority of the mothers were between 25 to 34 years (56%) and did not work outside their homes (76%). About half of the women had delivered at a health facility (53%) or had been assisted by a skilled attendant (56%) at delivery; however, only 37% had visited an antenatal clinic at least 4 times during their last pregnancy. As many as 38% of the mothers and 49% of the children did not have a postnatal checkup record. Most of the fathers were older than 25 years (91%) and were involved in nonagricultural occupations (83%). The proportion of households having an improved source of drinking water and an improved toilet facility were 90% and 69%, whereas only 36% used efficient cooking fuel (electricity, LPG, natural gas, and biogas). The ICC of community characteristics ranged from 0.29 to 0.69.
Table 1.
Characteristics at individual, household, and community level in Pakistan, 2012–2013
| N | % or mean (SE) | |
|---|---|---|
| Child characteristics | ||
| Female | 2,806 | 48.8 |
| Currently breastfed | 2,806 | 75.7 |
| Age (months) | 2,806 | |
| 6–11 | 35.2 | |
| 12–17 | 41.3 | |
| 18–23 | 23.5 | |
| Birth order | 2,806 | |
| Firstborn | 24.6 | |
| Second to fourth | 49.6 | |
| Fifth and more | 25.9 | |
| Birth interval (month) | 2,806 | |
| No previous birth | 24.6 | |
| <24 | 24.6 | |
| ≥24 | 50.8 | |
| Perceived birth weight | 2,800 | |
| Smaller than average | 20.5 | |
| Average | 74.5 | |
| Larger than average | 5.1 | |
| Received vitamin A supplementation in the past 6 months | 2,769 | 71.6 |
| Received iron pills, sprinkles, or syrup in the last 7 days | 2,802 | 8.0 |
| Complete age‐appropriate vaccination | 2,778 | 49.5 |
| Child health: had the following symptom in the past 2 weeks | ||
| Diarrhea | 2,804 | 34.3 |
| Fever | 2,804 | 47.9 |
| Cough | 2,803 | 40.4 |
| Maternal characteristics | ||
| Age (years) | 2,806 | |
| 15–24 | 29.4 | |
| 25–34 | 56.1 | |
| 35–49 | 14.5 | |
| Smoker | 2,802 | 3.4 |
| Reproductive health care | ||
| Delivered at health facility | 2,805 | 52.6 |
| Type of delivery assistance | 2,799 | |
| Health professional | 56.3 | |
| Traditional birth attendant | 37.5 | |
| Other | 6.3 | |
| Caesarean delivery | 2,801 | 16.0 |
| Antenatal clinic (ANC) visits | 2,802 | |
| None | 21.7 | |
| 1–3 | 41.0 | |
| ≥4 | 37.3 | |
| Postnatal checkup on woman | 2,806 | |
| 0–1 day | 59.8 | |
| ≥2 day | 2.3 | |
| Missing or unknown | 37.9 | |
| Postnatal checkup on child | 2,806 | |
| 0–1 day | 41.0 | |
| ≥2 day | 10.4 | |
| Missing or unknown | 48.7 | |
| Highest educational level: primary or higher | 2,806 | 45.3 |
| Occupation | 2,806 | |
| Not working | 75.8 | |
| Agricultural | 9.1 | |
| Nonagricultural | 15.1 | |
| Working outside home | 2,802 | 12.5 |
| Currently married | 2,806 | 99.2 |
| Exposure to media: at least once a week | ||
| Reading newspaper | 2,796 | 2.9 |
| Listening to radio | 2,806 | 2.2 |
| Watching TV | 2,805 | 45.1 |
| Involved in decision making on | ||
| how man's income is used | 2,765 | 40.7 |
| large household purchases | 2,778 | 40.7 |
| visiting family and friends | 2,781 | 43.7 |
| regarding own health care | 2,779 | 47.5 |
| Attitude towards domestic violence: beating justified if | ||
| goes out without telling him | 2,802 | 33.0 |
| neglects the children | 2,800 | 35.1 |
| argues with him | 2,802 | 36.5 |
| refuses to have sex with him | 2,802 | 33.0 |
| burns the food | 2,800 | 20.2 |
| none above | 2,802 | 51.1 |
| Women's empowerment score (5 items) | 2,760 | 2.2 |
| Ethnicity | 2,806 | |
| Punjabi | 36.4 | |
| Pushto | 15.3 | |
| Siraiki | 16.1 | |
| Sindhi | 9.4 | |
| Urdu | 7.9 | |
| Balochi/Baraunhi | 6.3 | |
| Other | 8.6 | |
| Paternal characteristics | ||
| Age (years) | 2,775 | |
| 15–24 | 9.0 | |
| 25–34 | 49.1 | |
| ≥35 | 41.9 | |
| Highest educational level: primary or higher | 2,802 | 67.6 |
| Occupation | 2,805 | |
| Not working | 1.7 | |
| Agricultural | 15.4 | |
| Nonagricultural | 82.9 | |
| Household characteristics | ||
| Female household head | 2,806 | 8.2 |
| No. of HH members | 2,806 | 9.0 |
| No. of children younger than 5 years | 2,806 | 2.3 |
| Types of cooking fuel | 2,419 | |
| Electricity, LPG, natural gas, biogas | 35.7 | |
| Wood, straw/shrubs/grass, animal dung, and other | 64.3 | |
| Water source | ||
| Unimproved source of drinking water | 2,795 | 10.0 |
| Source for water not in own dwelling or yard/plot | 2,064 | 34.0 |
| Time to get to water source (minutes) | 2,786 | |
| 0 | 76.3 | |
| 1–59 | 20.9 | |
| ≥60 | 2.8 | |
| Toilet condition | ||
| Unimproved toilet facility | 2,806 | 30.7 |
| Shared toilet with other households | 2,648 | 37.7 |
| Household wealth | 2,806 | |
| Poorest | 21.2 | |
| Poorer | 22.4 | |
| Middle | 20.0 | |
| Richer | 20.7 | |
| Richest | 15.6 |
| Community characteristics | N of PSUs | % or mean (SE) |
|---|---|---|
| Rural residence | 489 | 65.8 |
| Geographical region | 489 | |
| Punjab | 59.5 | |
| Sindh | 22.4 | |
| Khyber pakhtunkhwa | 13.0 | |
| Balochistan | 3.8 | |
| Gilgit | 0.7 | |
| Islamabad | 0.6 | |
| % women completed primary or higher education | 489 | 44.3 (1.4) |
| Mean women's empowerment | 489 | 2.6 (0.04) |
| Access to health care | ||
| % children completed age‐appropriate vaccine | 489 | 48.5 (1.3) |
| % delivered at health facility | 489 | 55.3 (1.6) |
| % delivered with professional assistance | 489 | 59.1 (1.6) |
| % had caesarean delivery | 489 | 17.8 (0.9) |
| % had ≥4 ANC visits | 489 | 40.6 (1.4) |
| % postnatal checkup on woman within 1 day of delivery | 489 | 59.3 (1.5) |
| % postnatal checkup on child within 1 day of delivery | 489 | 42.7 (1.7) |
| % children 0–5 years received vitamin A in the last 6 months | 489 | 65.9 (1.3) |
| % children 0–5 years received iron pills, sprinkles, or syrup in the last 7 days | 489 | 8.2 (0.6) |
| % women given or bought iron tablets during pregnancy | 489 | 46.9 (1.1) |
| Average rank of access to health care | 489 | 227.2 (4.7) |
| Sanitation condition | ||
| % unimproved toilet | 489 | 27.7 (1.8) |
| % sharing toilet with other households | 489 | 32.0 (1.7) |
3.2. Complementary feeding practices
Figure 1 presents the distribution of complementary feeding practices by child age group and by feeding status. The overall weighted proportion (95% confidence interval, CI) of intro, MMF, MDD, and MAD were 67% (60%, 72%), 63% (60%, 66%), 22% (19%, 25%), and 15% (13%, 17%), respectively. Intro did not differ between breastfed [66% (59%, 72%)] and nonbreastfed [71% (52%, 85%)] children. However, the proportion of children who met the MMF criteria was significantly lower among breastfed than among nonbreastfed children regardless of child age. The proportion of children who met the MMF, MDD, and MAD criteria increased with age. Among children 6–11 months, 53% (49%, 57%), 12% (10%, 15%), and 10% (7%, 12%) met the MMF, MAD, and MAD criteria, respectively. The corresponding proportions for children 12–17 months of age were 64% (60%, 69%), 24% (20%, 28%), and 16% (13%, 19%). Among children 18–23 months of age, the proportion of meeting MMF, MDD, and MAD criteria was the highest: 74% (69%, 79%), 34% (28%, 40%), and 22% (17%, 27%), respectively.
Figure 1.
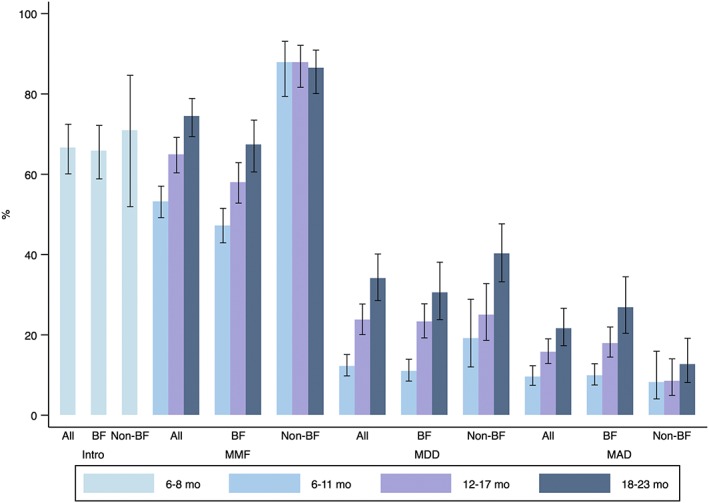
Distribution of complementary feeding practices among children aged 6–23 months by age group and feeding status. Error bars are the lower and upper 95% confidence bounds of the proportion. BF = breastfed; non‐BF = nonbreastfed; intro = introduction of solid, semi‐solid, and soft food; MMF = minimum meal frequency; MDD = minimum dietary diversity; MAD = minimum acceptable diet
Figure 2 shows the number (panel a) and kinds of food groups (panel b) consumed by children in the three age groups. The mean (95% CI) dietary diversity score was 1.8 (1.7, 1.9), 2.6 (2.5, 2.7), and 3.0 (2.8, 3.1) in children aged 6–11, 12–17, and 18–23 months, respectively (Figure 2a). In children aged 6–11 months, the cumulative proportion of children being fed 0 (16%), 1 (28%), or 2 (29%) food groups reached 73%. In our sample, the most commonly consumed food groups were grains, roots, and tubers (81%) and dairy products (59%). Eggs and other fruits and vegetables were consumed by only 25% and 34% of the sampled children, respectively. Legumes and nuts (6%), flesh foods (18%), and vitamin A‐rich fruits and vegetables (19%) were least consumed by children aged 6–23 months (Figure 2b).
Figure 2.
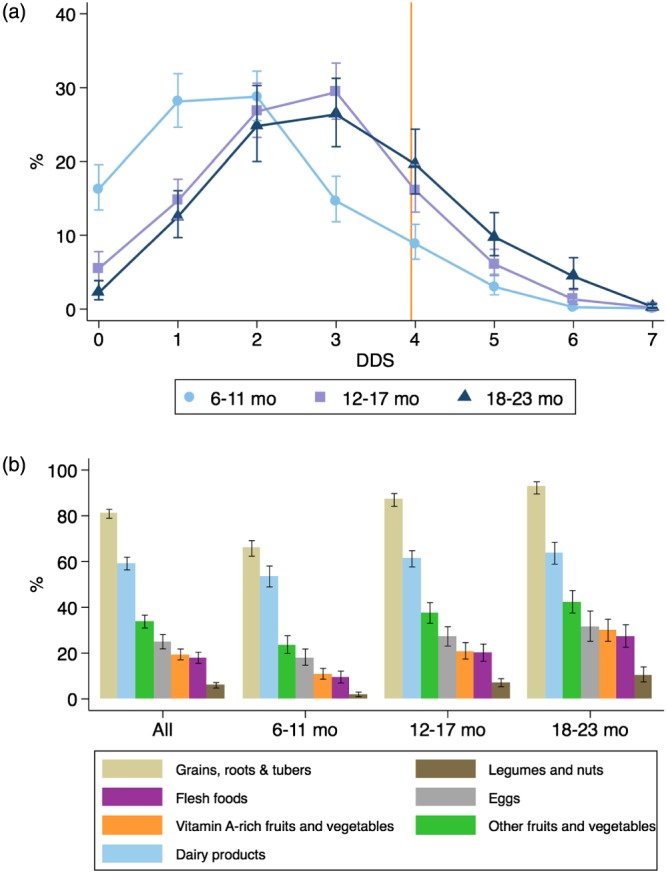
Patterns of food group consumption by child age group. (a) Proportion of children consuming different number of food groups and (b) proportion of food group consumption by child age. The orange vertical line indicates the minimum required four food groups per WHO's recommendation. Error bars are the lower and upper 95% confidence bounds of the proportion. DDS = dietary diversity score
Among children who met the MDD criterion (consumed ≥4 food groups, Figure 3), grains, roots, and tubers (99%), dairy products (84%), other fruits and vegetables (77%), and eggs (65%) were commonly fed; however, legumes and nuts (15%), flesh foods (49%), and vitamin A‐rich fruit and vegetables (55%) were consumed less frequently. The most common combination of food groups, reported by 16% of those meeting the MDD criterion, was grains, roots, and tubers (G); dairy products (D); other fruits and vegetables (O), and eggs (E). The next common food group combinations included flesh foods (F) and were consumed by an additional 25% children who were fed the 4 or more groups (G + D + O + F, G + D + O + F + E, G + D + E + F). Although flesh foods and vitamin A‐rich fruits and vegetables tended to be more commonly fed to older children (p trend for both foods >.05), egg consumption showed an opposite trend: among children who consumed four food groups in the previous day, the proportion of egg consumption was 72%, 54%, and 52% in 6–11, 12–17, and 18–23 months old children (p trend = .003); a similar trend was seen in children who were fed five food groups; among these children the corresponding proportions were 91%, 86%, and 58%, respectively (p trend = .08). However, there was no significant trend in the proportions of children eating any eggs or flesh foods (when combining the two groups) in children over age.
Figure 3.
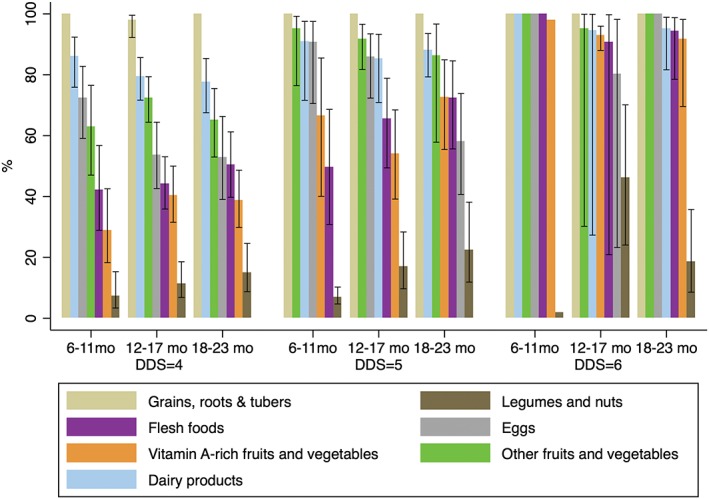
Proportion of food group consumption by child age among children with minimum dietary diversity (dietary diversity score [DDS] = 4, 5, or 6. Children with DDS = 7 was excluded because there is no variation). Error bars are the lower and upper 95% confidence bounds of the proportion
3.3. Independent factors in multivariable multilevel models
The adjusted odds ratios (95% CI) for positive complementary feeding practices in all eligible children are shown in Table 2 (see Table S1 for bivariate associations). Among the individual and household level factors, the odds of not meeting the intro criterion was significantly higher among mothers having insufficient antenatal clinic visits (1–3 times) and not having a recorded postnatal checkup. Fewer antenatal clinic visits and delayed, missing, or unknown postnatal checkups on mothers were independent factors for not meeting MMF. However, having a delayed postnatal checkup on the infant (beyond the second day after delivery) was associated with 59% increased odds to meet the MMF criterion, compared to children who had their postnatal checkup within 1 day of delivery [1.59 (1.11, 2.28)]. Older child age (12–17 and 18–23 months, as compared to 6–11 months) increased the odds of meeting MMF by about two folds [1.77 (1.44, 2.17) and 2.09 (1.63, 2.67), respectively]. Significant risk factors associated with not meeting MDD and MAD were similar, including younger maternal age, missing, or unknown postnatal checkup on women and living in a poorer household. Older child age was associated with a ~2.5 fold higher odds of meeting the MDD and MAD criteria in children aged 12–17 months and a ~4‐fold higher odds in children aged 18–23 months as compared to children aged 6–11 months (reference).
Table 2.
Significant factors (OR [95% CI]) to appropriate complementary feeding practices per WHO's indicators using multivariable multilevel logistic regression analysisa
| Intro | MMF | MDD | MAD | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Child characteristics | ||||||||
| Age (months) | ** | ** | ** | |||||
| 6–11 | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||||
| 12–17 | 1.77 (1.44, 2.17) | 2.69 (2.08, 3.48) | 2.50 (1.89, 3.32) | |||||
| 18–23 | 2.09 (1.63, 2.67) | 4.27 (3.22, 5.68) | 3.92 (2.87, 5.37) | |||||
| Birth interval (month) | 0.08 | |||||||
| No previous birth | 1.35 (1.02, 1.80) | |||||||
| <24 | 1.23 (0.95, 1.59) | |||||||
| ≥24 | 1.00 (referent) | |||||||
| Perceived birth weight | * | |||||||
| Smaller than average | 0.66 (0.49, 0.87) | |||||||
| Average | 1.00 (referent) | |||||||
| Larger than average | 0.82 (0.54, 1.26) | |||||||
| Maternal characteristics | ||||||||
| Age (years) | * | * | ||||||
| 15–24 | 0.67 (0.52, 0.87) | 0.71 (0.54, 0.92) | ||||||
| 25–34 | 1.00 (referent) | 1.00 (referent) | ||||||
| 35–49 | 0.91 (0.68, 1.23) | 0.74 (0.53, 1.02) | ||||||
| Antenatal clinic visits | ** | ** | ||||||
| None | 0.95 (0.44, 2.02) | 0.81 (0.60, 1.09) | ||||||
| 1–3 | 0.46 (0.26, 0.82) | 0.64 (0.50, 0.80) | ||||||
| ≥4 | 1.00 (referent) | 1.00 (referent) | ||||||
| Postnatal checkup on woman | ** | ** | ** | ** | ||||
| 0–1 day | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| ≥2 day | 0.49 (0.15, 1.66) | 0.53 (0.31, 0.90) | 0.62 (0.34, 1.14) | 0.51 (0.26, 1.03) | ||||
| Missing or unknown | 0.39 (0.22, 0.69) | 0.65 (0.51, 0.82) | 0.50 (0.38, 0.65) | 0.56 (0.42, 0.75) | ||||
| Postnatal checkup on child | * | |||||||
| 0–1 day | 1.00 (referent) | |||||||
| ≥2 day | 1.59 (1.11, 2.28) | |||||||
| Missing or unknown | 1.25 (0.97, 1.60) | |||||||
| Occupation | 0.09 | |||||||
| Not working | 1.00 (referent) | |||||||
| Agricultural | 0.31 (0.11, 0.91) | |||||||
| Nonagricultural | 1.07 (0.53, 2.17) | |||||||
| Household characteristics | ||||||||
| Household wealth | 0.07 | 0.15 | ** | * | ||||
| Richest | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | ||||
| Richer | 1.09 (0.46, 2.58) | 0.79 (0.57, 1.11) | 0.67 (0.49, 0.93) | 0.67 (0.47, 0.96) | ||||
| Middle | 0.69 (0.30, 1.57) | 0.78 (0.54, 1.12) | 0.59 (0.41, 0.85) | 0.60 (0.40, 0.88) | ||||
| Poorer | 0.36 (0.15, 0.86) | 0.61 (0.41, 0.91) | 0.56 (0.38, 0.83) | 0.49 (0.32, 0.75) | ||||
| Poorest | 0.48 (0.19, 1.23) | 0.63 (0.41, 0.97) | 0.44 (0.28, 0.69) | 0.47 (0.30, 0.76) | ||||
| Community characteristics | ||||||||
| Geographical region | ** | ** | ** | |||||
| Punjab | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||||
| Sindh | 1.98 (1.43, 2.74) |
1.16 (0.81, 1.66) |
1.22 (0.84, 1.76) | |||||
| Khyber pakhtunkhwa | 1.89 (1.37, 2.61) | 1.52 (1.06, 2.18) | 1.43 (0.98, 2.09) | |||||
| Balochistan | 1.83 (1.19, 2.81) | 0.94 (0.55, 1.62) | 1.34 (0.77, 2.34) | |||||
| Gilgit | 1.69 (1.09, 2.63) | 4.03 (2.48, 6.53) | 3.88 (2.38, 6.33) | |||||
| Islamabad | 2.79 (1.67, 4.66) | 2.17 (1.32, 3.55) | 1.88 (1.14, 3.08) | |||||
| Rank of access to health care | 0.16 | ** | ** | |||||
| Highest (best access) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | |||||
| Higher | 1.11 (0.75, 1.63) | 1.05 (0.71, 1.56) | 1.14 (0.76, 1.71) | |||||
| Medium | 1.56 (1.03, 2.37) | 0.88 (0.57, 1.36) | 0.98 (0.63, 1.53) | |||||
| Lower | 1.24 (0.80, 1.92) | 0.79 (0.50, 1.26) | 0.78 (0.48, 1.25) | |||||
| Lowest (worse access) | 1.11 (0.67, 1.83) | 0.38 (0.21, 0.67) | 0.41 (0.23, 0.75) | |||||
Note. intro = introduction of solid, semi‐solid, and soft foods; MMF = minimum meal frequency; MDD = minimum dietary diversity; MAD = minimum acceptable diet; OR = odds ratio; CI = confidence interval.
Both the bold and italics are to indicate significant (at p < 0.05 level) at individual level of categorical variables.
Models are controlled for breastfeeding status. p values are from the chi‐square tests of the overall significance for categorical variables.
p < .05.
p < .01.
At the community level, regional differences were evident in MDD and MAD. Punjab (the referent group) was the region with relatively poorer MMF, MDD, and MAD indices. Even after controlling for factors at individual and household level, including household wealth, low general access to health and nutrition services at community level was significantly associated with poor feeding practices according to WHO's MDD and MAD indicators.
4. DISCUSSION
Using the most recent nationally representative data collected in 2012–2013, we examined risk factors to poor complementary feeding practices in Pakistan, where about two thirds of the children have met the criteria for timely introduction of complementary foods and MMF and only about one in five children have met the MDD and MAD criteria. Poor diet diversity is a bigger problem than feeding frequency in Pakistani children aged 6–23 months. Using the most recent national data, cross‐comparison among countries in South Asia on MAD has ranked Pakistan (15%) the lowest among Nepal (32%; Central Bureau of Statistics, 2015), Bangladesh (23%; National Institute of Population Research and Training, Mitra and Associates, & ICF International, 2014), and India (21%; International Institute for Population Sciences, 2007). The overall poor quality of Pakistani children's complementary is alarming.
Unpacking dietary diversity by the food groups consumed by children, we find a universally low intake of all food groups except grains, roots, and tubers and dairy products, especially among infants aged 6–11 months. Even among children who were fed the minimum required four food groups, legumes and nuts, vitamin A‐rich fruits and vegetables, and flesh foods were not included in the diet of 45–85% of the children. The decreasing trend in egg consumption with age was unlikely due to chance and may be due to a substitution effect between eggs and flesh foods in older children. These less consumed foods are important dietary sources of essential fatty acids, multiple micronutrients, bioactive compounds, and animal‐source foods of high digestibility, which are known to be critical for optimal child growth and development (Allen, Peerson, & Olney, 2009; Iannotti, Lutter, Bunn, & Stewart, 2014; Koletzko et al., 2008). Indicators of greater food group diversity are strongly associated with micronutrient adequacy of the diet (Moursi et al., 2008; Steyn, Nel, Nantel, Kennedy, & Labadarios, 2006; Working Group on Infant and Young Child Feeding Indicators, 2006) and with a lower risk of stunted growth in developing countries (Arimond & Ruel, 2004; Rah et al., 2010). Eating a variety of foods in early infancy and childhood is also important to help shape healthy eating behaviors in adolescence and adult life (Nicklaus, Boggio, Chabanet, & Issanchou, 2005). Policies aiming at addressing the dietary diversity gap in children's diets should emphasize intake of key nutrient‐dense food groups at all ages, with emphasis on the latter half of infancy.
Among the individual and household independent risk factors, younger child age consistently increased the odds of not meeting the criteria of MMF, MDD, and MAD. Our finding is in line with previous studies in which younger children had higher odds of being fed inadequately in terms of frequency and variety in Bangladesh (Kabir et al., 2012), India (Patel et al., 2012), and Sri Lanka (Senarath et al., 2012). Perceived child size at birth was associated with less variety of foods given to the child and it may be explained by specific cultural beliefs that a “weak” child cannot digest many different kinds of foods. Younger maternal age and poorer household wealth were strong predictors in relation to MDD and MAD. In resource‐limited settings, better household wealth has been positively associated with higher dietary diversity at both household and maternal levels (Na, Gross, & West, 2015; Na et al., 2016; Thorne‐Lyman et al., 2010). Studies conducted in Bangladesh have shown that children's dietary diversity was compromised in food insecure households (Ali et al., 2013), likely due to less nutritious foods under food stress (Saha et al., 2008). We did not see a strong relationship between household wealth and the timing and frequency of feeding complementary foods, which may suggest that these feeding practices are less‐resource dependent. Previous studies have identified household wealth as a consistent predictor of dietary diversity and/or overall diet adequacy in young children, but not of meal frequency (Joshi et al., 2012; Kabir et al., 2012; Senarath et al., 2012). Though children's energy intake is likely prioritized in the South Asian context (Abdullah & Wheeler, 1985; Kramer, Peterson, Rogers, & Hughes, 1997), achieving MMF alone may not be sufficient to meet energy requirements. Evidence from Bangladesh has shown that the amounts of complementary foods consumed is lower than required, especially in younger children (Arsenault & Brown, 2017; Kimmons et al., 2005).
Contrary to previous studies, maternal or paternal education, was not independently associated with any of the feeding practices examined (Senarath et al., 2012). Comparing to previous research, our study included more variables at the household and community levels that likely predict women's time management (e.g., time to get water, shared toilet, access to health care, and women's empowerment). These factors correlate with women's access to education and average education level, which may explain that in our analysis women's education was not independently associated with child feeding indicators as seen in other studies.
At community level, the odds of achieving minimum requirements on meal frequency, dietary diversity, and overall acceptable diet varied significantly by geographic region. Children from communities with poor access to health care were at lower odds to be fed with MDD and MAD, even after adjusting for individual and household level factors. Indicators of maternal access to health care, such as the number of antenatal clinic visits and timing of a postnatal checkup on women were strong predictors of several of the complementary feeding practices examined, even after adjustment for demographic and socioeconomic factors. These findings suggest that caregivers may have acquired child feeding advice through health care system (World Health Organization & UNICEF, 2003). The identification of independent community‐level indicators has programing and policy implications in targeting and intervening the vulnerable at larger scale. For example, effective nutrition education and counselling delivered in the at‐risk regions and communities may generate large impact in improving feeding knowledge and practices. In Bangladesh, for example, intensive counseling combined with a nationwide mass media campaign improved the proportion of meeting MAD substantially from 16% to 50% (Menon et al., 2016). In Pakistan, community health workers may provide an effective channel to deliver feeding education at scale. There are 90,000 lady health workers (LHWs) serving 70% of the rural population, with an average 1,000 people per LHW (Bhutta, Lassi, Pariyo, & Huicho, 2010). Integrating feeding education into LHW's routine work is culturally acceptable and feasible (Rahman et al., 2012) and a pilot study has proved its effectiveness in improving initiation of early and exclusive breast feeding in Pakistan (Bhutta et al., 2008).
The strengths of our study include the use of nationally representative data from the PDHS 2012–2013, which was well designed and implemented. The risk factor analysis was conducted by using rigorous multilevel models, which took into account the multistage structure of the PDHS data. The moderate‐to‐high (0.3–0.7) ICC in community‐level characteristics posed low risk of estimate bias of the multilevel models (Kravdal, 2006) and consistent results from sensitivity analyses indicated the robustness of our results (data not shown). Lack of differences in key characteristics between the included and the not included (data not shown) implies that our findings likely apply to the general population of infants and young children in Pakistan. However, our analysis is limited by the cross‐sectional nature of the PDHS 2012–2013, which does not allow one to draw casual inference or to fully understand the pathways leading to the observed results. We cannot rule out possibilities of reverse‐causality, bidirectional relationships, or residual confounding from unmeasured factors. Associations may be attenuated due to the day‐to‐day variability presented in a cross‐sectional data and there may be true associations that were not identified. In addition, the community‐level access to maternal and child health and nutrition services was created using available data. Given that this indicator appears to have some predictive validity in this study, future studies are necessary to provide evidence of construct validity, by comparing it to other measures of health service availability, quality, and utilization. There is a lack of information on many political, economic, and societal factors in PDHS 2012–2013 that prevents us from examining the full picture of risk factors of poor complementary feeding.
Despite these limitations, our study has a few important implications in terms of nutrition policy and program action. In terms of timing within the “window of opportunity,” our results suggest that complementary feeding practices in the second half of infancy (i.e., for children aged 6–11 months) should be given priority attention. At the individual level, interventions and programs addressing dietary diversity and the overall complementary diet should focus efforts on children born small, to younger mothers, and in poor households. At the community level, communities with lower access to maternal and child health and nutrition services should be prioritized. Enhancing the training of health and nutrition professionals and integrating educational interventions into the existing LHW Program may effectively improve complementary feeding practices among the most vulnerable populations at scale and at a low or marginal cost. Among the poorest communities, however, education and counselling alone may not be sufficient and has to be coupled with effective interventions that leverage resources to overcome economic barriers to accessing a diverse diet, through cash or other social transfer programs (Bhutta et al., 2013).
Poor complementary feeding is prevalent in Pakistan, where only about one in five children between 6 and 23 months are fed diets that meet the MDD and MAD criteria. The proportion of young children who meet the timely introduction of complementary food or MMF criteria were higher but below 70% in both cases. Consistent risk factors across multiple poor feeding indicators indicate the need to prioritize future interventions and programs for infants and children aged 6–11 months, whose mothers have lower or no access to prenatal and postnatal care services, from poorer households and from communities with poorer general access to health and nutrition services. A variety of strategies might be needed to improve practices and remove barriers to healthy, diverse diets for young children in Pakistan.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
MN, VMA, MA and CPS conceptualized the research question. MN requested data, conducted literature review, data analysis and prepared the first draft of the manuscript. VMA, MA and CPS provided technical support on study methods and insights on results interpretation. All authors read and approved the final manuscript.
Supporting information
Supplementary Table 1: Factors [OR(95%CI)] for positive complementary feeding practice per WHO's indicator using bivariate multilevel logistic regression analysis
ACKNOWLEDGEMENTS
We thank Shari S. Brown, Alessandra T. Mine and Srishti Tewari for their support on literature review and reference management.
Na M, Aguayo VM, Arimond M, Stewart CP. Risk factors of poor complementary feeding practices in Pakistani children aged 6–23 months: A multilevel analysis of the Demographic and Health Survey 2012–2013. Matern Child Nutr. 2017;13(S2):e12463 10.1111/mcn.12463
REFERENCES
- Abdullah, M. , & Wheeler, E. F. (1985). Seasonal variations, and the intra‐household distribution of food in a Bangladeshi village. The American Journal of Clinical Nutrition, 41, 1305–1313. [DOI] [PubMed] [Google Scholar]
- Ali, D. , Saha, K. K. , Nguyen, P. H. , Diressie, M. T. , Ruel, M. T. , Menon, P. , & Rawat, R. (2013). Household food insecurity is associated with higher child undernutrition in Bangladesh, Ethiopia, and Vietnam, but the effect is not mediated by child dietary diversity. The Journal of Nutrition, 143, 2015–2021. [DOI] [PubMed] [Google Scholar]
- Allen, L. H. , Peerson, J. M. , & Olney, D. K. (2009). Provision of multiple rather than two or fewer micronutrients more effectively improves growth and other outcomes in micronutrient‐deficient children and adults. The Journal of Nutrition, 139, 1022–1030. [DOI] [PubMed] [Google Scholar]
- Arimond, M. , & Ruel, M. T. (2004). Dietary diversity is associated with child nutritional status: Evidence from 11 demographic and health surveys. The Journal of Nutrition, 134, 2579–2585. [DOI] [PubMed] [Google Scholar]
- Arsenault, J. E. & Brown, K. H. (2017). Dietary protein intake in young children in selected low‐income countries is generally adequate in relation to estimated requirements for healthy children, except when complementary food intake is low. The Journal of Nutrition, jn239657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar, M. , Organization W.H . & Organization W.H . (1986). Physiological development of the infant and its implications for complementary feeding. [PMC free article] [PubMed]
- Bhutta, Z. A. , Das, J. K. , Rizvi, A. , Gaffey, M. F. , Walker, N. , Horton, S. , … Maternal and Child Nutrition Study Group (2013). Evidence‐based interventions for improvement of maternal and child nutrition: What can be done and at what cost? The Lancet, 382, 452–477. [DOI] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Lassi, Z. S. , Pariyo, G. , & Huicho, L. (2010). Global experience of community health workers for delivery of health related millennium development goals: a systematic review, country case studies, and recommendations for integration into national health systems. Global Health Workforce Alliance, 1, 249–261. [Google Scholar]
- Bhutta, Z. A. , Memon, Z. A. , Soofi, S. , Salat, M. S. , Cousens, S. , & Martines, J. (2008). Implementing community‐based perinatal care: Results from a pilot study in rural Pakistan. Bulletin of the World Health Organization, 86, 452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, R. E. , Cousens, S. , Johnson, H. L. , Lawn, J. E. , Rudan, I. , Bassani, D. G. , … Eisele, T. (2010). Global, regional, and national causes of child mortality in 2008: A systematic analysis. The Lancet, 375, 1969–1987. [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , De Onis, M. , … Uauy, R. (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. The Lancet, 382, 427–451. [DOI] [PubMed] [Google Scholar]
- Central Bureau of Statistics (2015). Nepal Multiple Indicator Cluster Survey 2014, Final Report.
- Dewey K. (2003). Guiding principles for complementary feeding of the breastfed child.
- Dewey, K. G. , & Adu‐Afarwuah, S. (2008). Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition, 4, 24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frongillo, E. A. , Nguyen, P. H. , Saha, K. K. , Sanghvi, T. , Afsana, K. , Haque, R. , … Menon, P. (2017). Large‐scale behavior‐change initiative for infant and young child feeding advanced language and motor development in a cluster‐randomized program evaluation in Bangladesh. The Journal of Nutrition, 147(2), 256–263. [DOI] [PubMed] [Google Scholar]
- Gross, R. , Giay, T. , Sastroamidjojo, S. , Schultink, W. , & Lang, N. T. (2000). Premature complementary feeding is associated with poorer growth of Vietnamese children. The Journal of Nutrition, 130, 2683–2690. [DOI] [PubMed] [Google Scholar]
- Horta, B. L. & Victora, C. G. (2013). Long‐term effects of breastfeeding‐a systematic review.
- Iannotti, L. L. , Lutter, C. K. , Bunn, D. A. , & Stewart, C. P. (2014). Eggs: The uncracked potential for improving maternal and young child nutrition among the world's poor. Nutrition Reviews, 72, 355–368. [DOI] [PubMed] [Google Scholar]
- Imdad, A. , Yakoob, M. Y. , & Bhutta, Z. A. (2011). Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health, 11, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Institute for Population Sciences (IIPS) M.I . (2007). India National Family Health survey (NFHS‐3), 2005–2006, volume I.
- Joshi, N. , Agho, K. E. , Dibley, M. J. , Senarath, U. , & Tiwari, K. (2012). Determinants of inappropriate complementary feeding practices in young children in Nepal: Secondary data analysis of Demographic and Health Survey 2006. Maternal & Child Nutrition, 8, 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir, I. , Khanam, M. , Agho, K. E. , Mihrshahi, S. , Dibley, M. J. , & Roy, S. K. (2012). Determinants of inappropriate complementary feeding practices in infant and young children in Bangladesh: Secondary data analysis of Demographic Health Survey 2007. Maternal & Child Nutrition, 8, 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal, V. , Sauer, K. , & Zhao, Y. (2013). Determinants of complementary feeding practices among Nepalese children aged 6–23 months: Findings from demographic and health survey 2011. BMC Pediatrics, 13, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmons, J. E. , Dewey, K. G. , Haque, E. , Chakraborty, J. , Osendarp, S. J. , & Brown, K. H. (2005). Low nutrient intakes among infants in rural Bangladesh are attributable to low intake and micronutrient density of complementary foods. The Journal of Nutrition, 135, 444–451. [DOI] [PubMed] [Google Scholar]
- Koletzko, B. , Lien, E. , Agostoni, C. , Böhles, H. , Campoy, C. , Cetin, I. , … Hoesli, I. (2008). The roles of long‐chain polyunsaturated fatty acids in pregnancy, lactation and infancy: Review of current knowledge and consensus recommendations. Journal of Perinatal Medicine, 36, 5–14. [DOI] [PubMed] [Google Scholar]
- Kramer, E. , Peterson, K. , Rogers, B. , & Hughes, M. (1997). Intrahousehold allocation of energy intake among children under five years and their parents in rural Bangladesh. European Journal of Clinical Nutrition, 51, 750–756. [DOI] [PubMed] [Google Scholar]
- Kravdal, Ø. (2006). A simulation‐based assessment of the bias produced when using averages from small DHS clusters as contextual variables in multilevel models. Demographic Research, 15, 1–20. [Google Scholar]
- Menon, P. , Nguyen, P. H. , Saha, K. K. , Khaled, A. , Sanghvi, T. , Baker, J. , … Rawat, R. (2016). Combining intensive counseling by frontline workers with a nationwide mass media campaign has large differential impacts on complementary feeding practices but not on child growth: Results of a cluster‐randomized program evaluation in Bangladesh. The Journal of Nutrition, 146, 2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health Government of Pakistan (2006). National maternal newborn and child health (MNCH) program 2006–2012. Islamabad, Pakistan: Government of Pakistan Ministry of Health. [Google Scholar]
- Moursi, M. M. , Arimond, M. , Dewey, K. G. , Trèche, S. , Ruel, M. T. , & Delpeuch, F. (2008). Dietary diversity is a good predictor of the micronutrient density of the diet of 6‐ to 23‐month‐old children in Madagascar. The Journal of Nutrition, 138, 2448–2453. [DOI] [PubMed] [Google Scholar]
- Na, M. , Gross, A. L. , & West, K. P. (2015). Validation of the food access survey tool to assess household food insecurity in rural Bangladesh. BMC Public Health, 15, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na, M. , Jennings, L. , Talegawkar, S. A. , & Ahmed, S. (2015). Association between women's empowerment and infant and child feeding practices in sub‐Saharan Africa: An analysis of Demographic and Health Surveys. Public Health Nutrition, 18, 3155–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na, M. , Mehra, S. , Christian, P. , Ali, H. , Shaikh, S. , Shamim, A. A. , … West, K. P. (2016). Maternal dietary diversity decreases with household food insecurity in rural Bangladesh: A longitudinal analysis. The Journal of Nutrition, 146, 2109–2116. [DOI] [PubMed] [Google Scholar]
- National Institute of Population Research and Training, Mitra and Associates, & ICF International (2016). Bangladesh demographic and health survey 2014. Dhaka, Bangladesh; Rockville, Maryland, USA. [Google Scholar]
- National Institute of Population Studies & ICF International (2013). Pakistan demographic and health survey 2012–2013. Islamabad, Pakistan; Calverton, Maryland, USA. [Google Scholar]
- Nicklaus, S. , Boggio, V. , Chabanet, C. , & Issanchou, S. (2005). A prospective study of food variety seeking in childhood, adolescence and early adult life. Appetite, 44, 289–297. [DOI] [PubMed] [Google Scholar]
- Patel, A. , Pusdekar, Y. , Badhoniya, N. , Borkar, J. , Agho, K. E. , & Dibley, M. J. (2012). Determinants of inappropriate complementary feeding practices in young children in India: secondary analysis of National Family Health Survey 2005–2006. Maternal & Child Nutrition, 8, 28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce, J. , Taylor, M. , & Langley‐Evans, S. (2013). Timing of the introduction of complementary feeding and risk of childhood obesity: a systematic review. International Journal of Obesity, 37, 1295–1306. [DOI] [PubMed] [Google Scholar]
- Rah, J. H. , Akhter, N. , Semba, R. D. , De Pee, S. , Bloem, M. W. , Campbell, A. A. , … Kraemer, K. (2010). Low dietary diversity is a predictor of child stunting in rural Bangladesh. European Journal of Clinical Nutrition, 64, 1393–1398. [DOI] [PubMed] [Google Scholar]
- Rahman, A. , Haq, Z. , Sikander, S. , Ahmad, I. , Ahmad, M. , & Hafeez, A. (2012). Using cognitive‐behavioural techniques to improve exclusive breastfeeding in a low‐literacy disadvantaged population. Maternal & Child Nutrition, 8, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutstein, S. O. , & Johnson, K. (2004). The DHS wealth index. Calverton, Maryland, USA: ORC Macro. [Google Scholar]
- Rutstein, S. O. , & Rojas, G. (2006). Guide to DHS statistics. Calverton, Maryland, USA: ORC Macro. [Google Scholar]
- Saha, K. K. , Frongillo, E. A. , Alam, D. S. , Arifeen, S. E. , Persson, L. Å. , & Rasmussen, K. M. (2008). Household food security is associated with infant feeding practices in rural Bangladesh. The Journal of Nutrition, 138, 1383–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem, A. F. , Mahmud, S. , Baig‐Ansari, N. , & Zaidi, A. K. (2014). Impact of maternal education about complementary feeding on their infants' nutritional outcomes in low‐and middle‐income households: a community‐based randomized interventional study in Karachi, Pakistan. Journal of Health, Population, and Nutrition, 32, 623. [PMC free article] [PubMed] [Google Scholar]
- Sawadogo, P. S. , Martin‐Prével, Y. , Savy, M. , Kameli, Y. , Traissac, P. , Traoré, A. S. , & Delpeuch, F. (2006). An infant and child feeding index is associated with the nutritional status of 6‐to 23‐month‐old children in rural Burkina Faso. The Journal of Nutrition, 136, 656–663. [DOI] [PubMed] [Google Scholar]
- Senarath, U. , Agho, K. E. , Akram, D. E. S. , Godakandage, S. S. , Hazir, T. , Jayawickrama, H. , … Pusdekar, Y. (2012). Comparisons of complementary feeding indicators and associated factors in children aged 6–23 months across five South Asian countries. Maternal & Child Nutrition, 8, 89–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senarath, U. , Godakandage, S. S. , Jayawickrama, H. , Siriwardena, I. , & Dibley, M. J. (2012). Determinants of inappropriate complementary feeding practices in young children in Sri Lanka: Secondary data analysis of demographic and health survey 2006–2007. Maternal & Child Nutrition, 8, 60–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart, C. P. , Iannotti, L. , Dewey, K. G. , Michaelsen, K. F. , & Onyango, A. W. (2013). Contextualising complementary feeding in a broader framework for stunting prevention. Maternal & Child Nutrition, 9, 27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn, N. , Nel, J. , Nantel, G. , Kennedy, G. , & Labadarios, D. (2006). Food variety and dietary diversity scores in children: are they good indicators of dietary adequacy? Public Health Nutrition, 9, 644–650. [DOI] [PubMed] [Google Scholar]
- Thorne‐Lyman, A. L. , Valpiani, N. , Sun, K. , Semba, R. D. , Klotz, C. L. , Kraemer, K. , … Bloem, M. W. (2010). Household dietary diversity and food expenditures are closely linked in rural Bangladesh, increasing the risk of malnutrition due to the financial crisis. The Journal of Nutrition, 140, 182S–188S. [DOI] [PubMed] [Google Scholar]
- UNICEF (2013). Improving child nutrition. The achievable imperative for global progress. [Google Scholar]
- Wolter, K. (2007). Introduction to variance estimation. New York: Springer. [Google Scholar]
- Working Group on Infant and Young Child Feeding Indicators (2006). Developing and validating simple indicators of dietary quality of infants and young children in developing countries: Additional analysis of 10 data sets. Washington, DC, USA: Food and Nutrition Technical Assistance Project (FANTA). [Google Scholar]
- World Bank (2014). Pakistan enhanced nutrition for mothers and children project.
- World Health Organization (2010). Indicators for assessing infant and young child feeding practices: part 2: measurement.
- World Health Organization & UNICEF (2003). Global strategy for infant and young child feeding. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Zaman, S. , Ashraf, R. N. , & Martines, J. (2008). Training in complementary feeding counselling of healthcare workers and its influence on maternal behaviours and child growth: A cluster‐randomized controlled trial in Lahore, Pakistan. Journal of Health, Population and Nutrition, 26(2), 210–222. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Factors [OR(95%CI)] for positive complementary feeding practice per WHO's indicator using bivariate multilevel logistic regression analysis


