Abstract
Patients with schizophrenia do not usually achieve remission state even after adequate antipsychotics treatment. Previous studies found significant difference in white matter integrity between patients with good outcomes and those with poor outcomes, but difference is still unclear at individual tract level. This study aimed to use a systematic approach to identify the tracts that were associated with remission state in patients with schizophrenia. We evaluated 91 patients with schizophrenia (remitted, 50; nonremitted, 41) and 50 healthy controls through diffusion spectrum imaging. White matter tract integrity was assessed through an automatic tract‐specific analysis method to determine the mean generalized fractional anisotropy (GFA) values of the 76 white matter tract bundles in each participant. Analysis of covariance among the 3 groups revealed 12 tracts that were significantly different in GFA values. Post‐hoc analysis showed that compared with the healthy controls, the nonremission group had reduced integrity in all 12 tracts, whereas the remission group had reduced integrity in only 4 tracts. Comparison between the remission and nonremission groups revealed 4 tracts with significant difference (i.e., the right fornix, bilateral uncinate fasciculi, and callosal fibers connecting the temporal poles) even after adjusting age, sex, education year, illness duration, and medication dose. Furthermore, all the 4 tracts were correlated with negative symptoms scores of the positive and negative syndrome scale. In conclusion, our study identified the tracts that were associated with remission state of schizophrenia. These tracts might be a potential prognostic marker for the symptomatic remission in patients with schizophrenia.
Keywords: diffusion MRI, generalized fractional anisotropy, schizophrenia, tract‐based analysis, treatment outcome
1. INTRODUCTION
Antipsychotic drugs have become the standard treatment for schizophrenia, but the treatment outcomes vary. Under medication, some patients achieve symptomatic remission (45%–47%) and others continue to experience some or most symptoms (Lambert et al., 2006; San, Ciudad, Alvarez, Bobes, & Gilaberte, 2007). To date, treatment effectiveness in different patients remains unclear (Lieberman et al., 1996). Different treatment outcomes may be attributed to the genetic and molecular heterogeneity of patients (Kaur et al., 2014; Mirnics, Middleton, Marquez, Lewis, & Levitt, 2000), which may have signatures in the brain structures and functions (Egan et al., 2001). Therefore, a brain correlate that is characteristic of the treatment outcomes is desirable to avoid unnecessary treatment of nonremission, to ensure an adequate trial for patients who are likely to benefit from taking antipsychotics, and to improve the long‐term treatment outcomes (Correll, Malhotra, Kaushik, McMeniman, & Kane, 2003).
Volumetric structural magnetic resonance imaging (MRI) studies have revealed alterations in the gray matter of the brains in patients with schizophrenia. Many studies assessing the brain structure and its relationship with treatment outcomes have reported that smaller volumes of gray matter are associated with subsequently poor clinical or functional outcomes (Dazzan, 2014). For example, a longitudinal study by Jaaskelainen et al. (2014) reported that individuals with a higher gray matter density in the frontal and limbic regions have more favorable clinical and functional outcomes. In addition, Van Haren et al. (2007) reported significantly reduced gray matter volumes in the frontal and temporal cortices of the individuals with poor outcomes. Bodnar, Harvey, Malla, Joober, and Lepage (2010) examined the parahippocampal gyrus in patients with first‐episode psychosis and observed that the parahippocampal volume is significantly smaller in non‐remitted patients than in remitted patients with schizophrenia. The findings of the aforementioned studies suggest that the treatment outcomes might be associated with the structural alterations in the gray matter of specific brain regions, and these alterations might occur early in the course of schizophrenia.
As schizophrenia is characterized by alterations of structure and function in specific gray matter brain regions, schizophrenia has been considered as a dysconnectivity syndrome, a disorder arising from abnormal neuronal connectivity, which leads to associated symptoms and cognitive impairments (Stephan, Friston, & Frith, 2009). White matter comprises numerous neuronal axons and forms structural connections between brain regions, so alteration in white matter integrity has been a key focus of schizophrenia research (Chiappelli et al., 2015; Kubicki, McCarley, & Shenton, 2005; Oh et al., 2009). The fractional anisotropy (FA) value, an index derived from diffusion tensor imaging (DTI), is considered to indicate the composite properties of the underlying white matter microstructure, including the axonal density, axonal diameter, and myelination (Basser, Mattiello, & LeBihan, 1994; Beaulieu, 2002; Johansen‐Berg, 2013). Reduced FA in white matter of the frontal and temporal lobes has been frequently reported in previous DTI studies for schizophrenia (Pettersson‐Yeo, Allen, Benetti, McGuire, & Mechelli, 2011). Recent studies using advanced analysis techniques have reported reduced FA values not only in the frontal and temporal regions but also in the parietal and occipital cortices (Fujino et al., 2014; Roalf et al., 2013; Walther et al., 2011). Apparent FA reduction has also been observed in patients with first‐episode schizophrenia (Hao et al., 2006) and probands' siblings (Camchong, Lim, Sponheim, & Macdonald, 2009). Furthermore, diffusion MRI findings have suggested that alterations in white matter tract integrity may serve as endophenotypic or trait markers for schizophrenia (Skudlarski et al., 2013).
Although diffusion MRI has provided crucial insights regarding the alterations in white matter tract integrity in patients with schizophrenia, only four studies have reported their associations with treatment outcomes (Luck et al., 2011; Mitelman et al., 2006, 2007; Reis Marques et al., 2014; Table 1). As shown in Table 1, these four studies differ in patient populations, follow‐up periods, and analytic approaches. The two studies by Mitelman et al. were performed on patients with chronic schizophrenia with follow‐up periods of at least 5 years, whereas the other two studies were conducted on patients with first episode psychosis with follow‐up periods of 3 and 6 months, respectively. Three studies used a region‐of‐interest (ROI) approach to investigate white matter FA values in 40 Brodmann's areas (Mitelman et al., 2006), 5‐by‐5‐pixel boxes with the coordinates extracted from the literature (Mitelman et al., 2007), and pixels specific to 3 pairs of association fibers (Luck et al., 2011). One study resorted to a whole‐brain approach using an FA skeleton‐based technique (i.e., tract‐based spatial statistics, TBSS) (Reis Marques et al., 2014). Despite different populations and approaches employed by the limited number of studies as mentioned above, these studies repeatedly reported significantly altered white matter regions containing associative fibers connecting the frontal, temporal, and occipital lobes and commissural fibers. It seems to imply that there are distinct white matter tracts with altered integrity in patients with poor treatment outcome as compared to those with good outcome. To date, however, no studies have been conducted to systematically investigate white matter tracts over the whole brain to clarify the findings.
Table 1.
Previous DTI studies comparing white matter microstructural integrity between good and poor outcomes following antipsychotics treatment
| Citations | Mitelman et al. (2006) | Mitelman et al. (2007) | Luck et al. (2011) | Reis Marques et al. (2014) |
|---|---|---|---|---|
| Approaches | ROI | ROI | TSA | TBSS |
| Brain regions | WB, Brodmann's areas | 5 × 5‐pixel boxes at pre‐ selected stereotactic coordinates | UF, SLF, fornix | WB, FA Skeleton |
| Patients | Chronic schizophrenia | Chronic schizophrenia | FEP | FEP |
| Follow‐up periods | At least 5 years | At least 5 years | 6 months | 12 weeks |
| Reduced FA in poor outcome compared with good outcome at baseline | left lateral prefrontal, left temporal‐occipital junction, right cingulate white matter | posterior CC, fronto‐occipital fasciculus, left optic radiation, fronto‐temporal white matter | UF, SLF | UF, fornix, CC, internal capsule, external capsule, and corona radiata |
Note. Abbreviations: CC = corpus callosum; DTI = diffusion tensor imaging; FA = fractional anisotropy; FEP = first‐episode psychosis; ROI = region of interest; SLF = superior longitudinal fasciculus; TBSS = tract‐based spatial statistics; TSA = tract‐specific analysis; UF = uncinate fasciculus; WB = whole brain.
Therefore, this study conducted a tract‐based automatic analysis (TBAA) over the whole brain (Chen et al., 2015) to determine the tracts of which white matter tract integrity differs between the remission and nonremission groups. The TBAA method can analyze microstructural integrity of the 76 white matter tracts automatically and provide a standardized output for between‐groups comparisons. Using TBAA, we observed that some white matter tracts were altered in the siblings with less generalized fractional anisotropy (GFA) reduction than in the probands (Wu et al., 2015b), suggesting that alterations in white matter tract integrity may indicate a genetic predisposition to schizophrenia. Furthermore, the severity of white matter tract alterations in the patients with first‐episode schizophrenia remained similar to those in the patients with chronic schizophrenia (Wu et al., 2015a). Our findings suggest that alterations in white matter tract integrity might be inherent to the disease, and these inherent alterations might be reflected in the severity of tract impairment between remission and nonremission groups.
Based on this discussion, this study investigated white matter tract integrity in 3 groups, namely, patients in remission, those in nonremission, and healthy controls. We aimed to demonstrate that the remission and nonremission groups had considerably distinct white matter tracts with altered tract integrity compared with the healthy controls. We hypothesized that (1) as compared with the control group, the remission and nonremission groups would exhibit distinct sets of tracts that had significant reduction in white matter tract integrity; and (2) compared with the remission group, the nonremission group would show a specific set of tracts with significant reduction in white matter tract integrity, even after adjustment for major clinical variables.
2. METHODS
2.1. Participants
Patients with schizophrenia were consecutively recruited from the outpatient clinic of Department of Psychiatry at National Taiwan University Hospital consecutively from 2010 to 2015. Patients received a diagnosis of schizophrenia in accordance with the Diagnostic and Statistical Manual of Mental Disorders‐IV criteria, as determined through comprehensive chart review and personal interviews conducted by experienced psychiatrists. Patients with schizoaffective or bipolar disorder, or had a history of substance use disorder, intellectual disability, and major systemic and neurological diseases were excluded. All patients had duration of illness of at least 2 years. They were followed up regularly for at least 6 months to ensure that they were symptomatically stable during this period. Psychiatrists (C.M.L., T.J.H., H.G.H., Y.T.L., M.H.H., C.C.L., and Y.L.C., each with more than 10 years of experience) assessed the patients using the Positive and Negative Syndrome Scale (PANSS) at the initial recruitment and at the 6‐month follow up. The intraclass correlation (ICC) of the 30 items of PANSS ranged from 0.4 to 0.86, of which 9 items' ICCs were above 0.7 (satisfactory level), and 21 items' ranged from 0.4 to 0.7 (acceptable level). The means of coefficient of agreement (COA) of positive, negative, and general psychopathology subscales were 0.77, 0.76, and 0.78, respectively. Because several confounding factors may influence the remission of schizophrenia patients, such as poor drug compliance, postacute exacerbation illness, and unreliable course clarification using retrospective chart review, we took a prospective design to ensure that these patients were not in postacute exacerbation illness and had regular medication compliance during the 6‐month follow‐up period. Patients who had acute exacerbation, irregular medication compliance, or dropped out of the study were excluded from the study. Healthy controls were recruited from hospital and university staff and community people through publicly posting posters and internet advertising. Healthy controls were screened by trained research assistants using the Mandarin version of Diagnostic Interview for Genetic Studies (DIGS) (Chen, Hsiao, Hsiao, & Hwu, 1998; Nurnberger et al., 1994). Controls were excluded if they had any of the following diagnoses: (1) schizophrenia, schizoaffective disorder, bipolar affective disorder, or major depressive disorder and other psychotic disorders; (2) substance use and addictive disorder; (3) mental retardation; (4) major systemic and neurological disorders, such as systemic lupus erythematosus, seizure, stroke, and major head injury; and (5) significant neurotic disorders, such as generalized anxiety disorder, obsessive compulsive disorder, and posttraumatic stress disorder. In addition, controls whose first‐degree relatives had psychotic disorders were excluded. All participants were assessed for handedness by using the Edinburgh Handedness Inventory (Oldfield, 1971). This study was approved by the Institutional Review Board of the hospital, and the participants provided informed consent prior to recruitment in the study.
In this study, participants were categorized into 3 groups, namely, healthy control, remission, and nonremission groups. Patients were categorized into the remission and nonremission groups according to the Remission in Schizophrenia Working Group (RSWG) criteria, including both severity and duration criteria, on the basis of PANSS scores (Andreasen et al., 2005). The RSWG published consensus criteria to define remission of psychotic symptoms in patients with schizophrenia (Andreasen et al., 2005). The criteria for remission are to measure both a symptom criterion for severity of symptoms and a time criterion for duration of sustained mild symptoms or lack of symptoms. The RSWG remission criteria have been widely used in clinical settings, and have been shown to have good correlations between symptomatic remission and functional outcome (Dunayevich, Sethuraman, Enerson, Taylor, & Lin, 2006; Emsley et al., 2007). Remission was defined as a score of ≤3 on each of the 8 specific items of the PANSS (i.e., P1, P2, P3, N1, N4, N6, G5, and G9) at the initial recruitment and 6‐month follow‐up, and nonremission was defined as a score of >3 on any 1 of the 8 items at either of the two assessment time points (Andreasen et al., 2005). All antipsychotic doses were converted to chlorpromazine equivalents (Andreasen, Pressler, Nopoulos, Miller, & Ho, 2010; Woods, 2003).
2.2. Image acquisition
All images were acquired on a 3 T MRI system (Trio, Siemens, Erlangen, Germany) with a 32‐channel phased‐array head coil. T1‐weighted images were obtained using a three‐dimensional magnetization‐prepared rapid gradient‐echo (3D MPRAGE) sequence with the following parameters: repetition time (TR) = 2,000 ms, echo time (TE) = 3 ms, flip angle = 9°, acquisition matrix size = 256 × 192 × 208, field of view (FOV) = 256 × 192 × 208 mm3, and spatial resolution = 1 × 1 × 1 mm3. To acquire a better angular resolution of the diffusion signal, diffusion MRI data were obtained through diffusion spectrum imaging (DSI) (Wedeen, Hagmann, Tseng, Reese, & Weisskoff, 2005). DSI was performed using a pulsed‐gradient twice‐refocused spin‐echo echo‐planar imaging sequence (EPI) with the following parameters: TR = 9,600 ms, TE = 130 ms, FOV = 200 × 200 mm2, matrix size = 80 × 80, slice number = 56, and slice thickness = 2.5 mm (Reese, Heid, Weisskoff, & Wedeen, 2003). A total of 102 image volumes, comprising 101 diffusion‐weighted images and one baseline (B0) image, were acquired. These images corresponded to 102 grid points filled in the half sphere of diffusion‐encoding space. The maximum diffusion sensitivity (b max) was 4,000 s mm−2 (Kuo, Chen, Wedeen, & Tseng, 2008). Altogether, the acquired DSI data sets comprised 5,712 image slices. The total scan time for the 3D MPRAGE and DSI acquisition was ∼20 min.
2.3. Image quality assurance and DSI data reconstruction
During relatively long scanning time of DSI, head motion may occur, which induces signal dropout in the DSI images. Our assessment showed that the diffusion anisotropy values derived from DSI, referred to as GFA values, would deviate from the accurate value if the number of images with signal dropout increased. Specifically, the DSI data with more than 90 image slices of signal dropout would cause an error of ∼6% in the GFA estimation. Therefore, a DSI data set with more than 90 image slices of signal dropout was discarded (Chen et al., 2015).
For each voxel of the DSI data set, the 102 diffusion MR signals of a half‐sphere were projected to fill the other half of the sphere based on the fact that the data in diffusion‐encoding space are symmetric around the origin. 3D Fourier transform was performed to obtain the average propagator at each voxel (Callaghan, Coy, MacGowan, Packer, & Zelaya, 1991). The orientation distribution function (ODF) was computed by calculating the second moment of the average propagator in 362 radial directions (Wedeen et al., 2008). To indicate the local tract orientation in each voxel, a decomposition method was used to decompose the ODF into several constituent Gaussian ODFs (Yeh, Verstynen, Wang, Fernandez‐Miranda, & Tseng, 2013). The GFA value at each voxel was computed using the following formula: (standard deviation of the ODF)/(root mean square of the ODF) (Tuch, 2004).
2.4. Tract‐based automatic analysis (TBAA)
We conducted TBAA to assess the integrity of 76 major white matter tracts in the brain (Chen et al., 2015). The TBAA method uses 3 pieces of information, a DSI template, a tract atlas, and a nonlinear transformation registering individual diffusion data sets to the template (Hsu, Hsu, & Tseng, 2012). The DSI template, named NTU‐DSI‐122 (Hsu, Lo, Chen, Wedeen, & Isaac Tseng, 2015), was constructed by registering 122 DSI data sets of healthy adults in the Montreal Neurological Institute (MNI) space (Hsu et al., 2012). The tract atlas contains 76 major white matter tract bundles segmented in the template through deterministic tractography. TBAA was performed as described previously (Chen et al., 2015). (1) A study‐specific template (SST) was created through two‐step registration of the DSI data sets of all the study participants by performing nonlinear transformations of the T1WI and DSI (Hsu et al., 2012). (2) The SST was registered to the DSI template following the same two‐step registration method. (3) A deformation map was created by combining the transformations obtained from steps 2 and 3. (4) The sampling coordinates of the 76 white matter tract bundles were transformed from the DSI template to the individual DSI data sets by using the deformation map obtained at step 3. (5) The GFA values were sampled along the sampling coordinates in the native space to obtain the GFA profiles of each tract bundle. (6) The mean GFA values were calculated for each tract bundle to obtain 76 mean GFA values for each participant (Chen et al., 2015).
2.5. Statistical analysis
To verify whether the demographic variables satisfy the assumptions of the parametric statistical tests, data normality was examined using the Kolmogorov–Smirnov test, and the results were expressed as the means and standard deviations. To assess the between‐groups differences in the demographic variables, the chi‐squared test was conducted for categorical variables, and one‐way analysis of variance was performed for continuous variables exhibiting a normal distribution. To compare the mean GFA values of the 76 white matter tract bundles between the remission, non‐remission, and healthy control groups, we used one‐way analysis of covariance (ANCOVA) with age (Zhang et al., 2013), sex (Walder et al., 2006), and education year as the covariates to minimize their effects on the study variables. Bonferroni method was performed for multiple comparisons (McDonald, 2009). As the significant differences in some tracts between the two diseased groups may be caused by the differences in duration of illness and medication dose between the two groups, ANCOVA was conducted again to compare the ANCOVA‐positive tracts between the two diseased groups by including duration of illness and medication dose as covariates in addition to age, sex, and education year. Bonferroni method was performed to correct for multiple comparisons. Before ANCOVA, we evaluated the collinearity between the clinical variables. We estimated variance inflation factor (VIF) for each clinical variables in 91 patients using the collinearity diagnostics in SPSS. To reassure that the ANCOVA results still remained, we performed a sensitivity analysis by excluding patients with comorbid disorders. We performed ANCOVA again on this group of subjects with the same procedures as described above. Partial correlation analysis was performed to investigate the associations between the GFA values of the tracts with significant differences between remitted and nonremitted patients and the PANSS positive and negative scores, controlling the duration of illness and medication dose. Statistical comparisons were conducted using SPSS version 20.0 (SPSS Inc., Chicago, IL).
3. RESULTS
3.1. Demographic variables
One hundred and nine patients received MRI scanning and first PANSS assessment initially. Thirteen patients were excluded because their images failed to pass image quality control (please see Section 2.3). The remaining 96 patients received a 6‐month follow‐up. Five patients were excluded because they dropped out of the study or had acute exacerbation of psychosis. There were 91 patients (50 remitted patients and 41 nonremitted patients) entering the final analysis. Fifty‐six control subjects were recruited, and 6 were excluded because of poor image quality. There were 50 controls in the final analysis (Fig. 1). Subsequently, 50, 41, and 50 participants were categorized in the remission, nonremission, and healthy control groups, respectively (Table 2). Among the 3 groups, age, sex, and handedness showed no significant differences; however, years of education differed significantly among the 3 groups. The scores of PANSS positive symptom subscale, negative symptom subscale, general psychopathology subscale, duration of illness, daily antipsychotics dose, and the use of second generation antipsychotics or the combined differed significantly between the remission and nonremission groups. There were 5, 42, and 3 patients in the remission group receiving first‐generation, second‐generation, and combined antipsychotics, respectively, and 3, 22 and 16 patients in the nonremission group receiving the abovementioned antipsychotic drugs, respectively. There were 3 patients comorbid with depressive disorder, 1 patient with obsessive compulsive disorder in the remission group, and 2 patients with depressive disorder and 2 patients with obsessive compulsive disorder in the nonremission group.
Figure 1.
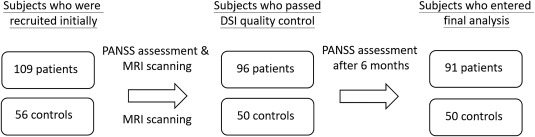
Diagram of recruitment process
Table 2.
Demographics and clinical characteristics of the study group
| Remission (n‐50) | Nonremission (n‐4I) | Control (n = 50) | p value | |
|---|---|---|---|---|
| Age (year) mean ± SD | 32.30 ± 8.03 | 35.17 ± 9.96 | 33.1 ± 8.74 | .796a |
| Sex, male (%) | 19(38%) | 22(54%) | 22(44%) | .325b |
| Education (year), mean ± SD | 14.68 ± 2.01 | 13.44 ± 0.37 | 15.72 ± 2.59 | .001a* |
| Right‐handed (%) | 49(98%) | 41(100%) | 50(100%) | .400b |
| Duration of illness (year), mean ± SD | 8.18 ± 6.79 | 11.66 ± 7.52 | — | .023c* |
| Daily antipsychotic dose, mean CPZ equivalent (mg) ± SD | 240.71 ± 150.24 | 418.79 ± 216.38 | — | <.001'c |
| PANSS positive score, mean ± SD at baseline | 10.08 ± 0.65 | 16.34 ± 5.34 | — | <.001c* |
| at 6‐month follow‐up | 10.02 ± 2.8 | 15.93 ± 4.49 | — | <.001”c |
| PANSS negative score, mean ± SD at baseline | 11.56 ± 4.02 | 20.88 ± 7.22 | — | <.001c * |
| at 6‐month follow‐up | 11.88 ± 4.06 | 20.56 ± 6.56 | — | <.001c* |
| PANSS general score, mean ± SD at baseline | 21.02 ± 5.33 | 29.32 ± 8.53 | — | .001c* |
| at 6‐month follow‐up | 22.94 ± 5.48 | 30.563.29 | — | <.001c* |
Note. Abbreviations: CPZ = chlorpromazine; PANSS = Positive and Negative Syndrome Scale.
Statistical comparison of the demographics across groups by ANOVA.
Statistical comparison of the demographics across groups by Chi‐square test.
Statistical comparison of the demographics across groups by two‐sample t test.
*Statistically significant.
3.2. White matter tract integrity
ANCOVA was performed using the mean GFA values of the 76 white matter tracts as the within‐subjects variables and the study groups as the between‐subjects variables. We observed that 12 tracts varied significantly among the 3 groups after Bonferroni correction for multiple comparisons. Table 3 lists the tracts with significant difference among the 3 groups. In the association fibers, the tracts included the right arcuate fasciculus, bilateral fornices, bilateral uncinate fasciculi, and right perpendicular fasciculus, and those in the commissure fibers included the callosal fibers (CFs) connecting the temporal poles, hippocampi, amygdalae, dorsolateral prefrontal cortices, and orbitofrontal gyri (genu), and those in the projection fibers included the left thalamic radiation of optic radiation (p < .001). In these 12 tracts, a stepwise decrement in the GFA values was observed from the healthy control to remission to nonremission groups. Figure 2 shows the effect size of each pair of the study groups. All of the 12 tracts found in ANCOVA had effects sizes of 0.98 or above in the post‐hoc comparison between nonremission and control groups.
Table 3.
Comparison of the mean GFA values of white matter tracts among three groups
| Post hoc | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C (n = 50) | R (n = 50) | NR (n = 41) | ANCOVA | C vs. R | C vs Nit | R vs Nit | ||||
| Fiber tracts | GFA value, mean (SD) | GFA value, mean (SD) | GFA value, mean (SD) | p value | p value | p value | p value | |||
| Rt arcuate fasciculus | 0.276 | (0.014) | 0.271 | (0.014) | 0.261 | (0.016) | a<.001* | .286 | <.001* | .022* |
| Lt fornix | 0.176 | (0.017) | 0.158 | (0.022) | 0.139 | (0.025) | a<.001* | <.001* | <.001* | .003* |
| Rt fornix | 0.215 | (0.016) | 0.201 | (0.021) | 0.182 | (0.022) | a<.001* | <.001* | <.001* | .001* |
| Rt perpendicular fasciculus | 0.213 | (0.018) | 0.205 | (0.018) | 0.195 | (0.018) | a<.001* | .072 | <.001* | .053 |
| Lt uncinate fasciculus | 0.234 | (0.014) | 0.233 | (0.020) | 0.215 | (0.019) | a<.001* | 1 | <.001* | <.001* |
| Rt uncinate fasciculus | 0.248 | (0.016) | 0.246 | (0.017) | 0.225 | (0.022) | a<.001* | 1 | <.001* | <.001* |
| Lt TR of optic radiation | 0.324 | (0.012) | 0.321 | (0.013) | 0.309 | (0.015) | a<.001* | .778 | <.001* | .005* |
| CF to genu | 0.318 | (0.020) | 0.312 | (0.019) | 0.297 | (0.023) | a<.001* | .394 | <.001* | .005* |
| CF to DLPFC | 0.338 | (0.014) | 0.333 | (0.014) | 0.319 | (0.020) | a<.001* | .207 | <.001* | .001* |
| CF to temporal poles | 0.206 | (0.012) | 0.195 | (0.015) | 0.181 | (0.017) | a<.001* | <.001* | <.001* | .001* |
| CF to hippocampi | 0.200 | (0.021) | 0.183 | (0.023) | 0.166 | (0.026) | a<.001* | .001* | <.001* | .024* |
| CF to amygdalae | 0.268 | (0.014) | 0.264 | (0.015) | 0.252 | (0.015) | a<.001* | .418 | <.001* | .004* |
Note. Abbreviations: C = control; CF = callosal fibers; DLPFC = dorsolateral prefrontal cortex; GFA = generalized fractional anisotropy; Lt = left; NR = nonremission; R = remission; Rt = right; TR = thalamic radiation.
Covariates: age, sex, and education year. Bonferroni method was conducted to correct for multiple comparisons of 76 tracts.
*Statistically significant.
Figure 2.
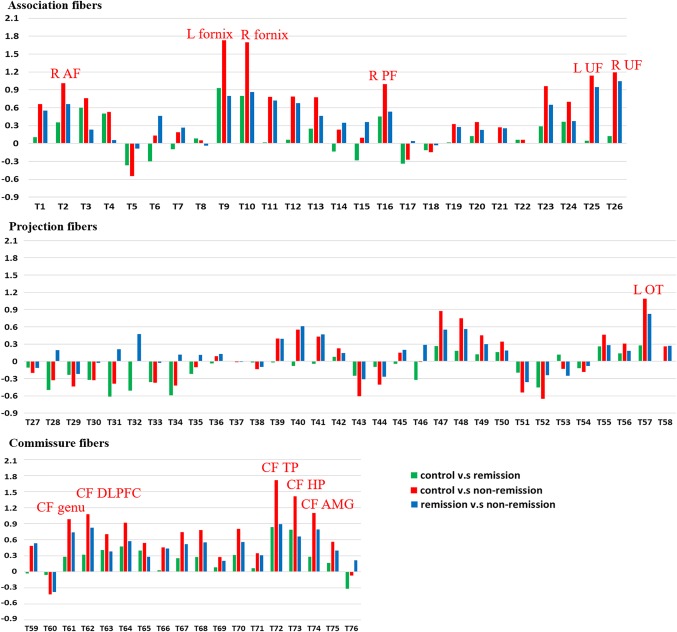
The effect sizes of post‐hoc between‐groups comparison. X coordinates are the serial numbers of 76 white matter tract bundles from T1 to T76, and Y coordinates are the effect size of each pair of the study groups. All the tracts with significant difference in ANCOVA had an effect size of 0.9 or above. As indicated in the figure, these tracts include the right arcuate fasciculus (R AF), bilateral fornices, bilateral uncinate fasciculi (L UF, R UF), right perpendicular fasciculus (R PF), the left optic tract of the thalamic radiation (L OT), the callosal fibers (CF) connecting the temporal poles (TP), hippocampi (HP), amygdalae (AMG), dorsolateral prefrontal cortices (DLPFC), and orbitofrontal gyri (genu) [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Post‐hoc between‐groups analysis
Post‐hoc between‐groups analysis of each pair of groups was performed to discriminate the differences in the alterations in the 12 tracts. The nonremission group had significantly lower GFA values in all 12 white matter tracts than did the healthy controls. In contrast, the remission group had significantly lower GFA values than did the control group in only 4 tracts (i.e., the bilateral fornices and the CFs connecting the temporal poles, and hippocampi). The nonremission group had lower GFA in all of the 12 tracts except right perpendicular fasciculus than did the remission group (Table 3).
3.4. ANCOVA between remission and nonremission groups
The collinearity analysis showed that VIF values for age, education year, duration of illness, and medication dose were 1.755, 1.084, 2.007, and 1.166, respectively. These values were smaller than 10, the diagnostic threshold of collinearity. With the age, sex, education year, duration of illness, and medication dose as covariates in the comparison between the two diseased groups, the results of ANCOVA showed 4 tracts that remained significantly different; these tracts were the right fornix, left and right uncinate fasciculi, and callosal fibers connecting the temporal poles (Table 4 and Figure 3).
Table 4.
Comparison of the mean GFA values of white matter tracts between remission and nonremission groups
| R (n = 50) | NR (n = 41) | ANCOVA | |
|---|---|---|---|
| Fiber tracts | GFA value | GFA value | |
| Mean (SD) | Mean (SD) | p value | |
| Rt. Fornix | 0.200 (0.020) | 0.182 (0.022) | .001* |
| U. uncinate fasciculus | 0.233 (0.020) | 0.215 (0.019) | .001* |
| Rt. uncinatc fasciculus | 0.246 (0.017) | 0.225 (0.022) | <.00l* |
| CF to temporal poles | 0.195 (0.015) | 0.181 (0.017) | .002* |
Note. Abbreviations: CF = callosal fibers; GFA = generalized fractional anisotropy; Lt = left; NR = nonremission; R = remission; RT = right.
Covariates: age, sex, education year, duration of illness, and medication dose.
Bonferroni method was conducted to correct for multiple comparisons of 76 tracts.
*Statistically significant.
Figure 3.
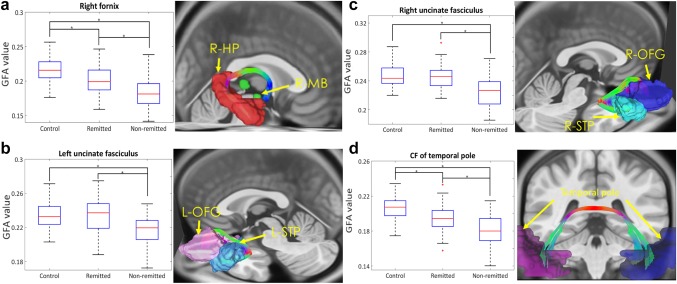
Box plots and three‐dimensional fiber tracking of the four fibers that were significantly different between remission and nonremission groups. (a) Right fornix: ROIs, R‐MB, and R‐HP. (b) Left uncinate fasciculus: ROIs, L‐STP, and L‐OFG. (c) Right uncinate fasciculus: ROIs, R‐STP, and R‐OFG. (d) CF of the temporal pole: ROIs, bilateral temporal poles. Abbreviation: CF = callosal fibers; HP = hippocampus; L = left; MB, mammillary body; OFG = orbitofrontal gyrus; ROIs = regions of interest; R = right; STP = superior temporal pole [Color figure can be viewed at http://wileyonlinelibrary.com]
After excluding the patients with comorbid disorders, the sensitivity analysis showed similar results (Supporting Information). In ANCOVA, among the three groups (i.e., controls, remission, and nonremission), only 3 out of 12 tracts (i.e., the right arcuate fasciculus, right perpendicular fasciculus, and left optic radiation) became insignificant. In ANCOVA between the remission and nonremission groups, only the left uncinate fasciculus became insignificant.
3.5. Correlation between GFA in white matter tracts and PANSS items
We investigated the correlations between the tracts that remained altered in the non‐remission group as compared with the remission group (i.e., right fornix, bilateral uncinate fasciculi, and callosal fibers connecting the temporal poles) with PANSS positive and negative symptoms scores (Fig. 4). We found that GFA values of the right fornix showed significant correlation with the positive symptom scores (r = −.22, p = .039) and the negative symptom scores (r = −.45, p < .001), whereas bilateral UF and the CF connecting the temporal poles were significantly correlated with the negative symptom scores (r = −.31, p = .004 for the left UF; r = −.35, p = .001 for the right UF; r = −.37, p < .001 for the CF connecting the temporal poles).
Figure 4.
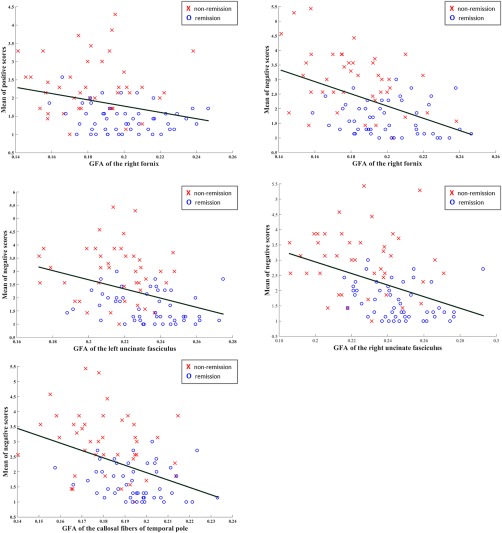
Correlations between PANSS positive and negative symptom scales and tract integrity of the tracts that were significantly different between remission and nonremission groups. Abbreviation: CF = callosal fibers; GFA = generalized fractional anisotropy, L = left; R = right; TP = temporal pole; UF = uncinate fasciculus [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
To investigate whether remitted and nonremitted patients with schizophrenia showed considerably distinct white matter tract alterations, we performed the first systematic comparisons of the tract integrity over the whole brain in 141 participants (remission, 50; nonremission, 41; healthy controls, 50) by adjusting age, sex, and education year. ANCOVA revealed 12 fiber tract bundles that had significantly different alterations among the 3 groups. Furthermore, post‐hoc between‐groups analysis showed that compared with the healthy controls, the nonremission group had reduced integrity in all 12 tracts, whereas the remission group showed reduced integrity in only 4 tracts. Compared with the remission group, the nonremission group showed reduced integrity in 11 tracts. ANCOVA between the remission and nonremission groups was further performed by adding illness duration and medication dose together with age, sex, and education year as covariates. The results showed 4 tracts that remained significantly different; these tracts were the right fornix, bilateral uncinate fasciculi, and CF connecting the temporal poles. The sensitivity analysis further confirmed that the results still remained even after excluding the patients with comorbid disorders. The results support our hypothesis that the remission and nonremission groups showed considerably distinct sets of altered white matter tracts as compared with controls. More importantly, nonremitted patients exhibited 4 fiber tracts that were more severely impaired than remitted patients.
Our ANCOVA results showed 12 tracts that were significantly different in GFA values among the 3 groups. Post‐hoc analysis further showed that these 11 tracts were significantly reduced in GFA in the nonremission group as compared with the remission group (Table 3). Our findings are consistent with previous DTI studies on chronic schizophrenia using different analysis approaches. Using 40 Brodmann's areas as ROIs, Mitelman et al. (2006) found that patients with poor outcomes had widespread FA reductions in both hemispheres at baseline. Subsequently, the same authors refined the analysis using stereotactic ROIs preselected from previous voxel‐based analyses, and found FA reduction in the posterior corpus callosum, fronto‐occipital fasciculus, left optic radiation, and fronto‐temporal white matter at baseline (Mitelman et al., 2007). Consistently, the 11 tracts found in our results comprise associative fibers mainly connecting the frontal and temporal lobes (the right arcuate fasciculus, bilateral fornices, and bilateral uncinate fasciculi), projection fibers of the left optic radiation, and commissural fibers connecting bilateral frontal lobes (the CF to the genu and DLPFC) or temporal lobes (the CF to the temporal poles, hippocampi, and amygdalae).
Although treatment outcome was associated with FA reduction at baseline in patients with chronic schizophrenia, the association is confounded by the possible effect of illness duration and medication dose. To address this problem, two studies on patients with first‐episode psychosis have been conducted recently. As compared with responders following a 12‐week antipsychotic treatment, Reis Marques et al. (2014) found that nonresponders had marked reduction of FA in the tracts interconnecting the frontal and temporal cortices (e.g., the uncinate and fornix) at baseline. Luck et al. (2011) studied the association of the treatment outcome after 6 months of medication with three pairs of fronto‐temporal white matter tracts (i.e., the cingulum, superior longitudinal fasciculus, and uncinate fasciculus). As compared with patients with good outcomes, patients with poor outcomes had reduced FA in the superior longitudinal fasciculus and uncinate fasciculus at baseline. The results of these studies imply that fronto‐temporal white matter tracts are impaired early in the course of disease, and these tracts might be a prognostic marker of antipsychotic treatment. Our comparison in chronic schizophrenia with age, sex, education year, illness duration, and medication dose as covariates revealed four tracts (i.e., the right fornix, bilateral uncinate fasciculi, and the CF connecting the temporal poles) with significant FA reduction in patients with poor outcomes. Interestingly, our findings largely agree with the two studies in first‐episode psychosis. The minor inconsistency might be due partly to the difference in patient selection, namely, chronic schizophrenia in our study and first‐episode psychosis in other studies.
In the 4 tracts that showed significantly impaired in nonremitted patients as compared with remitted patients, we found that the right fornix and the CF connecting the temporal poles also showed significantly impaired in remitted patients as compared with controls (Tables 3 and 4). In contrast, bilateral uncinate fasciculi showed significant difference only in the comparison between remitted and nonremitted patients, but not in the comparison between remitted patients and controls (Tables 3 and 4). The results indicate that the right fornix and the CF connecting the temporal poles exhibit alterations of tract integrity in schizophrenia disregarding the state of remission, whereas abnormality of bilateral uncinate fasciculi only occurs in nonremitted patients. In other words, impairment of bilateral uncinate fasciculi might signal the nonremission of treatment outcome.
In this study, we found that PANSS negative symptom scores were negatively correlated with GFA of all of the 4 tracts (Figure 4). Note that these 4 tracts are the tracts connecting ipsilateral temporal and prefrontal lobes (right fornix and bilateral uncinate fasciculi) or connecting bilateral temporal lobes (CF connecting the temporal poles). Our findings are consistent with prior studies reporting that negative symptoms were associated with white matter alterations in the frontal and temporal regions (Anderson et al., 2002; Sanfilipo et al., 2000; Sigmundsson et al., 2001; Wible et al., 2001; Wolkin et al., 2003). In our study, we also found that nonremitted patients had more severe negative symptoms than remitted patients (Table 2). This finding is consistent with clinical observation that patients with more severe negative symptoms had poorer treatment outcome (Ezeme et al., 2017). Based on the previous reports and our findings, we speculate that impairment of these 4 tracts might aggravate negative symptoms which results in poor treatment outcome (Cahn et al., 2002; Gur et al., 1998; Rossi et al., 2000).
In the correlation analysis, one might notice a floor effect in remitted patients. This is because remitted patients had an average negative scores lower than 3 (Figure 4). The floor effect, along with the small sample size, could make the correlation analysis within each group insignificant. Disregarding remission and nonremission states, clinical relevance of these four tracts is revealed by the presence of significant negative correlations with the PANSS negative scores.
In this study, we also found that PANSS positive symptom scores were negatively correlated with the GFA values of the right fornix (Figure 4). Many studies have investigated the associations between structural data and the PANSS positive symptom scores, but the results vary. Samartzis et al. (2014) conducted a review of 18 DTI studies on schizophrenia and noted that, in general, there was a positive correlation between white matter FA and positive symptoms and a negative correlation between white matter FA and negative symptoms. However, some studies found negative correlations between white matter FA and positive symptoms (Ashton Acton, 2013; Skelly et al., 2008). For instance, Skelly et al. (2008) reported that positive symptoms were inversely correlated with FA in diverse regions, including the left uncinate fasciculus, right sagittal striatum, and left superior longitudinal fasciculus. The inconsistency might be reflected partly in the low value of correlation coefficient (r = −.22) in our results.
When performing group‐based comparisons between remitted and nonremitted patients with schizophrenia, confounders including age, sex, and education year are often considered because these factors may covary with white matter tract integrity (Walder et al., 2006; Zhang et al., 2013). However, previous studies did not consider duration of illness and antipsychotic dose as covariates. Some of previous studies reported that FA is associated with duration of illness in various white matter tract alterations in chronic patients with schizophrenia (Friedman et al., 2008; Ho et al., 2003; Mori et al., 2007). However, Wu et al. conducted TBAA to investigate white matter tract alterations in chronic patients with schizophrenia, those with first‐episode schizophrenia, and healthy controls, and observed that most tracts that were altered in chronic patients were also altered in first‐episode patients (Kanaan, Picchioni, McDonald, Shergill, & McGuire, 2017; Wu et al., 2015a). The effect of antipsychotic drugs on the integrity of white matter also remains debatable. Kanaan et al. (2009) revealed that the FA of white matter did not differ between briefly‐medicated and chronic patients with schizophrenia. Kyriakopoulos et al. (2011) observed no relationship between the antipsychotic dose or cumulative drug exposure and white matter tract integrity. However, Ho, Andreasen, Ziebell, Pierson, and Magnotta (2011) revealed that increased antipsychotic drug exposure was associated with white matter volume reduction in first‐episode schizophrenia. In our study, there are 11 tracts with significant difference between remitted and nonremitted patients when age, sex, and education year are considered as covariates. The number of tracts with significant difference reduces to 4 when we add duration of illness and medication dose to the covariates. Our results indicate that duration of illness and medication dose explain a large portion of the variance between remitted and nonremitted patients, and should be considered.
When comparing the tract integrity between the remission and nonremission groups, we adjusted age, sex, education year, duration of illness, and medication dose, to minimize their effects on white matter tract integrity. Despite these adjustments, 4 tracts had significantly distinct alterations between the two groups. Schizophrenia is a mental disorder with varying degrees of symptoms determined by both genetic and environmental factors (Tsuang, 2000). This genetic variation may result in different antipsychotic outcomes and side‐effects, and consequently result in considerable disease heterogeneity and different treatment outcomes in individuals (Kirov, O'Donovan, & Owen, 2005; Zandi and Judy, 2010). Voineskos (2015) showed that the loci in genes intimately implicated in oligodendrocyte and myelin development, growth, and maintenance are associated with white matter microstructure, supporting the heritability of white matter phenotypes. In a DTI heritability study, Chiang et al. (2009) compared the monozygotic and dizygotic twin pairs and showed that genetic factors explain 75%–90% of the variance in FA in most white matter regions. Genetic effects have also been demonstrated in the heritability studies on schizophrenia, showing a stepwise GFA or FA decrease from healthy controls to siblings to probands, thus indicating that GFA or FA values are potential endophenotypic markers (Skudlarski et al., 2013; Wu et al., 2015b). Altogether, many results support that alterations in white matter tract integrity may be inherent to the disease; therefore, we suspect that the distinct severities of tract alterations observed in the remission and nonremission groups might also be an inherent prognostic marker of schizophrenia.
This study has several limitations. First, although the diagnoses of patients were determined through comprehensive chart review and personal interviews conducted by experienced psychiatrists, we did not use structured interview such as Structure Clinical Interview for DSM‐IV (SCID) (First, Spitzer, Williams, & Gibbon, 1995) to confirm their diagnoses. Second, the study was conducted in chronic patients with schizophrenia. Although we believe that the same difference in the integrity of white matter tracts can be observed early in the course of disease because tract alterations are inherent to the disease, it warrants a longitudinal study on drug naïve patients to validate our claims. Third, we defined the treatment outcomes by both RSWG symptom and time criteria (Andreasen et al., 2005), which do not consider the persistence of negative symptoms or the recovery of patient's daily life functioning (Oorschot et al., 2012). Categorization of the patients according to other criteria of treatment outcomes might yield different groups of altered fiber tracts. Fourth, different types of antipsychotic drugs may exert different effects on white matter volume (Bartzokis et al., 2007). The heterogeneous effects of different antipsychotic drugs were not considered in this study (Andreasen et al., 2010; Woods, 2003). In addition, a “dose/year” metric would be much better than current dose for antipsychotic exposure. However, because most of these patients had long duration of illness, it was very difficult to collect the dose/year variables accurately. Access to such information would improve the present analysis. In this study, we used current dose of medication and duration of illness to represent two facets of medication dose.
In conclusion, we observed different severities in white matter tract alterations between remitted and nonremitted patients with schizophrenia. The right fornix, bilateral uncinate fasciculi, and CF connecting bilateral temporal poles exhibit more impairment in nonremitted patients than in remitted patients. These tracts might be potential prognostic markers of the treatment outcomes in patients with schizophrenia. The generalization of our findings requires validation in multiple institutes using other analysis approaches or diffusion acquisitions.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Table 1
Supporting Information Table 2
ACKNOWLEDGMENT
This work was supported in part by the Ministry of Science and Technology, Taiwan (grant number: MOST103–2325‐B‐002–040; NSC 100–2321‐B‐002‐0‐16).
Huang J‐Y, Liu C‐M, Hwang T‐J, et al. Shared and distinct alterations of white matter tracts in remitted and nonremitted patients with schizophrenia. Hum Brain Mapp. 2018;39:2007–2019. 10.1002/hbm.23982
Funding information Ministry of Science and Technology, Taiwan, Grant/Award Numbers: MOST 103‐2325‐B‐002‐040, NSC 100‐2321‐B‐002‐0‐16
REFERENCES
- Anderson, J. E. , Wible, C. G. , McCarley, R. W. , Jakab, M. , Kasai, K. , & Shenton, M. E. (2002). An MRI study of temporal lobe abnormalities and negative symptoms in chronic schizophrenia. Schizophrenia Research, 58(2), 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen, N. C. , Carpenter, W. T., Jr , Kane, J. M. , Lasser, R. A. , Marder, S. R. , & Weinberger, D. R. (2005). Remission in schizophrenia: Proposed criteria and rationale for consensus. 162, 441–449. (2005). [DOI] [PubMed]
- Andreasen, N. C. , Pressler, M. , Nopoulos, P. , Miller, D. , & Ho, B. C. (2010). Antipsychotic dose equivalents and dose‐years: A standardized method for comparing exposure to different drugs. Biological Psychiatry, 67, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis, G. , Lu, P. H. , Nuechterlein, K. H. , Gitlin, M. , Doi, C. , Edwards, N. , … Mintz, J. (2007). Differential effects of typical and atypical antipsychotics on brain myelination in schizophrenia. Schizophrenia Research, 93, 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser, P. J. , Mattiello, J. , & LeBihan, D. (1994). MR diffusion tensor spectroscopy and imaging. Biophysical Journal, 66, 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system–a technical review. NMR in Biomedicine, 15, 435–455. [DOI] [PubMed] [Google Scholar]
- Bodnar, M. , Harvey, P. O. , Malla, A. , Joober, R. , & Lepage, M. (2010). The parahippocampal gyrus as a neural marker of early remission in first‐episode psychosis: A voxel‐based morphometry study. Clinical Schizophrenia & Related Psychoses, 4, 217–228. [DOI] [PubMed] [Google Scholar]
- Cahn, W. , Pol, H. E. , Lems, E. B. , van Haren, N. E. , Schnack, H. G. , van der Linden, J. A. , … Kahn, R. S. (2002). Brain volume changes in first‐episode schizophrenia: A 1‐year follow‐up study. Archives of General Psychiatry, 59(11), 1002–1010. [DOI] [PubMed] [Google Scholar]
- Callaghan, P. T. , Coy, A. , MacGowan, D. , Packer, K. J. , & Zelaya, F. O. (1991). Diffraction‐like effects in NMR diffusion studies of fluids in porous solids. Nature, 351, 467–469. [Google Scholar]
- Camchong, J. , Lim, K. O. , Sponheim, S. R. , & Macdonald, A. W. (2009). Frontal white matter integrity as an endophenotype for schizophrenia: Diffusion tensor imaging in monozygotic twins and patients' nonpsychotic relatives. Frontiers in Human Neuroscience, 3, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. J. , Hsiao, C. K. , Hsiao, L. L. , & Hwu, H. G. (1998). Performance of the continuous performance test among community samples. Schizophrenia Bulletin, 24, 163–174. [DOI] [PubMed] [Google Scholar]
- Chen, Y. J. , Lo, Y. C. , Hsu, Y. C. , Fan, C. C. , Hwang, T. J. , Liu, C. M. , … Tseng, W. Y. (2015). Automatic whole brain tract‐based analysis using predefined tracts in a diffusion spectrum imaging template and an accurate registration strategy. Human Brain Mapping, 36, 3441–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, M. C. , Barysheva, M. , Shattuck, D. W. , Lee, A. D. , Madsen, S. K. , Avedissian, C. , … Thompson, P. M. (2009). Genetics of brain fiber architecture and intellectual performance. Journal of Neuroscience, 29, 2212–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappelli, J. , Hong, L. E. , Wijtenburg, S. A. , Du, X. , Gaston, F. , Kochunov, P. , & Rowland, L. M. (2015). Alterations in frontal white matter neurochemistry and microstructure in schizophrenia: Implications for neuroinflammation. Translational Psychiatry, 5, e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll, C. U. , Malhotra, A. K. , Kaushik, S. , McMeniman, M. , & Kane, J. M. (2003). Early prediction of antipsychotic response in schizophrenia. American Journal of Psychiatry, 160, 2063–2065. [DOI] [PubMed] [Google Scholar]
- Dazzan, P. (2014). Neuroimaging biomarkers to predict treatment response in schizophrenia the end of 30 years of solitude? Dialogues in Clinical Neuroscience, 16, 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunayevich, E. , Sethuraman, G. , Enerson, M. , Taylor, C. C. , & Lin, D. (2006). Characteristics of two alternative schizophrenia remission definitions: Relationship to clinical and quality of life outcomes. Schizophrenia Research, 86, 300–308. [DOI] [PubMed] [Google Scholar]
- Egan, M. F. , Goldberg, T. E. , Kolachana, B. S. , Callicott, J. H. , Mazzanti, C. M. , Straub, R. E. , … Weinberger, D. R. (2001). Effect of COMT Val108158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences, 98, 6917–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley, R. , Rabinowitz, J. , & Medori, R. Early Psychosis Global Working, G . (2007). Remission in early psychosis: Rates, predictors, and clinical and functional outcome correlates. Schizophrenia Research, 89, 129–139. [DOI] [PubMed] [Google Scholar]
- Ezeme, M. S. , Uwakwe, R. , Ndukuba, A. C. , Igwe, M. N. , Odinka, P. C. , Amadi, K. , & Obayi, N. O. (2017). Clinical correlates of treatment response among patients with schizophrenia in a tertiary Nigerian hospital. Journal of Health Care Poor Underserved, 28, 721–738. [DOI] [PubMed] [Google Scholar]
- First, M. B. , Spitzer, R. L. , Williams, J. B. , & Gibbon, M. (1995). Structured clinical interview for DSM‐IV‐patient edition (SCID‐P). Washington, DC: American Psychiatric Press. [Google Scholar]
- Friedman, J. I. , Tang, C. , Carpenter, D. , Buchsbaum, M. , Schmeidler, J. , Flanagan, L. , … Davis, K. L. (2008). Diffusion tensor imaging findings in first‐episode and chronic schizophrenia patients. American Journal of Psychiatry, 165, 1024–1032. [DOI] [PubMed] [Google Scholar]
- Fujino, J. , Takahashi, H. , Miyata, J. , Sugihara, G. , Kubota, M. , Sasamoto, A. , … Murai, T. (2014). Impaired empathic abilities and reduced white matter integrity in schizophrenia. Progress in Neuropsychopharmacology & Biological Psychiatry, 48, 117–123. [DOI] [PubMed] [Google Scholar]
- Gur, R. E. , Cowell, P. , Turetsky, B. I. , Gallacher, F. , Cannon, T. , Bilker, W. , & Gur, R. C. (1998). A follow‐up magnetic resonance imaging study of schizophrenia. Archives of General Psychiatry, 55(2), 145–152. [DOI] [PubMed] [Google Scholar]
- Hao, Y. , Liu, Z. , Jiang, T. , Gong, G. , Liu, H. , Tan, L. , … Zhang, Z. (2006). White matter integrity of the whole brain is disrupted in first‐episode schizophrenia. Neuroreport, 17, 23–26. [DOI] [PubMed] [Google Scholar]
- Ho, B. C. , Andreasen, N. C. , Nopoulos, P. , Arndt, S. , Magnotta, V. , & Flaum, M. (2003). Progressive structural brain abnormalities and their relationship to clinical outcome. Archives of General Psychiatry, 60, 585–594. [DOI] [PubMed] [Google Scholar]
- Ho, B. C. , Andreasen, N. C. , Ziebell, S. , Pierson, R. , & Magnotta, V. (2011). Long‐term antipsychotic treatment and brain volumes. Archives of General Psychiatry, 68, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu, Y. C. , Hsu, C. H. , & Tseng, W. Y. (2012). A large deformation diffeomorphic metric mapping solution for diffusion spectrum imaging datasets. NeuroImage, 63, 818–834. [DOI] [PubMed] [Google Scholar]
- Hsu, Y. C. , Lo, Y. C. , Chen, Y. J. , Wedeen, V. J. , & Isaac Tseng, W. Y. (2015). NTU‐DSI‐122: A diffusion spectrum imaging template with high anatomical matching to the ICBM‐152 space. Human Brain Mapping, 36, 3528–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaskelainen, E. , Juola, P. , Kurtti, J. , Haapea, M. , Kyllonen, M. , Miettunen, J. , … Isohanni, M. (2014). Associations between brain morphology and outcome in schizophrenia in a general population sample. European Psychiatry, 29, 456–462. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg, H. B. T. (2013). Diffusion MRI: From quantitative measurement to in vivo neuroanatomy. Academic Press. [Google Scholar]
- Kanaan, R. , Barker, G. , Brammer, M. , Giampietro, V. , Shergill, S. , Woolley, J. , … McGuire, P. (2009). White matter microstructure in schizophrenia: Effects of disorder, duration and medication. British Journal of Psychiatry, 194, 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan, R. A. , Picchioni, M. M. , McDonald, C. , Shergill, S. S. , & McGuire, P. K. (2017). White matter deficits in schizophrenia are global and don't progress with age. Australian New Zealand Journal of Psychiatry, 4867417700729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur, H. , Jajodia, A. , Grover, S. , Baghel, R. , Gupta, M. , Jain, S. , & Kukreti, R. (2014). Genetic variations of PIP4K2A confer vulnerability to poor antipsychotic response in severely ill schizophrenia patients. PLoS One, 9, e102556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov, G. , O'Donovan, M. C. , & Owen, M. J. (2005). Finding schizophrenia genes. The Journal of Clinical Investigation, 115, 1440–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubicki, M. , McCarley, R. W. , & Shenton, M. E. (2005). Evidence for white matter abnormalities in schizophrenia. Current Opinion in Psychiatry, 18, 121–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo, L. W. , Chen, J. H. , Wedeen, V. J. , & Tseng, W. Y. (2008). Optimization of diffusion spectrum imaging and q‐ball imaging on clinical MRI system. NeuroImage, 41, 7–18. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos, M. , Samartzis, L. , Dima, D. , Hayes, D. , Corrigall, R. , Barker, G. , … Frangou, S. (2011). P03–111 ‐ Does antipsychotic medication affect white matter in schizophrenia and bipolar disorder? A review of diffusion tensor imaging literature. European Psychiatry, 26, 1280. [Google Scholar]
- Lambert, M. , Schimmelmann, B. G. , Naber, D. , Schacht, A. , Karow, A. , Wagner, T. , & Czekalla, J. (2006). Prediction of remission as a combination of symptomatic and functional remission and adequate subjective well‐being in 2960 patients with schizophrenia. The Journal of Clinical Psychiatry, 67, 1690–1697. [DOI] [PubMed] [Google Scholar]
- Lieberman, J. A. , Alvir, J. M. , Koreen, A. , Geisler, S. , Chakos, M. , Sheitman, B. , & Woerner, M. (1996). Psychobiologic correlates of treatment response in schizophrenia. Neuropsychopharmacology, 14, 13S–21S. [DOI] [PubMed] [Google Scholar]
- Luck, D. , Buchy, L. , Czechowska, Y. , Bodnar, M. , Pike, G. B. , Campbell, J. S. , … Lepage, M. (2011). Fronto‐temporal disconnectivity and clinical short‐term outcome in first episode psychosis: A DTI‐tractography study. Journal of Psychiatry Research, 45, 369–377. [DOI] [PubMed] [Google Scholar]
- McDonald, J. H. (2009). Handbook of biological statistics (Vol. 2, pp. 173–181). Baltimore, MD: Sparky House Publishing. [Google Scholar]
- Mirnics, K. , Middleton, F. A. , Marquez, A. , Lewis, D. A. , & Levitt, P. (2000). Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron, 28, 53–67. [DOI] [PubMed] [Google Scholar]
- Mitelman, S. A. , Newmark, R. E. , Torosjan, Y. , Chu, K. W. , Brickman, A. M. , Haznedar, M. M. , … Buchsbaum, M. S. (2006). White matter fractional anisotropy and outcome in schizophrenia. Schizophrenia Research, 87, 138–159. [DOI] [PubMed] [Google Scholar]
- Mitelman, S. A. , Torosjan, Y. , Newmark, R. E. , Schneiderman, J. S. , Chu, K. W. , Brickman, A. M. , … Buchsbaum, M. S. (2007). Internal capsule, corpus callosum and long associative fibers in good and poor outcome schizophrenia: A diffusion tensor imaging survey. Schizophrenia Research, 92, 211–224. [DOI] [PubMed] [Google Scholar]
- Mori, T. , Ohnishi, T. , Hashimoto, R. , Nemoto, K. , Moriguchi, Y. , Noguchi, H. , … Kunugi, H. (2007). Progressive changes of white matter integrity in schizophrenia revealed by diffusion tensor imaging. Psychiatry Research, 154, 133–145. [DOI] [PubMed] [Google Scholar]
- Nurnberger, J. I. , Blehar, M. C. , Kaufmann, C. A. , York‐Cooler, C. , Simpson, S. G. , Harkavy, F. J. , … Reich, T. (1994). Diagnostic interview for genetic studies rationale, unique features, and training. Archives of General Psychiatry, 51, 849–859. [DOI] [PubMed] [Google Scholar]
- Oh, J. S. , Kubicki, M. , Rosenberger, G. , Bouix, S. , Levitt, J. J. , McCarley, R. W. , … Shenton, M. E. (2009). Thalamo‐frontal white matter alterations in chronic schizophrenia. Human Brain Mapping, 30, 3812–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9, 97–113. [DOI] [PubMed] [Google Scholar]
- Oorschot, M. , Lataster, T. , Thewissen, V. , Lardinois, M. , van Os, J. , Delespaul, P. A. , & Myin‐Germeys, I. (2012). Symptomatic remission in psychosis and real‐life functioning. The British Journal of Psychiatry: The Journal of Mental Science, 201, 215–220. [DOI] [PubMed] [Google Scholar]
- Pettersson‐Yeo, W. , Allen, P. , Benetti, S. , McGuire, P. , & Mechelli, A. (2011). Dysconnectivity in schizophrenia: Where are we now? Neuroscience & Biobehavioral Reviews, 35, 1110–1124. [DOI] [PubMed] [Google Scholar]
- Ashton Acton, Q. (2013). Advances in telencephalon research and application (2013 ed.). ScholarlyBrief. [Google Scholar]
- Reese, T. G. , Heid, O. , Weisskoff, R. M. , & Wedeen, V. J. (2003). Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magnetic Resonance in Medicine, 49, 177–182. [DOI] [PubMed] [Google Scholar]
- Reis Marques, T. , Taylor, H. , Chaddock, C. , Dell'acqua, F. , Handley, R. , Reinders, A. A. , … Dazzan, P. (2014). White matter integrity as a predictor of response to treatment in first episode psychosis. Brain, 137, 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roalf, D. R. , Ruparel, K. , Verma, R. , Elliott, M. A. , Gur, R. E. , & Gur, R. C. (2013). White matter organization and neurocognitive performance variability in schizophrenia. Schizophrenia Research, 143, 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, A. , Bustini, M. , Prosperini, P. , Marinangeli, M. G. , Splendiani, A. , Daneluzzo, E. , & Stratta, P. (2000). Neuromorphological abnormalities in schizophrenic patients with good and poor outcome. Acta Psychiatrica Scandinavica, 101(2), 161–166. [DOI] [PubMed] [Google Scholar]
- Samartzis, L. , Dima, D. , Fusar‐Poli, P. , & Kyriakopoulos, M. (2014). White matter alterations in early stages of schizophrenia: a systematic review of diffusion tensor imaging studies. Journal of Neuroimaging, 24(2), 101–110. [DOI] [PubMed] [Google Scholar]
- San, L. , Ciudad, A. , Alvarez, E. , Bobes, J. , & Gilaberte, I. (2007). Symptomatic remission and social/vocational functioning in outpatients with schizophrenia: Prevalence and associations in a cross‐sectional study. European Psychiatry, 22(8), 490. [DOI] [PubMed] [Google Scholar]
- Sanfilipo, M. , Lafargue, T. , Rusinek, H. , Arena, L. , Loneragan, C. , Lautin, A. , … Wolkin, A. (2000). Volumetric measure of the frontal and temporal lobe regions in schizophrenia: Relationship to negative symptoms. Archives of General Psychiatry, 57(5), 471–480. [DOI] [PubMed] [Google Scholar]
- Sigmundsson, T. , Suckling, J. , Maier, M. , Williams, S. C. , Bullmore, E. T. , Greenwood, K. E. , … Toone, B. K. (2001). Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. American Journal of Psychiatry, 158(2), 234–243. [DOI] [PubMed] [Google Scholar]
- Skelly, L. R. , Calhoun, V. , Meda, S. A. , Kim, J. , Mathalon, D. H. , & Pearlson, G. D. (2008). Diffusion tensor imaging in schizophrenia: relationship to symptoms. Schizophrenia research, 98(1), 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski, P. , Schretlen, D. J. , Thaker, G. K. , Stevens, M. C. , Keshavan, M. S. , Sweeney, J. A. , … Pearlson, G. D. (2013). Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. The American Journal of Psychiatry, 170, 886–898. [DOI] [PubMed] [Google Scholar]
- Stephan, K. E. , Friston, K. J. , & Frith, C. D. (2009). Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self‐monitoring. Schizophrenia Bulletin, 35, 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang, M. (2000). Schizophrenia genes and environment. Biological Psychiatry, 47, 210–220. [DOI] [PubMed] [Google Scholar]
- Tuch, D. S. (2004). Q‐ball imaging. Magnetic Resonance in Medicine, 52, 1358–1372. [DOI] [PubMed] [Google Scholar]
- van Haren, N. E. , Hulshoff Pol, H. E. , Schnack, H. G. , Cahn, W. , Mandl, R. C. , Collins, D. L. , … Kahn, R. S. (2007). Focal gray matter changes in schizophrenia across the course of the illness: A 5‐year follow‐up study. Neuropsychopharmacology, 32, 2057–2066. [DOI] [PubMed] [Google Scholar]
- Voineskos, A. N. (2015). Genetic underpinnings of white matter 'connectivity': Heritability, risk, and heterogeneity in schizophrenia. Schizophrenia Research, 161, 50–60. [DOI] [PubMed] [Google Scholar]
- Walder, D. J. , Seidman, L. J. , Cullen, N. , Su, J. , Tsuang, M. T. , & Goldstein, J. M. (2006). Sex differences in language dysfunction in schizophrenia. American Journal of Psychiatry, 163, 470–477. [DOI] [PubMed] [Google Scholar]
- Walther, S. , Federspiel, A. , Horn, H. , Razavi, N. , Wiest, R. , Dierks, T. , … Muller, T. J. (2011). Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiology of Disease, 42, 276–283. [DOI] [PubMed] [Google Scholar]
- Wedeen, V. J. , Hagmann, P. , Tseng, W. Y. , Reese, T. G. , & Weisskoff, R. M. (2005). Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magnetic Resonance in Medicine, 54, 1377–1386. [DOI] [PubMed] [Google Scholar]
- Wedeen, V. J. , Wang, R. P. , Schmahmann, J. D. , Benner, T. , Tseng, W. Y. , Dai, G. , … de Crespigny, A. J. (2008). Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. NeuroImage, 41, 1267–1277. [DOI] [PubMed] [Google Scholar]
- Wible, C. G. , Anderson, J. , Shenton, M. E. , Kricun, A. , Hirayasu, Y. , Tanaka, S. , … McCarley, R. W. (2001). Prefrontal cortex, negative symptoms, and schizophrenia: An MRI study. Psychiatry Research: Neuroimaging, 108(2), 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkin, A. , Choi, S. J. , Szilagyi, S. , Sanfilipo, M. , Rotrosen, J. P. , & Lim, K. O. (2003). Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: A diffusion tensor imaging study. American Journal of Psychiatry, 160(3), 572–574. [DOI] [PubMed] [Google Scholar]
- Woods, S. W. (2003). Chlorpromazine equivalent doses for the newer atypical antipsychotics. The Journal of Clinical Psychiatry, [DOI] [PubMed] [Google Scholar]
- Wu, C. H. , Hwang, T. J. , Chen, Y. J. , Hsu, Y. C. , Lo, Y. C. , Liu, C. M. , … Isaac Tseng, W. Y. (2015a). Primary and secondary alterations of white matter connectivity in schizophrenia: A study on first‐episode and chronic patients using whole‐brain tractography‐based analysis. Schizophrenia Research, 169, 54–61. [DOI] [PubMed] [Google Scholar]
- Wu, C. H. , Hwang, T. J. , Chen, Y. J. , Hsu, Y. C. , Lo, Y. C. , Liu, C. M. , … Tseng, W. Y. (2015b). Altered integrity of the right arcuate fasciculus as a trait marker of schizophrenia: A sibling study using tractography‐based analysis of the whole brain. Human Brain Mapping, 36, 1065–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, F. C. , Verstynen, T. D. , Wang, Y. , Fernandez‐Miranda, J. C. , & Tseng, W. Y. (2013). Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One, 8, e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandi, P. P. , & Judy, J. T. (2010). The promise and reality of pharmacogenetics in psychiatry. The Psychiatric Clinics of North America, 33, 181–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Li, B. , & Shan, B. (2013). Age‐related white matter degradation rule of normal human brain: The evidence from diffusion tensor magnetic resonance imaging. Chinese Medical Journal, 127, 532–537. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information Table 1
Supporting Information Table 2


