Abstract
Background
Childhood adversity (CA) leads to greater vulnerability for psychopathology by causing structural as well as functional brain abnormalities. Recent findings on gray matter effects point towards the importance of identifying CA outcome as a function of different CA types, varying in the dimensions of threat and deprivation. Using diffusion tensor imaging, we investigate whether different forms of CA impact differently on white matter connectivity in a healthy cohort not confounded by other aspects of disease.
Methods
In 120 healthy young males, we assessed different forms of maltreatment during childhood with the Childhood Trauma Questionnaire (CTQ). Fractional anisotropy (FA) and mean diffusivity (MD) images were generated and projected onto a white matter skeleton using tract‐based spatial statistics. Correlational analysis between FA, MD, and CTQ subscores was then performed using voxelwise statistics.
Results
Of all CTQ‐subscores, only physical neglect (PN) predicted a decrease of FA but not MD in the bilateral anterior thalamic radiation around the middle frontal gyrus and the right inferior fronto‐occipital fasciculus, the inferior longitudinal fasciculus, the cingulum and precuneus. Reduced FA in the posterior cingulum was related to the effects of PN during childhood on anxiety levels at trend level.
Conclusions
PN may have severe consequences and should be considered equally important to more active forms of abuse. FA changes, particularly in the cingulum, actually appear to a functional consequence and are linked to trait anxiety, a personality dimension that is suggested to be a transdiagnostic risk factor of affective disorders. Potentially this reveals a mechanistic chain that forms one pathyway from CA to disease.
Keywords: childhood adversity, diffusion tensor imaging (DTI), fractional anisotropy, healthy controls, tract‐based spatial statistics (TBSS)
1. INTRODUCTION
There are significant relationships between adverse life events, psychosocial resources and well‐being. The general consensus is that childhood adversities (CA) can have a profound influence on behavioral, emotional, physical and cognitive functioning (Carr, Martins, Stingel, Lemgruber, & Juruena, 2013), leading to greater vulnerability for psychopathology. Indeed, CA is a consistently documented risk factor for psychiatric disorders (Cuijpers et al., 2011) whereby an estimated 30% of all psychiatric disorders is explained by exposure to childhood adversities (McLaughlin et al., 2012). The chance of developing psychiatric problems is highest in case of severely traumatic interpersonal childhood events, such as aggression or sexual‐ and emotional abuse (EA) (Spinhoven et al., 2010; Vrijsen et al., 2014). Frequent occurrence of childhood trauma increases the risk for psychiatric disorders even more (Hovens et al., 2010).
The experience of childhood trauma seems to cause several structural and functional brain differences as it hits the developing brain (Teicher & Samson, 2013). As such it is not surprising that extensive differences in adults can be found in gray matter (GM) volume of the amygdala, hippocampus, insula, caudate nucleus, orbitofrontal cortex, frontal‐ and postcentral gyri, anterior cingulate gyrus, and cerebellum when compared with controls with no history of adverse childhood experiences (Lim, Radua, & Rubia, 2014). Several studies have also taken into account that CA may as well affect interconnecting white matter structures and hence the communication between regions in addition to isolated brain areas as for example the corpus callosum (Jackowski et al., 2008). White matter structure can be measured by means of diffusion tensor imaging (DTI) and tractography. Fractional anisotropy (FA) measures the directionality of diffusion whilst mean diffusivity (MD) quantifies free diffusion of water within a voxel (Basser, 1995; Beaulieu, 2002). FA is commonly used in diffusion imaging and is sensitive to microstructural changes. It is less specific in regards to the type of change since myelination and other sources, including the axon itself, contribute to FA. MD is a measure of membrane density and is susceptible to changes in both grey and white matter tissue.
For example, Eluvathingal et al. (2006) investigated postinstitutionalized children who experienced socioemotional deprivation compared to controls by means of DTI and fiber tractography. They found lower values of FA in the left uncinate fasciculus, which connects the emotion regulation pathway between the orbitofrontal cortex and the anterior temporal lobe including the amygdala.
Recent findings point towards the importance of identifying CA outcome as a function of different CA subtypes, varying in the dimensions of threat and deprivation. Using tract‐based spatial statistics (TBSS), Choi, Jeong, Rohan, Polcari, and Teicher (2009) reported that high levels of exposure to parental verbal abuse most significantly led to reduced FA of the arcuate fasciculus that interconnects Broca and Wernicke's area, whereas visually witnessing domestic violence specifically affected the inferior longitudinal fasciculus, which connects visual and limbic systems. Notably, these CA‐dependent changes appear to be in the same region than in adolescents with major depressive disorder (Bessette, Nave, Caprihan, & Stevens, 2014). In this regard, Benedetti et al. (2014) investigated the impact of CA on the white matter integrity in bipolar patients and revealed changes in axial diffusivity in cortico‐limbic networks including the corona radiata, thalamic radiations, corpus callosum, cingulum bundle, superior longitudinal fasciculus, inferior fronto‐occipital fasciculus, and uncinate fasciculus.
Moreover, Tomoda et al. (2011) showed that parental verbal abuse was associated with GM volume changes in superior temporal gyrus/auditory cortex, whereas witnessing domestic violence was associated with reduced GM volume and thinning of portions of the visual cortex. Heim, Mayberg, Mletzko, Nemeroff, and Pruessner (2013) found that women who experienced childhood sexual abuse (SA) had thinning in the somatosensory cortex potentially overlapping with the region representing the genitals, whereas women reporting EA did not. These findings support a recent review from Teicher et al. (2016) showing that pathways that process and convey the aversive experience are subsequently affected. In this regard, the findings of Everaerd et al. (2016) are less clear. The authors compared voxel‐based morphometry data of young, healthy subjects reporting specific childhood exposure to abuse (n = 127) or deprivation (n = 126) and a similar sized group of controls (n = 129) without reported CA. Subjects were matched on age, gender, and educational level. Differences between CA types were found in the fusiform gyrus and middle occipital gyrus, where subjects with a history of deprivation showed reduced GM compared with subjects with a history of abuse. Taken together these studies provide an indication that also the CA‐related white matter effects have distinct signatures for different CA types.
Thus, we set out to investigate whether in line with the aforementioned GM changes different forms of CA also have a different impact on white matter connectivity in a healthy sample not confounded by other aspects of disease. We speculated that in line with the adversity‐specific changes in higher order cortical sensory areas, white matter bundles connecting these areas with emotion regulation areas, particularly the prefrontal cortex may be affected differentially depending on the form of CA.
2. METHODS AND MATERIALS
2.1. Participants
We opted to include males only in this exploratory study and not confound this exploratory study by the additional factor of gender because the menstrual cycle has been linked to fluctuations of white matter structure (Teicher & Samson, 2016).
We included 120 healthy men (sociodemographic characteristics described in Table 1). Candidates for participation were recruited using a local participant database and advertisements. Screening was conducted by self‐report questionnaires before participation, which was then reviewed by the investigator together with the subject prior to participation.Participants were excluded if the screening revealed a history of somatic disease potentially affecting the brain, current or past psychiatric or neurological disorder, medication or illicit drug use during the preceding 6 months, history of substance abuse, current or past alcohol dependence, or MRI contraindications. Three subjects were excluded based on technical failure with the MRI acquisition. Participants all signed informed consent before participation and received 60 Euros reimbursement for participating. The study was approved by the local ethics committee (CMO Region Arnhem‐Nijmegen, The Netherlands).
Table 1.
Sociodemographic and questionnaire data of study participants
| Age | 21.9 ± 2.9 (18–30) |
|---|---|
| BDI | 4.3 ± 4.1 (0–18) |
| STAI | 35.7 ± 7.8 (21–60) |
| CTQ total | 32.7 ± 6.3(25–56) |
| CTQ emotional neglect | 9.4 ± 3.4 (5–14) |
| CTQ emotional abuse | 6.7 ± 2.1 (5–14) |
| CTQ physical neglect | 6.4 ± 1.8 (5–15) |
| CTQ physical abuse | 5.3 ± 1.0 (5–13) |
| CTQ sexual abuse | 5.1 ± 0.5 (5–7) |
N = 120 representing the total sample included in the study; mean ± SD (range).
BDI, Beck Depression Inventory; STAI, State‐Trait Anxiety Inventory; CTQ, Childhood Trauma Questionnaire.
2.2. Questionnaires
In the present study, we specifically focus on the impact of CA, but the study was performed in the context of a larger project on individual differences in how stress affects the brain (e.g., Lisofsky et al., 2015). Maltreatment during childhood was assessed by administering the Dutch version of the Childhood Trauma Questionnaire (CTQ) (Everaerd, Klumpers, van Wingen, Tendolkar, & Fernández, 2015). The CTQ‐Short Form (CTQ‐SF), a short version of the CTQ, is a 25‐item self‐report questionnaire designed to assess five types of childhood maltreatment, namely EA, emotional neglect (EN), physical abuse (PA), physical neglect (PN), and SA. Participants rate statements about childhood experiences on a five‐point scale (1 = “never true” to 5 = “very often true”). Scores of the five individual items on the corresponding subscale were summed for each subscale, ranging from 5 to 25. The CTQ‐SF has been proven as a standardized and adequately validated instrument for retrospective assessment of maltreatment experiences (Everaerd et al., 2015). The Beck Depression Inventory (BDI) (Bernstein et al., 2003) and Spielberger's State‐Trait Anxiety Inventory (STAI) (Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) were included to further characterize subjects.
2.3. MR methods
DTI data were acquired using a 1.5 T Siemens Magnetom Avanto syngo MR B17 (Siemens Medical, Erlangen, Germany) using a 32‐channel head coil system. An 2d echo planar diffusion sequence was used with the following imaging parameters: 56 transversal slices, FOV 220 × 220 mm, TR/TE 8,000/98 ms, voxel size 2.5 mm3 isotropic, bandwidth 2,030 Hz/Px, diffusion sensitive gradient direction (diffusion directions) of 34 and a diffusion encoding gradient strength of b = 1,000 s/mm2.
2.4. DTI data analysis
Diffusion weighted images were preprocessed and analysed using the FSL software library 5.0 (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/, Smith et al., 2004). FA and MD images were created by fitting a tensor model to the raw diffusion weighted images using FDT (FMRIB's Diffusion Toolbox). Brain extraction was done using BET (Smith, 2002). Brain extracted data was then aligned into common space using FNIRT (FMRIB's nonlinear image registration tool (Anderson, Jenkinson, & Smith, 2007a, Anderson, Jenkinson, & Smith, 2007b) and a mean FA images was created and thinned to create a skeletonised image representing the centres of all tracts common to the group. The FA and MD images were then projected onto this skeleton and the resulting data fed into voxelwise statistics using TBSS (Smith et al., 2006).
To investigate whether the different CTQ subscales were associated with values of FA and MD analysis using each of the subscales as covariates and age as a nuisance variable was carried out. The same analysis was conducted for the total score of all the CTQ subscales. Demeaned values of these measures for each participant were added and voxelwise permutation based analysis using Randomise was performed with 5,000 permutations. Only images that were fully corrected for multiple comparisons using threshold‐free cluster enhancement and at p < .05 were considered.
2.5. Mediation analysis
Though the cross‐sectional nature of our study withheld us from drawing any conclusions on causality, we made use of a single‐level mediation analysis to investigate whether any potential regional white matter alterations associated with CTQ would be related to the impact of CTQ on trait anxiety. We performed the analysis with accelerated bias‐corrected bootstrap significance testing (10,000 bootstrap samples) as implemented in the M3 toolbox (http://wagerlab.colorado.edu/tools).
3. RESULTS
3.1. Behavioral results
Mean scores on CTQ, STAI, and BDI (Table 1) for this study population indicate a considerable variance within a subclinical range, despite a low incidence of perceived traumatic experiences. The CTQ was evaluated for each subscale separately and revealed that participants generally scored below the cutoff for moderate to severe trauma (Majer, 2010). As the subscales PN, EN, and EA show the most variance in this sample, they were used for further analysis.
Correlational analyses showed that CTQ‐sum scores correlated with STAI scores (rs(120) = .373; p = .000; see also Figure 1). Even after correcting for multiple comparisons, three of the five subscales of the CTQ correlated positively with the STAI score: EA (rs(120) = .333; p =.000), EN (rs(120) = .318; p = .000), and PN (rs(120) = .276; p = .002). PA (rs(120) = .170; p = .063) and SA (rs(120) = .161; p = .079) showed a statistical trend towards a positive correlation that do not survive correction for multiple comparisons. Moreover, when correlating the subscales with each other, EA correlated significantly with PA (rs(120) = .372; p = .000) and EN (rs(120) = .463; p = .000). PN was significantly correlated with EN. Though BDI scores correlated with CTQ‐total scores (rs(120) = .243; p = .008), they were not included in further analyses, since BDI scores show little variance in this sample.
Figure 1.
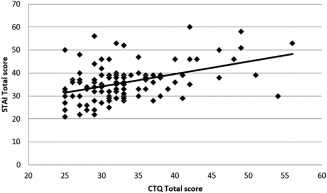
Correlation between sum scores of trait anxiety and childhood adversity. STAI = Spielberger's Trait Anxiety Inventory, CTQ = childhood trauma questionnaire
3.2. DTI results
Bearing in mind the differential effects of CA type on brain function and structure, we explored whether differences in white matter were associated with specific kinds of CA. Therefore, DTI data was related to the different CTQ scores indexing EA, EN, PA, PN, and SA. This was performed for the full CTQ scale using both FA and MD within the skeletonised areas following TBSS preprocessing with no results. Next, FA and MD were analyzed for correlations with CTQ‐subscale scores PN, EN, and EA, which showed the most variance in this sample.
Whole brain TBSS analyses revealed a negative correlation between CTQ‐PN and FA in the genu of the corpus callosum and the left anterior thalamic radiation. This can be taken as an indication that PN in childhood is reflected later in life as less directed diffusivity in these areas. However, taken into account that the distribution of CTQ‐PN scores was skewed, the analysis was repeated without three apparent outliers (=CTQ‐PN > 11). The area in corpus callosum found previously no longer showed a significant correlation with PN scores. Higher CTQ‐PN was, however, seen to predict FA reductions in several regions (Figure 2): bilateral anterior thalamic radiation around the middle frontal gyrus, which included the forceps minor and unciate fasciculus (Figure 2A, further mentioned as “forceps minor”) on the left side and the inferior fronto‐occipital fasciculus on the right side (Figure 2B, further mentioned as “atr”). Additional regions include the left inferior fronto‐occipital fasciculus and inferior longitudinal fasciculus (Figure 2C), and the posterior cingulum near the precuneus (Figure 2D, further mentioned as “cingulum”). No other CTQ‐subscales were found to correlate with FA, nor where there any findings for MD in relation to CTQ scores.
Figure 2.
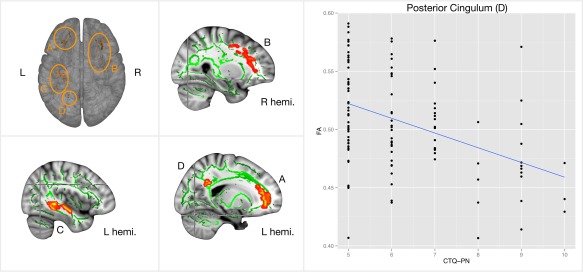
Displayed are fractional anisotropy images of all subjects (n = 114), which are projected onto a skeletonised image representing the centres of all tracts common to the group. Images are fully corrected for multiple comparisons using threshold‐free cluster enhancement at p < .05. The color‐coded areas (subfigure 1–4) depict the negative correlation between the physical neglect subscale of the CTQ in the bilateral anterior thalamic radiation around the middle frontal gyrus, which included the forceps minor and unciate fasciculus (area A) on the left side and the inferior fronto‐occipital fasciculus on the right side (area B). The regions also include the left inferior fronto‐occipital fasciculus and inferior longitudinal fasciculus (area C), and the posterior cingulum near the precuneus (area D). All regions were voxelwise correlated to CTQ‐PN, but only FA in the posterior cingulum mediated trait anxiety (subfigure 5, also see Figure 3) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Mediation analysis
We subsequently tested whether STAI score (Y) as an outcome would be mediated by the CTQ‐PN score through its effects on FA value (M). Indeed, adding FA‐values as a covariate in a lineair regression reduced the association between the CTQ‐PN and STAI, which was left only borderline significant when controlling for FA values in the different regions (forceps minor: p = .051, anterior thalamic radiation: p = .080, inferior fronto‐occipital fasciculus: p = .079, cingulum: p = .092). This is initial evidence for a potential mediation of the impact of CTQ‐PN on STAI via white matter differences.
Given the strongest relationship between emotion regulation, stress reactivity, and psychiatric disorders is known for the posterior cingulum, a formal mediation analysis using hierarchical linear regression (see also Figure 3) of FA data from the posterior cingulum confirmed that participants with a higher CTQ‐PN score showed decreased FA (path a: t(113) = –4.98, p < .001), and that decreased FA in this region was negatively associated with STAI score at trend level (path b: t(113) = –1.79, p = .053). Again, elevated CTQ‐PN was associated with increased current trait anxiety (path c: t(113) = 1.96, p = .036), critically controlling for individual differences in posterior cingulum FA values rendered this relation insignificant (path c′: t(113) = 1.05, p = .252) indicative of mediation. In line with this, our formal test showed that FA in the posterior cingulum mediated effects of PN during childhood on anxiety levels at trend level (path ab: t(113) = 1.65, p = .057). We observed that the white matter differences in anterior thalamic radiation (p = .715), forceps minor (p = .358) and inferior fronto‐occipital fasciculus (p = .408) did not mediate the relationship between childhood PN and anxiety later in life. In sum, we show intitial evidence for a relation between CTQ‐related FA differences and differences in trait anxiety, with the strongest evidence for effects in the posterior cingulum.
Figure 3.
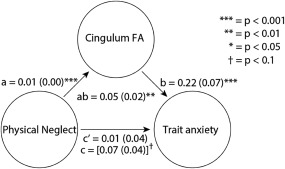
Displayed is the single‐level mediation analysis. The following factors were used: X = CTQ‐PN score, M = FA value cingulum and precuneus, Y = STAI total score. Three different paths depict the relation between the different factors. Path a: relationship between X–M, path b: relationship M–Y, path ab: mediation of relationship X–Y by M. The lines are labeled with path coefficients, and standard errors are shown in parentheses. ***p = 0.0001
4. DISCUSSION
Early adversities in the life of a child can have detrimental effects on its mental health later in life. The aim of the present study was to extend studies investigating differences in GM density as a function of distinct subtypes of CA, varying in the dimensions of threat and deprivation. Here, we explored differences in white matter that are potentially associated with childhood maltreatment by analyzing DTI data for correlations with CTQ‐subscale scores. Notably, of all the subscales, only PN predicted FA in the anterior thalamic radiation, the inferior fronto‐occipital fasciculus, the inferior longitudinal fasciculus and the posterior cingulum. Our correlational analyses support earlier findings (Ferguson & Dacey, 1997; Teicher & Samson, 2013; Teicher, Samson, Polcari, & McGreenery, 2006) by showing that having experienced maltreatment early in life potentially leads to higher levels of trait anxiety in later life. In particular, white matter microstructure integrity of the cingulum appeared to be related to effects of PN during childhood on anxiety levels in adulthood, though the cross‐sectionality of our data withholds us from drawing any conclusions on causality.
Childhood adversity (CA) has been associated with numerous differences in adult brain structures (Teicher & Samson, 2013; Teicher & Samson, 2016). These differences have not only been found in clinical samples, but also in healthy samples with vulnerability characteristics indicating that they are related to CA independent of disease. For instance, in a sample of 148 healthy subjects, which was quite comparable to our own group, CA was associated with remarkable functional and structural changes in adulthood, including amygdala responsiveness and reduced hippocampal volumes (Dannlowski et al., 2012). Several studies report white matter tract changes in individuals exposed to CA (Huang, Guandapuneedi, & Rao, 2012; Peng et al., 2013). Findings converge to a pattern of affected tracts with reduced FA mainly in pathways connecting brain regions involved in emotion regulation.
In the current study, we tried to extend prior findings by relating different kinds of CA to white matter differences. Surprisingly, but in line with a recent study by Tatham and colleagues (Tatham et al., 2016), only increased scores on the PN subscale were associated with decreased connectivity in a broad set of brain tracts associated with emotional appraisal and regulation. Neglect is the failure to provide for the shelter, safety, supervision and nutritional needs of the child (English, Thompson, Graham, & Briggs, 2005). While below we will relate these findings to other similar ones from recent studies, we have to report that sexual and PA were rather uncommon in the present sample, which may have limited us to detect differences related to these subscales.
PN was associated with a decreased FA in the anterior thalamic radiation, which connects the thalamus with the prefrontal and occipital cortex. This white matter bundle is implicated in the expression of emotion, mediation of reward seeking behavior and regulation of affective states (Coenen, Panksepp, Hurwitz, Urbach, & Madler, 2012). Indeed, white matter changes in the anterior thalamic radiation as indexed by lower FA values have been observed in patients with major depressive disorder (Lai & Wu, 2014). The uncinate fasciculus was affected. This tract connects the anterior temporal lobe, amygdala and hippocampus to the medial prefrontal cortex and is critically involved in emotional appraisal, emotion regulation and memory (Schmahmann et al., 2007). Abnormalities in the uncinate fasciculus have been associated with several affective disorders such as social anxiety (Phan et al., 2009), depression and bipolar disorder (Benedetti et al., 2014).
Moreover, the integrity of the white matter microstructure of the inferior fronto‐occipital fasciculus as well as the inferior longitudinal fasciculus was reduced as a function of the amount of PN. The inferior fronto‐occipital fasciculus is a direct pathway between the posterior temporal, occipital, and orbitofrontal areas (Ashtari, 2012) thereby linking emotional appraisal and visual perception (Schmahmann et al., 2007). Alterations in the inferior fronto‐occipital fasciculus have indeed been implicated in the pathophysiology of depression (Cullen et al., 2010). The inferior longitudinal fasciculus is an indirect pathway connecting the occipital cortex to the frontal brain, but forms a direct connection of the occipital cortex to the temporal lobe. It joins the uncinate fasciculus anteriorly to deliver information to the orbito‐frontal areas (Ashtari, 2012). Its long fibres therefore connect visual areas to the main components of the limbic system and it is not surprising that changes in the integrity of the inferior longitudinal fasciculus have been found in a meta‐analysis of DTI studies in major depressive disorder (Liao et al., 2013). Finally, we found lower FA as a function of PN in the posterior cingulum, a bundle of white matter tracts that connects the cingulate cortex with prefrontal and (para)hippocampal cortices, ending in the amygdala. The posterior cingulum is important in motivation and emotional behavior (Fani et al., 2014), especially during stressful episodes. The posterior cingulum innervates the dorsal hippocampus with serotonin fibers from the midbrain raphe. DTI studies have shown abnormalities of the posterior cingulum in a variety of psychiatric disorders including depression (Schermuly et al., 2010), bipolar disorder (Wessa et al., 2009), and post‐traumatic stress disorder (Fani et al., 2012). Fani et al. (2012) examined microstructural integrity of white matter tracts in a group of highly traumatized African‐American women with and without PTSD. The authors also used a tract‐based spatial statistical approach to show that PTSD patients, relative to traumatized controls, exhibited decreased integrity (measured by FA) of the posterior cingulum. Changes in the integrity of the posterior cingulum have also been found in relation to childhood abuse (Choi et al., 2009). Our FA findings are further supported by data from Huang et al. (2012) who revealed reduced FA values in left inferior fronto‐occipital fasciculus and the right cingulum projecting to the hippocampus in adolescents who did not have a psychiatric disorder but were previously exposed to CA. Moreover, Everaerd et al., (2016) found less GM in subjects with EN compared to abuse in brain regions connecting frontal and limbic areas of emotional appraisal and regulation. The findings from Everaerd and colleagues as well as the present data support the results of Schalinski et al. (2016) showing that in particular physical and EN at an early age were related to an increased vulnerability to develop a post‐traumatic stress disorder later.
Finally, we found a trend‐significant effect indicating that FA changes, particularly in the posterior cingulum, may have a functional consequence and are linked to trait anxiety, a personality dimension that is suggested to be a transdiagnostic risk factor of affective disorder (see Brown, Chorpita, and Barlow, 1998; Min, Lee, Lee, Lee, and Chae, 2012). While this has to be confirmed in future studies, potentially our results provide initial evidence for a mechanistic chain that forms one pathway from CA to disease. Ideally, our DTI measure should have been taken prior to the abuse as well in a longitudinal design to rule out that the WM effect preceded the abuse. In the case of childhood maltreatment, this is however, due to the early age of scanning as well as the highly dynamic nature of the neural architecture at this age, practically challenging and to our knowledge never performed. Therefore, we have taken a carefully weighed decision that, despite the cross‐sectional nature of the study, the mediation model can still be applied to get an initial indication of whether these effects may be interrelated. Nevertheless, we clearly recognize that there could be other interpretations to our data in the discussion.
This study has several limitations that should be mentioned to be able to interpret our findings accordingly. First, exclusion of participants with a psychiatric diagnosis was only screened by means of self‐report excluding any somatic disease potentially affecting the brain, current or past psychiatric or neurological disorder, medication or illicit drug use during the preceding 6 months, history of substance abuse, current or past alcohol dependence. Moreover, the assessment of various types of maltreatment during childhood was performed retrospectively by means of a self‐report questionnaire. This might introduce uncertainty in the measurement, since it can be questioned to which extent retrospective reports of childhood maltreatment reflect reality; they might be biased by the subjective experience of the victim and the current affective state. Nonetheless, this particular questionnaire has been demonstrated to be a reliable and valid measure of childhood maltreatment. Internal consistency of the items (Lisofsky et al., 2015) and test–retest reliability (Scher, Stein, Asmundson, McCreary, & Forde, 2001) has been shown. Additionally, retrospective reports converge with clinical ratings of childhood maltreatment (Widom, Dutton, Czaja, & DuMont, 2005) and correlate with prospectively collected data (Williams, 1994), whereby adults minimize their degree of exposure on retrospective report (see also Sheikh, 2017). Thus, retrospective exposure rates are lower than prospective rates and this might lead to false negative reports but not false positive reports. Moreover, as we investigated a “healthy group”, the variation in the levels of childhood maltreatment was limited although it is comparable to community based samples (Jones & Cercignani, 2010). Despite the relatively large sample size, the number of participants with scores in the higher range was rather low. Even though some of the subscales, like PN, did show relevant variance, other scores remained in the moderate range. Moreover, there is a limitation of all instruments used in adults to retrospectively assess exposure to CA since none of the available questionnaires collect detailed information on how exposure levels changed across development. However, there may be sensitive periods when experience exerts maximal effects on the developmental trajectory of specific brain regions and risk for psychopathology. Our cross‐sectional approach does not rule out that white matter deficit might have been more present in the group with maltreatment before the maltreatments began. In the current study, we used a standard approach for voxel‐based analysis of DTI data at a field strength of 1.5 T using 34 directions. 30 directions were for a long time (and at the time of acquisition) considered optimal for DTI (Jones & Cercignani, 2010) with directions above 32 pushing into so‐called HARDI territory. It should also be noted that DTI sequences in general have limitations in regards to interpretation in areas of crossing fibers. Recent developments such CODIVIDE or NODDI help alleviate these problems (Lampinen et al., 2017), but were not accessible at the time of acquisition. Despite these limitations, our findings show that even low to moderate levels of childhood maltreatment have structural and functional consequences later in life. Finally, this study only included male participants. Consequently, our findings are not generalizable to women since research indicates that early adverse experience may produce different effects in males and females (Teicher et al., 2003). However, we show that differences in brain connectivity, anxiety and stress response are associated with PN in men, ergo, it would be of great interest to explore these effects in women as well.
In sum, our data suggest that PN may have more severe consequences than previously thought and should be considered equally important to more active forms of abuse depending on the time of exposure. Structural and functional abnormalities initially attributed to psychiatric illness may be a more direct consequence of CA. CA exerts a potent influence on brain development and has been an unrecognized confound in almost all psychiatric neuroimaging studies. Given that we investigated a young healthy cohort, these brain changes may be understood as adaptive responses in the face of adversity. Future studies should address differences in adaptive and maladaptive responses of the brain to CA.
Tendolkar I, Mårtensson J, Kühn S, Klumpers F, Fernández G. Physical neglect during childhood alters white matter connectivity in healthy young males. Hum Brain Mapp. 2018;39:1283–1290. 10.1002/hbm.23916
REFERENCES
- Anderson, J. L. R. , Jenkinson, M. , & Smith, S. (2007a). Non‐linear optimization. FMRIB technical report TR07JA1. http://www.fmrib.ox.ac.uk/analysis/techrep
- Anderson, J. L. R. , Jenkinson, M. , & Smith, S. (2007b). Non‐linear registration, aka Spatial normalization. FMRIB technical report TR07JA2. http://www.fmrib.ox.ac.uk/analysis/techrep
- Ashtari, M. (2012). Anatomy and functional role of the inferior longitudinal fasciculus: A search that has just begun. Developmental Medicine & Child Neurology, 54, 6–7. [DOI] [PubMed] [Google Scholar]
- Basser, P. J. (1995). Inferring microstructural features and the physiological state of tissues from diffusion‐weighted images. NMR in Biomedicine, 8(7), 333–344. [DOI] [PubMed] [Google Scholar]
- Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system—A technical review. NMR in Biomedicine, 15(7), 435–455. [DOI] [PubMed] [Google Scholar]
- Beck, A. T. , Ward, C. H. , Mendelson, M. , Mock, J. , & Erbaugh, J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Benedetti, F. , Bollettini, I. , Radaelli, D. , Poletti, S. , Locatelli, C. , Falini, A. , … Colombo, C. (2014). Adverse childhood experiences influence white matter microstructure in patients with bipolar disorder. Psychological Medicine, 44(14), 3069–3082. [DOI] [PubMed] [Google Scholar]
- Benedetti, F. , Bollettini, I. , Radaelli, D. , Poletti, S. , Locatelli, C. , Falini, A. , … Colombo, C. (2014). Adverse childhood exerpiences influence white matter microstructure in patients with bipolar disorder. Psychology Medicine, 27, 1–14. [DOI] [PubMed] [Google Scholar]
- Bernstein, D. P. , Stein, J. A. , Newcomb, M. D. , Walker, E. , Pogge, D. , Ahluvalia, T. , … Zule, W. (2003). Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse & Neglect, 27, 169–190. [DOI] [PubMed] [Google Scholar]
- Bessette, K. L. , Nave, A. M. , Caprihan, A. , & Stevens, M. C. (2014). White matter abnormalities in adolescents with major depressive disorder. Brain Imaging & Behavior, 8(4), 531–541. [DOI] [PubMed] [Google Scholar]
- Brown, T. A. , Chorpita, B. F. , & Barlow, D. H. (1998). Structural relationships among dimensions of the DSM‐IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology, 107(2), 179–192. [DOI] [PubMed] [Google Scholar]
- Carr, C. P. , Martins, C. M. , Stingel, A. M. , Lemgruber, V. B. , & Juruena, M. F. (2013). The role of early life stress in adult psychiatric disorders: A systematic review according to childhood trauma subtypes. The Journal of Nervous & Mental Disease, 201, 1007–1020. [DOI] [PubMed] [Google Scholar]
- Choi, J. , Jeong, B. , Rohan, M. L. , Polcari, A. M. , & Teicher, M. H. (2009). Preliminary evidence for white matter tract abnormalities in young adults exposed to parental verbal abuse. Biological Psychiatry, 65, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coenen, V. A. , Panksepp, J. , Hurwitz, T. A. , Urbach, H. , & Madler, B. (2012). Human medial forebrain bundle (MFB) and anterior thalamic radiation (ATR): Imaging of two major subcortical pathways and the dynamic balance of opposite affects in understanding depression. The Journal of Neuropsychiatry & Clinical Neurosciences, 24, 223–236. [DOI] [PubMed] [Google Scholar]
- Cuijpers, P. , Smit, F. , Unger, F. , Stikkelbroek, Y. , Have ten, M. , & de Graaf, R. (2011). The disease burden of childhood adversities in adults: A population‐based study. Child Abuse & Neglect, 35, 937–945. [DOI] [PubMed] [Google Scholar]
- Cullen, K. R. , KlimesDougan, B. , Muetzel, R. , Mueller, B. A. , Camchong, J. , Houri, A. , … Lim, K. O (2010). Altered white matter microstructure in adolescents with major depression: A preliminary study. Journal of the American Academy of Child & Adolescent Psychiatry, 49, 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski, U. , Stuhrmann, A. , Beutelmann, V. , Zwangzer, P. , Lenzen, T. , Grotegerd, D. , … Kugel, H. (2012). Limbic scars: Long‐term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry, 71, 286–293. [DOI] [PubMed] [Google Scholar]
- Eluvathingal, T. J. , Chugani, H. T. , Behen, M. E. , Juhasz, C. , Muzik, O. , Maqbool, M. , et al. (2006). Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics, 117, 2093–2100. [DOI] [PubMed] [Google Scholar]
- English, D. J. , Thompson, R. , Graham, J. C. , & Briggs, E. C. (2005). Toward a definition of neglect in young children. Child Maltreatment, 10, 190–206. [DOI] [PubMed] [Google Scholar]
- Everaerd, D. , Klumpers, F. , van Wingen, G. , Tendolkar, I. , & Fernández, G. (2015). Association between neuroticism and amygdala responsivity emerges under stressful conditions. Neuroimage, 112, 218–224. [DOI] [PubMed] [Google Scholar]
- Everaerd, D. , Klumpers, F. , Zwiers, M. , Guadalupe, T. , Franke, B. , van Oostrom, I. , … Tendolkar, I. (2016). Childhood abuse and deprivation are associated with distinct sex‐dependent differences in brain morphology. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 41(7), 1716–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani, N. , King, T. Z. , Jovanovic, T. , Glover, E. M. , Bradley, B. , Choi, K. , … Ressler, K. J. (2012). White matter integrity in highly traumatized adults with and without post‐traumatic stress disorder. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 37(12), 2740–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani, N. , King, T. Z. , Reiser, E. , Binder, E. B. , Jovanovic, T. , Bradley, B. , & Ressler, K. J. (2014). FKBP5 genotype and structural integrity of the posterior cingulum. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 39(5), 1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, K. S. , & Dacey, C. M. (1997). Anxiety, depression, and dissociation in women health care providers reporting a history of childhood psychological abuse. Child Abuse & Neglect, 21, 941–952. [DOI] [PubMed] [Google Scholar]
- Heim, C. M. , Mayberg, H. S. , Mletzko, T. , Nemeroff, C. B. , & Pruessner, J. C. (2013). Decreased cortical representation of genital somatosensory field after childhood sexual abuse. The American Journal of Psychiatry, 170(6), 616–623. [DOI] [PubMed] [Google Scholar]
- Hovens, J. G. , Wiersma, J. E. , Giltay, E. J. , van Oppen, P. , Spinhoven, P. , Penninx, B. W. , … Zitman, F. G. (2010). Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatrica Scandinavica, 122(1), 66–77. [DOI] [PubMed] [Google Scholar]
- Huang, H. , Guandapuneedi, T. , & Rao, U. (2012). White matter disruptions in adolescents exposed to childhood maltreatment and vulnerability to psychopathology. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology, 37, 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski, A. P. , Douglas‐Palumberi, H. , Jackowski, M. , Win, L. , Schultz, R. T. , Staib, L. W. , … Kaufman, J. (2008). Corpus callosum in maltreated children with posttraumatic stress disorder: A diffusion tensor imaging study. Psychiatry Research, 162, 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. K. , & Cercignani, M. (2010). Twenty‐five pitfalls in the analysis of diffusion MRI data. NMRi Biomedicine, 23(7), 803–820. [DOI] [PubMed] [Google Scholar]
- Lai, C. H. , & Wu, Y. T. (2014). Alterations in white matter micro‐integrity of the superior longitudinal fasciculus and anterior thalamic radiation of young adult patients with depression. Psychological Medicine, 6, 1–8. [DOI] [PubMed] [Google Scholar]
- Lampinen, B. , Szczepankiewicz, F. , Mårtensson, J. , van Westen, D. , Sundgren, P. C. , & Nilsson, M. (2017). Neurite density imaging versus imaging of microscopic anisotropy in diffusion MRI: A model comparison using spherical tensor encoding. Neuroimage, 147, 517–531. [DOI] [PubMed] [Google Scholar]
- Liao, Y. , Huang, X. , Wu, Q. , Yang, C. , Kuang, W. , Du, M. , … Gong, Q. (2013). Is depression a disconnection syndrome? Meta‐analysis of diffusion tensor imaging studies in patients with MDD. Journal of Psychiatry & Neuroscience, 38(1), 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, L. , Radua, J. , & Rubia, K. (2014). Gray matter abnormalities in childhood maltreatment: A voxel‐wise meta‐analysis. The American Journal of Psychiatry, 171(8), 854–863. [DOI] [PubMed] [Google Scholar]
- Lisofsky, N. , Mårtensson, J. , Eckert, A. , Lindenberger, U. , Gallinat, J. , & Kühn, S. (2015). Hippocampal volume and functional connectivity changes during the female menstrual cycle. NeuroImage, 118, 154–162. [DOI] [PubMed] [Google Scholar]
- McLaughlin, K. A. , Greif Green, J. , Gruber, M. J. , Sampson, N. A. , Zaslavsky, A. M. , & Kessler, R. C. (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, J. A. , Lee, N. B. , Lee, C. U. , Lee, C. , & Chae, J. H. (2012). Low trait anxiety, high resilience, and their interaction as possible predictors for treatment response in patients with depression. Journal of Affective Disorder, 137(1–3), 61–69. [DOI] [PubMed] [Google Scholar]
- Peng, H. , Ning, Y. , Zhang, Y. , Yang, H. , Zhang, L. , He, Z. , … Li, L. (2013). White‐matter density abnormalities in depressive patients with and without childhood neglect: A voxel‐based morphometry (VBM) analysis. Neuroscience Letters, 550, 23–28. [DOI] [PubMed] [Google Scholar]
- Phan, K. L. , Orlichenko, A. , Boyd, E. , Angstadt, M. , Coccaro, E. F. , Liberzon, I. , … Arfanakis, K. (2009). Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biological Psychiatry, 66, 691–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalinski, I. , Teicher, M. H. , Nischk, D. , Hinderer, E. , Müller, O. , & Rockstroh, B. (2016). Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry, 16, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher, C. D. , Stein, M. B. , Asmundson, G. J. , McCreary, D. R. , & Forde, D. R. (2001). The childhood trauma questionnaire in a community sample: Psychometric properties and normative data. Journal of Traumatic Stress, 14(4), 843–857. [DOI] [PubMed] [Google Scholar]
- Schermuly, I. , Fellgiebel, A. , Wagner, S. , Yakushev, I. , Stoeter, P. , Schmitt, R. , … Beutel, M. E. (2010). Association between cingulum bundle structure and cognitive performance: An observational study in major depression. European Psychiatry: The Journal of the Association of European Psychiatrists, 25(6), 355–6047. [DOI] [PubMed] [Google Scholar]
- Schmahmann, J. D. , Pandya, D. N. , Wang, R. , Dai, G. , D'arceuil, H. E. , de Crespigny, A. J. , … Wedeen, V. J. (2007). Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain: A Journal of Neurology, 130, 630–653. [DOI] [PubMed] [Google Scholar]
- Sheikh, M. A. (2017). Confounding and statistical significance of indirect effects: Childhood adversity, education, smoking, and anxious and depressive symptomatology. Frontiers in Psychology, 8, 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Johansen‐Berg, H. , Rueckert, D. , Nichols, T. E. , Mackay, C. E. , … Behrens, T. E. (2006). Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage, 31, 1487–1505. [DOI] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Woolrich, M. W. , Beckman, C. F. , Behrens, T. E. J. , Johansen‐Berg, H. , … Matthews, P. M. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, 208–219. [DOI] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , & Lushene, R. E. (1970). The State‐Trait Anxiety Inventory (test manual). Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Spinhoven, P. , Elzinga, B. M. , Hovens, J. G. , Roelofs, K. , Zitman, F. G. , van Oppen, P. , & Penninx, B. W. (2010). The specificity of childhood adversities and negative life events across the lifespan to anxiety and depressive disorders. Journal of Affective Disorders, 126, 103–112. [DOI] [PubMed] [Google Scholar]
- Tatham, E. L. , Ramasubbu, R. , Gaxiola‐Valdez, I. , Cortese, F. , Clark, D. , Goodyear, B. , … Hall, G. B. (2016). White matter integrity in major depressive disorder: Implications of childhood trauma, 5‐HTTLPR and BDNF polymorphisms. Psychiatry & Research, 253, 15–25. [DOI] [PubMed] [Google Scholar]
- Teicher, M. H. , & Samson, J. A. (2013). Childhood maltreatment and psychopathology: A case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. The American Journal of Psychiatry, 170(10), 1114–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , & Samson, J. A. (2016). Annual research review: Enduring neurobiological effects of childhood abuse and neglect. Journal of Child Psychology & Psychiatry, & Allied Disciplines, 57(3), 241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher, M. H. , Andersen, S. L. , Polcari, A. , Anderson, C. M. , Navalta, C. P. , & Kim, D. M. (2003). The neurobiological consequences of early stress and childhood maltreatment. Neuroscience & Biobehavioural Review, 27(1–2), 33–44. [DOI] [PubMed] [Google Scholar]
- Teicher, M. H. , Samson, J. A. , Polcari, A. , & McGreenery, C. Y. (2006). Sticks, stones, and hurtful words: Relative effects of various forms of childhood maltreatment. American Journal of Psychiatry, 163, 993–1000. [DOI] [PubMed] [Google Scholar]
- Tomoda, A. , Sheu, Y. S. , Rabi, K. , Suzuki, H. , Navalta, C. P. , Polcari, A. , … Teicher, M. H. (2011). Exposure to parental verbal abuse is associated with increased gray matter volume in superior temporal gyrus. Neuroimage, 54(Suppl 1), S280–S286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrijsen, J. N. , Becker, E. S. , Arias‐Vásquez, A. , van Dijk, M. K. , Speckens, A. , & Oostrom, I. (2014). What is the contribution of different cognitive biases and stressful childhood events to the presence and number of previous depressive episodes? Journal of Psychiatric Research, 217, 134–142. [DOI] [PubMed] [Google Scholar]
- Wessa, M. , Houenou, J. , Leboyer, M. , Chanraud, S. , Poupon, C. , Martinot, J. L. , & Paillère‐Martinot, M. L. (2009). Microstructural white matter changes in euthymic bipolar patients: A whole‐brain diffusion tensor imaging study. Bipolar Disorders, 11(5), 504–514. [DOI] [PubMed] [Google Scholar]
- Widom, C. S. , Dutton, M. A. , Czaja, S. J. , & DuMont, K. A. (2005). Development and validation of a new instrument to assess lifetime trauma and victimization history. Journal of Traumatic Stress, 18, 519–531. [DOI] [PubMed] [Google Scholar]
- Williams, L. M. (1994). Recall of childhood trauma: A prospective study of women's memories of child sexual abuse. Journal of Consulting & Clinical Psychology, 62(6), 1167–1176. [DOI] [PubMed] [Google Scholar]


