Abstract
Adolescence represents an important period during which considerable changes in the brain take place, including increases in integrity of white matter bundles, and increasing efficiency of the structural brain network. A more efficient structural brain network has been associated with higher intelligence. Whether development of structural network efficiency is related to intelligence, and if so to which extent genetic and environmental influences are implicated in their association, is not known. In a longitudinal study, we mapped FA‐weighted efficiency of the structural brain network in 310 twins and their older siblings at an average age of 10, 13, and 18 years. Age‐trajectories of global and local FA‐weighted efficiency were related to intelligence. Contributions of genes and environment were estimated using structural equation modeling. Efficiency of brain networks changed in a non‐linear fashion from childhood to early adulthood, increasing between 10 and 13 years, and leveling off between 13 and 18 years. Adolescents with higher intelligence had higher global and local network efficiency. The dependency of FA‐weighted global efficiency on IQ increased during adolescence (rph=0.007 at age 10; 0.23 at age 18). Global efficiency was significantly heritable during adolescence (47% at age 18). The genetic correlation between intelligence and global and local efficiency increased with age; genes explained up to 87% of the observed correlation at age 18. In conclusion, the brain's structural network differentiates depending on IQ during adolescence, and is under increasing influence of genes that are also associated with intelligence as it develops from late childhood to adulthood.
Keywords: adolescence, development, DTI, heritability, IQ, intelligence, longitudinal, MRI, network, puberty
1. INTRODUCTION
Adolescence represents a period of considerable brain development. During adolescence the cortex becomes thinner (Brown et al., 2012; Ducharme et al., 2012; Gogtay et al., 2004; Shaw et al., 2008; van Soelen et al., 2012a; Sowell, 2004) while its connecting white matter fibers increase in volume (Brouwer et al., 2012; Paus, 2010), which they continue into young adulthood (Lebel and Beaulieu, 2011; Peters et al., 2014; Schmithorst and Yuan, 2010; Simmonds, Hallquist, Asato, & Luna, 2014). Together, these white matter fibers form structural brain networks with an efficient organization that becomes increasingly more efficiently organized during development (Koenis et al., 2015). Determining the process of development of the brain network during adolescence is an important step in understanding developmental disorders that have their onset during this period of rapid changes (Paus, Keshavan, & Giedd, 2008). Because white matter network development and cognitive development go hand in hand (Koenis et al., 2015) and brain and experience may shape each other (Park and Friston, 2013; Paus, 2013), it is of importance to study both brain and cognitive development and their associations during adolescence.
Structural brain efficiency has been associated with cognitive functioning. Adults with a higher intelligence have a more efficient functional brain network (van den Heuvel, Stam, Kahn, & Hulshoff Pol, 2009; Langer et al., 2012) and structural white matter network (Bohlken et al., 2016b; Chiang et al., 2009; Li et al., 2009; Wen et al., 2011). Already in childhood (Kim et al., 2016) and early adolescence (Koenis et al., 2015; Schmithorst, Wilkes, Dardzinski, & Holland, 2005; Tamnes et al., 2011; Wang et al., 2012), positive associations between intelligence and structure of the white matter seem to be present, at least to some extent. How does the brain develop during this period of major maturational changes in cognition and social environment (Blakemore, Burnett, & Dahl, 2010; Luna, Marek, Larsen, Tervo‐Clemmens, & Chahal, 2015)? A longitudinal setup with measurements from late childhood and throughout adolescence allows us to study this question. By including twins and their siblings, the influences of genes and environment on these developmental changes can be assessed over time.
Recently we showed in a longitudinal twin study with measurements from late childhood to early adolescence that the FA‐weighted structural brain network becomes more efficient between the ages of 10 and 13, and that changes in intelligence were related to changes in structural efficiency (Koenis et al., 2015). We now rescanned these twins and their siblings for a third time at age 17 (twins) and age 15–23 years (siblings), which allowed us to examine the nonlinear development of FA‐based network efficiency and the possible relation between intelligence and this development. Previous studies showed nonlinear developmental patterns of FA (Lebel and Beaulieu, 2011; Peters et al., 2014; Schmithorst and Yuan, 2010; Simmonds et al., 2014) and network characteristics (Dennis et al. 2013; Zhao et al., 2015). One study found that the development of white matter integrity is related to intelligence (Tamnes et al., 2010) and in our previous work we observed a correlation between change in IQ and change in network efficiency. In addition, intelligence is related to trajectories of cortical thickness (Brans et al., 2010; Brouwer et al., 2014; Karama et al., 2014; Shaw et al., 2006; Schnack et al., 2015). Given the relationship between IQ and the development of several structural brain measures, this lead us to investigate the association between IQ and the developmental trajectory of network efficiency, hypothesizing that not only gray matter but also white matter microstructure shows a developmental trajectory that is associated with IQ level.
Because we found that FA‐weighted network efficiency was related to IQ whereas the streamlines‐based network efficiency was not, we chose to examine the relation between IQ and FA‐based network efficiency in this study. Thus, here we report on developmental patterns of white matter efficiency up to early adulthood, on the association between white matter network efficiency and intelligence, and on the extent to which genetic and the environmental factors influence this development of structural brain efficiency.
2. MATERIALS AND METHODS
2.1. Participants
A total of 226 twins (98 monozygotic subjects (48 boys and 50 girls), 128 dizygotic subjects (66 boys and 62 girls)) and 103 of their older siblings and 1 younger sibling (44 boys and 60 girls) were included in the BrainSCALE cohort (van Soelen et al., 2012b). The sample was recruited from the Netherlands Twin Register (van Beijsterveldt et al., 2013). At the first wave the mean (SD) age of the twins was 9.2 (0.24) years; at the second wave 12.2 (0.4) years; at the third wave 17.2 (0.3) years. The age difference between the twins and their siblings was 2.7 years on average. Interval between waves one and two was 2.93 (0.23) years, and between waves two and three, 5.02 (0.32) years (Figure 1 and Table 1). From 20 participants, no DTI scan with sufficient quality was collected. In total, 699 scans were collected from 310 participants: 51 participants had 1 scan; 129 participants had 2 scans; 130 participants had 3 scans. Zygosity of the twins was confirmed by genome‐wide SNP data. Written informed consents were obtained from all subjects and their parents. The Dutch Central Committee on Research involving Human Subjects (CCMO) approved the study.
Figure 1.
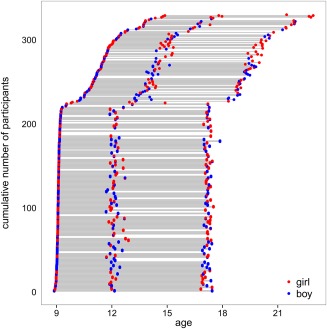
Ages of the individual participants from the twin families and their older singleton sibling at the three measurements. Individuals who have participated multiple times are connected by lines [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Demographics
| Wave 1 | Wave 2 | Wave 3 | |
|---|---|---|---|
| N (MZ/DZ/sibs) | 285 (90/115/80) | 179 (61/67/51) | 235 (71/97/67) |
| Age (range) | 10.0 [9.0–15.0] | 13.0 [9.8–17.9] | 18.0 [14.9–22.9] |
| IQ (sd) | 101.9 (14.7) | 101.7 (15.3) | 103.8 (12.7) |
2.2. Cognition
Intelligence was assessed based on the intelligence quotient (IQ) as measured with the Wechsler Intelligence Scale for Children III (WISC‐III, Dutch version, Wechsler et al., 2002) at measurement 1 and 2, and with the Wechsler Adult Intelligence Scale III (WAIS‐III, Dutch version) at measurement 3. At the first measurement, all subtasks of the WISC were included. At the second measurement, six subtasks of the WISC‐III were administered: four verbal subtests (similarities, arithmetic, vocabulary, and digit span), and two nonverbal subtests (picture completion and block design). At the third measurement, four subtasks of the WAIS‐III were administered: similarities, vocabulary, block design, and matrix reasoning. These tasks are similar to those in the WASI (Wechsler Abbreviated Scale of Intelligence, Wechsler 2011), which is a validated short version of the WAIS. The tasks in the WASI were selected as those with the highest factor loadings on general intelligence (Wechsler 2011). The IQs of the different measurements were highly correlated (0.77 between M1 and M2, 0.74 between M2 and M3). These correlations are of the same magnitude as test‐retest correlations with a similar time interval (Bartels, Rietveld, Van Baal, & Boomsma, 2002; Waber, Forbes, Almli, & Blood, 2012). Moreover, the genetic correlations between the measurements were 0.99 (95% Confidence Interval: 0.79–1.00) and 1.00(0.82–1.00), respectively, suggesting all IQ tests were driven by the same construct. Raw test scores on subtests from the WISC (Nederlands Instituut van Psychologen Dienstencentrum, 2003) or WAIS (Wechsler, 2004) were transformed into standardized scale scores against age‐specific norms, leading to a total IQ score. For the second and third measurement, a correction for the number of excluded subtests was applied (M2: 4 out of 5 verbal and 2 out of 5 nonverbal tests; M3: 2 out of 6 verbal and 2 out of 5 nonverbal tests) to obtain a total IQ score. For example for M3, the sum of the standardized scores of the verbal tests was multiplied by 3, the sum of the standardized scores of the nonverbal tests was multiplied by 2.5. The sum of these scores then corresponded to the full scale IQ score in the look up table.
2.3. MRI acquisition
All MRI brain scans were acquired at the University Medical Center Utrecht on a 1.5 Tesla Philips Achieva scanner (Philips, Best, The Netherlands) using the same protocol at all waves (Brouwer et al., 2012). For anatomy, a three‐dimensional T1‐weighted scan (Spoiled Gradient Echo; TE = 4.6 ms; TR = 30 ms; flip angle 30°; 160–180 contiguous coronal slices of 1.2 mm; in‐plane resolution 1 × 1 mm2; acquisition matrix 256 × 256) of the whole head was made of each individual. For white matter fiber tracking two Single Shot Echo Planar Imaging (SS‐EPI) DWI scans were acquired (32 diffusion‐weighted volumes with diffusion weighting b = 1000 s/mm2 and 32 noncollinear diffusion gradient directions; 8 diffusion‐unweighted (b = 0 s/mm2) scans; TE = 88 ms; TR = 9822 ms; parallel imaging SENSE factor 2.5; flip angle 90°; 60 transverse slices of 2.5 mm, no gap, FOV 240 mm; 128 × 128 reconstruction matrix; 96 × 96 acquisition matrix, no cardiac gating) for optimal signal‐to‐noise ratio.
2.4. MRI processing
White matter pathways, referred to as fibers or tracts, were reconstructed using streamline tractography. First, the two DWI measurements were concatenated—each scan in gradient direction being present twice, thereby reducing noise. Data were corrected for possible geometric distortions by estimating parameters characterizing subject movement and distortion, minimizing residual error when fitting data to the diffusion tensor model (Andersson and Skare, 2002). Next, the diffusion pattern in each voxel was fitted to a tensor matrix using a robust M‐estimator (Chang, Jones, & Pierpaoli, 2005), providing three eigenvectors (representing the three principal directions of diffusion) and corresponding eigenvalues. Fractional anisotropy (FA) values were calculated in each voxel as a measure of microstructural directionality from the eigenvalues (Basser and Pierpaoli, 1996). Then, the b0 scan was registered to the T1‐weighted scan using a rigid transformation (no scaling), based on optimization of a mutual information metric (Maes, Collignon, Vandermeulen, Marchal, & Suetens, 1997), and the T1‐weighted images were nonlinearly warped into model space per measurement up to a scale of 1 mm (Collins, Holmes, Peters, & Evans, 1995), and then to the model of the second measurement. All possible fiber tracts between two regions were reconstructed in individual space using the diffusion tensor images with an in‐house implementation of the fiber assignment by a continuous tracking (FACT) algorithm (Mori and Van Zijl, 2002) with 8 seed points per voxel, FA threshold of 0.1, and maximal angle of 45°.
Further computations were done in model space. Most available model brains are based on adults, which is not ideal for our cohort. We therefore created a study specific model brain of all the scans in the second wave, using methodology described in Peper et al. (2009). Individual brains were warped to the model brain using an iterative procedure with increasing level of precision using the ANIMAL software (Collins et al., 1995). The fiber tracts were warped into model space, using the concatenation of the transformations between the b0 scan and T1 scan, and between the T1 scan and model space. For network construction, the AAL template (Tzourio‐Mazoyer et al., 2002) was warped onto the model brain, segmenting the cortex in a parcellation map consisting of 90 regions.
2.5. Construction of structural brain networks
A network consists of a set of nodes and connections (edges) that can be mathematically expressed as a graph with a collection of nodes and a collection of edges between the nodes (Bullmore and Bassett, 2011). Whole brain networks were created based on the 90 AAL brain regions. Structural FA‐weighted brain networks were created for each individual, one for each measurement when available. Each individual network included bundles that were present in at least 60% of all participants in each wave (de Reus and van den Heuvel, 2013): Network nodes i and j were defined as structurally connected by an edge when from the total collection of reconstructed streamlines, in at least 60% of the participants of Wave 1, Wave 2, and Wave 3 at least one fiber connected region i and j. For each edge, each subject and each wave, weight wij was defined by the mean FA value of the traced fibers between region i and j.
2.6. Graph analysis
Mathematical representations of the structural connectivity of the human brain network have revealed that the brain is organized according to a highly efficient small‐world topology combining a high level of segregation (local efficiency) with a high level of global integration (global efficiency) (Achard and Bullmore, 2007). Global efficiency is a network attribute that quantifies how easy information can be exchanged over the network, providing information on the communication efficiency of a network as a whole. Local efficiency reflects how well information can travel in the direct neighborhood of a node, and is often interpreted as a metric of the local information processing capacity of a network (Bullmore and Sporns, 2012; van den Heuvel and Hulshoff Pol, 2010). To measure changes in efficiency, we computed global and local efficiency in the AAL regions for each individual at each measurement using the Brain Connectivity Toolbox (Rubinov and Sporns, 2010; http://www.brain-connectivity-toolbox.net). Figures were created with the BrainNet Viewer (Xia, Wang, & He, 2013).
2.7. Statistical analyses
Structural equation modeling, implemented in the OpenMx package (Boker et al., 2011) in R (R Core Team, 2014), was used in all analyses to account for dependency (both familial and temporal) of the data and to estimate the relative influences of genes and environment. In a first step, the best fitting age‐trajectory of global and local efficiency was determined as the model with the lowest Akaike information criterion (AIC): we entered efficiency data up to three measurements for each subject in a saturated model, modeling a regression of age on network measures, while allowing for nonzero covariances between measurements and between members of twin and twin‐sib pairs. As the upper level of complexity was clear given the age‐distribution of the data, we subsequently fitted a cubic, quadratic, linear, and constant age‐model, with age entered as continuous measure, for each network measure in a top–down fashion. Once the best‐fitting model for each network measure was determined, we proceeded in two ways:
(1) Based on the best‐fitting age model (cubic, quadratic, linear, or constant), we tested whether a model including regression terms for IQ fitted the data better than a model that included only regression terms for age. Nested models can be compared by computing minus twice the log‐likelihoods of these models, which is distributed as a chi‐square distribution, with n degrees of freedom, if n parameters are fixed to zero in the submodel. Thus, in the equation below, we tested whether a model in which β2, β3, β5, and β7 were constrained to zero fitted worse than a model in which they were freely estimated (p < 0.05 based on the differences between the −2 log likelihoods, with 4 degrees of freedom in the cubic model).
(2) We computed residuals of the network measures by taking out the effect of age based on the best fitting model determined above. The residuals were subsequently modeled in a 6‐variate genetic model, combining the network data and IQ per wave (for details please see the next paragraphs). This model allows us to simultaneously estimate (a) (genetic) correlations between network measures and IQ at each of the three waves; (b) heritability of network measures at the three waves to investigate possible quantitative changing influences of genetic factors explaining individual differences over time; (c) the genetic correlations between network measures at Waves 1 and 2, and between Waves 2 and 3, to investigate qualitative genetic differences influencing the network throughout development.
2.8. Genetic modeling of twin and sib data
Relative influences of genetic and environmental factors were examined in an extended twin design by comparing within‐pair correlations between monozygotic (MZ) and dizygotic (DZ) twins/twin sibling pairs. Differences between these correlations may arise because monozygotic twins share (almost) 100% of their genetic makeup and twin‐sibling pairs, like dizygotic pairs, share on average 50% of their segregating genes. When an MZ correlation is twice as high as a DZ correlation, this indicates that familial resemblance is largely accounted for by genetic factors (see Boomsma, Busjahn, & Peltonen, 2002 for an overview of twin research, and see Falconer and Mackay, 1992; Posthuma et al., 2003 for a more detailed description). In addition to genetic factors, resemblance between twins and sibs can arise from common environmental factors, which comprises those environmental factors that induce similarity in children growing up in the same family. The presence of common environmental factors is suggested when correlations between DZ twins and twins/sibs are larger than half the MZ correlation. When the MZ correlations are more than twice the DZ/sib correlations, there is a suggestion for nonadditive genetic influences (e.g., epistasis or dominance). Unique environmental influences are not shared with other family members and also contain the measurement error. In a SEM model for MZ and DZ data, the total variance of a trait (V) generally can be split into additive genetic variance (A), unique environmental variance (E), and either nonadditive genetic variance (D) or common environmental variance (C). In a twin‐sibling design, a model including both C and D are not identifiable and a choice for C or D is made after inspection of the pattern of the twin correlations. The proportion of the total variance that can be attributed to genetic or environmental factors gives estimates of (univariate) heritability (A/V = h 2), broad sense heritability ((A + D)/V = h 2 + d 2), common environmental influences (C/V = c 2), and unique environmental influence (E/V = e 2).
The same rationale as described for the univariate case can be applied to multivariate data. If a correlation exists between two variables, the cross‐trait cross‐twin/sibling correlations give an indication whether the same genes, or (shared) environment is responsible for the association. A genetic correlation r g between two variables is defined as the (broad) genetic covariance between two traits, standardized by the standard deviations of the two traits attributable to the genetic components (for details see Koenis et al., 2013 and formulas below). Because a genetic correlation does not take the heritability of the traits into account, one can also compute the r ph‐g which is defined as the (broad) genetic covariance between two traits, divided by the square roots of the variances for each trait (Toulopoulou et al., 2007 and formulas below). r ph‐g can be interpreted as the correlation that would be observed if only genetic factors are taken into account. The environmental correlations re and r ph‐e are defined similarly.
2.9. Genetic analyses
For each network measure, we fitted a 6‐variate model to the data including the three network measures (one at each wave) and IQ at each wave. Network metric was either global efficiency or local efficiency for each of the 90 brain regions. Based on our previous work, we fitted a model that allowed for additive and nonadditive genetic effects and unique environment for the network measures (Koenis et al., 2015) and additive genetic effects, and common and unique environment for mean IQ (van Soelen et al., 2011) (Figure 2). Following the path tracing rules, a variance is modeled as the sum of squares of paths connecting to a trait. For example, the variance of the network measure at wave 1 equals V 1 = ( + + ). Similarly, the variance of IQ at wave 1 equals V 4 = ( + + + + + + + + ) . Phenotypic correlations between IQ and network measures at e.g. wave 1 are defined as (a 11 × a 41 + e 11 × e 41)/(√V 1 × √V 4). Significance of this correlation was determined by constraining this quantity to zero, computing the −2 log likelihood difference which is distributed as a chi‐square distribution with 1 df. Similarly, r ph‐g and r ph‐e in this model are equal to (a 11 × a 41)/(√V 1 × √V 4) and (e 11 × e 41)/(√Vr1 × √Vr4), respectively, and can be tested likewise. A similar approach was used to investigate (genetic) correlations over time. As an example, the genetic correlation r g between network measures at Wave 1 and Wave 2 is defined as (a 11 × a 21 + d 11× d 21)/(√( + × √( + + + )). Broad heritability of network measures is defined as proportion of the additive and nonadditive genetic variance of the total variance. Hence broad heritability is modeled as ( + )/( + + ) for network measures at Wave 1, and as ( + + + )/( + + + + + ) for Wave 2 and similarly for Wave 3. For IQ measures, heritability at Wave 1 was defined as ( + + + )/( + + + + + + + ). Significance of the variance components h 2 + d 2 for network measures and h2 for IQ were determined through 95% confidence intervals.
Figure 2.
Six‐variate model to which the data were fitted. For simplicity, only the diagram for Twin 1 is shown. The additive genetic components A1 to A6 of Twin 1 are connected to those of Twin 2 with a correlation of 1 in MZ twins and 0.5 in DZ twins. The nonadditive genetic components D1 to D3 of Twin 1 are connected to those of Twin 2 with a correlation of 1 in MZ twins and 0.25 in DZ twins. The common environmental components C4 to C6 of Twin 1 are connected to those of Twin 2 with a correlation of 1 by definition for both MZ and DZ twins. For i in {1,6}, Ai represents the additive genetic factor acting on trait i. Di represents the dominant genetic factor acting on trait i. Similarly, Ci represents the common environmental factor acting on trait i and Ei represents the unique environmental factor acting on trait i. The additive and dominant genetic effects were combined to compute broad heritability of the individual traits as ( + )/( + + ) for network measure at Wave 1 and ( + + + )/( + + + + + ) for network measure at Wave 2. A phenotypic correlation between IQ and network measures at, for example, Wave 1 was defined as (a 11 × a 41 + e 11 × e 41)/√( + + ) × √( + + + + + + + + ). Furthermore, a (broad sense) genetic correlation between the broad sense genetic factors was computed as (a 11 × a 21 + d 11 × d21)/(√( + ) × √( + + + )
Network metric was either global efficiency or local efficiency for each of the 90 brain regions. When reporting results on local efficiency of the 90 regions, we used false discovery rate (FDR) corrections to control the expected proportion of discoveries that are false (Benjamini et al., 2010). The FDR was set to 5%. FDR corrections were done in R using p.adjust from the stat package.
2.10. Post‐hoc analyses
Because the IQ tests were different at each measurement, we investigated in a post‐hoc analysis whether that influenced our results. We recomputed the correlations between local efficiency and IQ using (a) a mean IQ derived by averaging all available IQ measures of an individual and (b) the full‐scale IQ score acquired at wave 1.
In our previous work, we found that changes in IQ are related to changes in FA‐based local efficiency between the age of 10 and 13 years (Koenis et al., 2015). Therefore, in a post‐hoc analysis, we investigated whether change in IQ and change in local efficiency were related between the ages of 13 and 18 years.
As another post‐hoc analysis, we tested whether the developmental pattern of the structural brain network was different for boys and girls. Subsequently, we tested whether the correlations between efficiency measures and IQ were different in boys and girls, by testing a genetic model that allows for qualitative sex differences. In such a model, the covariance between members of opposite‐sex twin and twin‐sibling pairs that is attributed to additive (or dominant) genetic factors is not assumed to be equal to 0.5 (0.25 for dominant factors), but estimated from the data. Sex differences in additive and dominant genetic influences on the covariance were tested in separate models. Quantitative sex effects were tested in a model in which the genetic factors influencing network measures in boys and girls were the same, but the path loadings (i.e., heritabilities) were allowed to be different.
3. RESULTS
3.1. Efficiency of adolescent brain networks develops in a non‐linear pattern
FA‐based global efficiency followed a cubic pattern (better fit compared to lower degree polynomial functions; AIC cubic = 4162; AIC quadratic = 4173) with age, characterized by an increase in efficiency from age 10 to around age 13, followed by a period of leveling off until around age 18 (Figure 3). On a local level, most regions showed this cubic pattern, although for some regions a quadratic or linear trajectory fitted the data better (Figure 4 and Supporting Information, Figure S1). Visually, regional differences in developmental trajectories were mostly observed between the ages of 13 and 18, where FA‐based local efficiency either decreased or remained stable: the decrease was most prominent in medial frontal areas; a milder decrease in efficiency was found in lateral frontal and subcortical areas, while decrease was minimal or absent in temporal, parietal and occipital areas (Figure 4 and Supporting Information, Figure S1).
Figure 3.
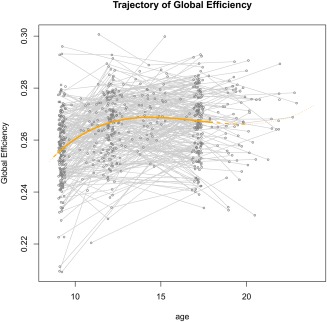
Development of FA‐weighted global efficiency during adolescence [Color figure can be viewed at http://wileyonlinelibrary.com]
Figure 4.
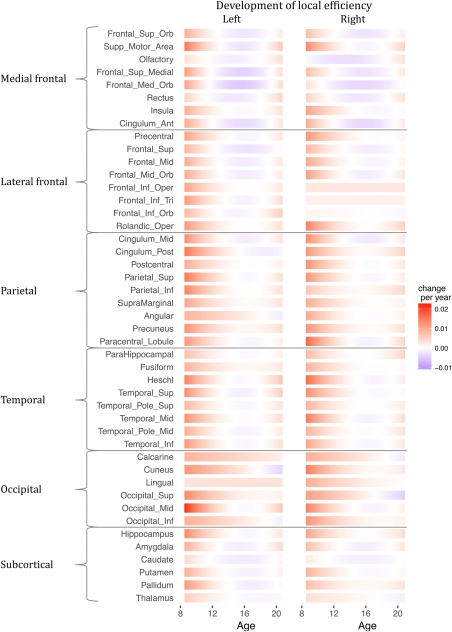
Development of FA‐weighted local efficiency during adolescence. The derivatives of the best fit function are displayed as change per year. Most regions followed a cubic developmental pattern. A quadratic pattern was found for: right inferior orbitofronal gyrus, left and right calcarine sulcus, left cuneus, right superior occipital gyrus, left inferior occipital gyrus, left angular gyrus, and right thalamus. A linear fit was found for right pars opercularis and triangularis, and left lingual gyrus [Color figure can be viewed at http://wileyonlinelibrary.com]
3.2. Adolescent brains differentiate in relation to IQ
A model of the developmental trajectory of global efficiency which included IQ regression terms fitted the data better than a model with only age regression terms (p = 0.03). More specifically, we found that a higher IQ was associated with higher and later peak of efficiency whereas an average and lower IQ was associated with a decline in global efficiency around the age of 14 (Figure 5a). A significant influence of IQ was also seen for local efficiency in numerous regions throughout the brain (31 regions p < 0.05; 23 regions FDR corrected) (Figure 5b).
Figure 5.
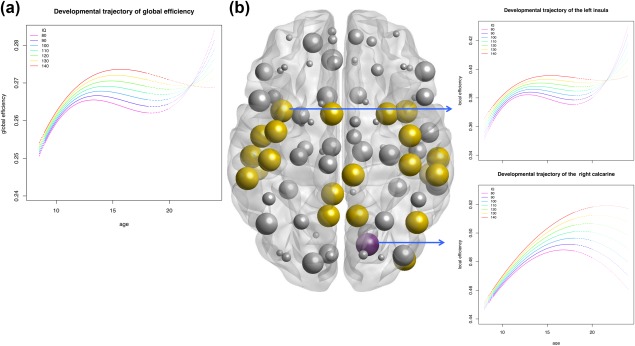
Development of efficiency depends in part on intelligence. (a) Global and (b) local FA‐weighted efficiency as a function of age and IQ. (a) Estimated age trajectories of global efficiency and its relationship with IQ. (b) Regions where IQ had a significant influence on the age trajectory of local efficiency (FDR corrected; in yellow, purple, and blue). Sizes of the spheres reflect relative p value (1 − FDR‐corrected p value). Yellow spheres indicate cubic fit; purple quadratic fit; blue a linear fit. Insets show an example of the local efficiency of the left insula and right calcarine sulcus [Color figure can be viewed at http://wileyonlinelibrary.com]
3.3. Correlations between IQ and efficiency emerge during adolescence
Over time, the association between IQ and global and local FA‐weighted network efficiency increased significantly (Figure 6): A model which allowed free estimations of phenotypic correlations between efficiency and IQ fitted better than a model which constrained the correlations to be equal across measurements (global efficiency p = 0.03; local efficiency in 33 regions, p < 0.05; 11 regions reached FDR corrected significance). More specifically, at age 10, efficiency of the structural network was not significantly correlated with IQ (global efficiency: r ph = 0.007; p = 0.92). At age 13, IQ was significantly correlated with global efficiency (r ph = 0.15, p = 0.04) and this correlation was even higher at age 18 (r ph = 0.23, p = 0.001).
Figure 6.
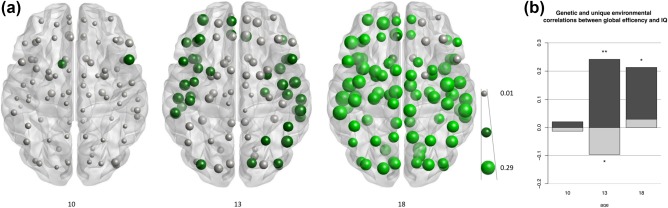
(a) Phenotypic correlation between IQ and local efficiency at age 10, 13, and 18. Size of the spheres reflects size of the correlation. Dark green nodes indicate significance at p < 0.05; light green spheres indicate FDR‐corrected significance. (b) Genetic (dark grey) and unique environmental light grey) contributions to the phenotypic correlation between global efficiency and IQ at age 10, 13, and 18. * significant at p < 0.05; ** significant at p < 0.005 [Color figure can be viewed at http://wileyonlinelibrary.com]
At the local level, there were three regions with a significant association between local efficiency and IQ at age 9 (r ph = 0.12–0.16, p < 0.05). At age 13, local efficiency was associated with IQ in 40 regions of the brain (mean local efficiency r ph = 0.16, p = 0.03; regionally in 40 regions up to r ph = 0.20, p = 0.005) but these correlations did not survive FDR‐corrected significance. At age 18, FDR corrected significant correlations between local efficiency and IQ were present in 80 regions (mean local efficiency: r ph = 0.24, p = 0.0005, regionally in 80 regions, up to r = 0.28, p < 0.0001; see Figure 6a and Supporting Information, Table SI).
Across measurements, the correlations between global efficiency and IQ were partly driven by genes (Figure 6b). The relative influence of genes implicated in the association between intelligence and FA‐weighted global efficiency increased significantly over time: a model which constrained the genetic part of the phenotypic correlation (r ph‐g) to be equal across all measurements fitted worse than a model which allowed the correlation to be fitted freely (global efficiency: p = 0.04). Investigating the three waves separately, r ph‐g between global efficiency and IQ was not significantly different from zero at the first wave (r ph‐g = 0.02, p = 0.76), but it was at waves 2 and 3 (wave 2: r ph‐g = 0.25, p = 0.004; wave 3: r ph‐g = 0.20, p = 0.016), to an equal extent (r ph‐g could be set equal at wave 2 and 3, p = 0.62). In addition, in wave 2, around the age of 13, but not in wave 3 (around age 18) there was a unique environmental influence that partly annulled the genetic association between FA‐weighted global efficiency and IQ (r ph‐e = −0.10; p = 0.04; Figure 6b).
Positive contributions of genes to the correlations between IQ and FA‐weighted local efficiency were found at ages 13 and 18 (age 13: 74 regions, p < 0.05 FDR corrected; age 18: 64 regions at p < 0.05, of which 13 regions reached FDR corrected significance; Supporting Information, Figure S2). The same pattern of an increase in the genetic contribution to the correlation between local efficiency and IQ from age 10 to age 13 (r ph‐g is not allowed to be equal across measurements; in 34 regions p < 0.05 uncorrected, of which 6 regions reached FDR corrected significance) and then a stable positive r ph‐g to age 18 (r ph‐g can be set equal in all 90 regions, p > 0.06 uncorrected) was seen throughout the brain. Similar to the correlation between IQ and global efficiency, locally, a negative unique‐environmental correlation between IQ and FA‐weighted local efficiency was found at age 13, throughout the brain in 30 regions (r ph‐e up to −0.16, p = 0.0004 uncorrected, see Supporting Information, Figure S2).
3.4. Genetic influences on structural network efficiency and IQ
Broad heritability of FA‐weighted global efficiency was 39% (95% CI: 14–59%), 20 (5–43)%, and 43 (19–62)% for ages 10, 13, and 18, respectively (Figure 7a). Broad heritability did not significantly differ at the respective ages for global and local efficiency (Figure 7b; Supporting Information, Table SII). Heritability of IQ at age 10 was estimated at 61% (95% CI: 40–79); at age 13 this was 65% (95% CI: 45–80%); and heritability of IQ at age 18 was estimated at 40% (95% CI: 22–67%). When, as according to the literature, an AE model was fitted to estimate heritability of IQ at age 18, heritability was estimated at 79% (68–86%). This is comparable to Swagerman et al. (2016), who estimated IQ in the twins only, in which case, C can be dropped from the model. In data from the twins and the siblings, a model without C fits worse than the full ACE model (p = 0.02).
Figure 7.
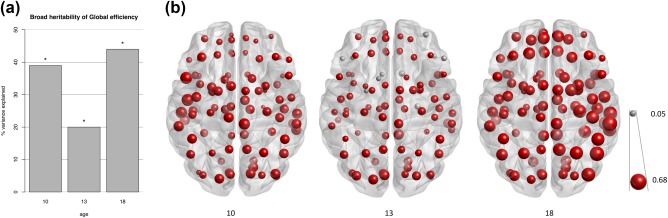
Broad heritability of (a) global and (b) local efficiency during adolescence. Broad heritability of global efficiency was significantly different from zero (*) on all three measurements. Size of the spheres reflects magnitude of broad heritability. Grey spheres indicate estimates of broad heritability were not significantly different from zero. See also Supporting Information, Table SII [Color figure can be viewed at http://wileyonlinelibrary.com]
A stable genetic factor influenced global efficiency at waves 1 and 2 (r g = 0.78 (0.09–0.98)). A model where r g was constrained to be zero fitted worse than a model that allowed r g to be estimated freely (p = 0.03). The same was found for the genetic correlation between global efficiency at Waves 2 and 3 (r g = 0.85 (0.25–1.00), p = 0.008).
For local efficiency, 48 regions were partly influenced by the same genetic factor at both wave 1 and 2 (p < 0.05 uncorrected, 40 regions FDR‐corrected, see Supporting Information, Table SII). For Waves 2 and 3, 47 regions were found to be influenced by the same genetic factor (p < 0.05, uncorrected; 5 regions were significant at p <0.05, FDR‐corrected). Regions where a stable genetic factor influenced local efficiency were distributed over the entire brain.
3.5. Post‐hoc analyses
Repeating the analyses by computing the associations between IQ and brain network measures using IQ averaged over all available measurements or full‐scale IQ from measurement 1, did not substantially change our findings, although the correlations between full scale IQ from measurement 1 and local efficiency at measurement 3 were less strong (Supporting Information, Figure S3).
Although 15% of the participants showed a change in IQ of more than 1 SD (15 IQ points) between wave 2 and 3, we did not find a correlation between change in IQ and change in global efficiency between age 13 and 18 (r = −0.11, p = 0.20). For local efficiency, an (uncorrected) significant correlation was found in two regions (left olfactory cortex: r = −0.17, p = 0.04; and left paracentral lobule, r = −0.19, p = 0.02).
There were no significant differences between boys and girls at each time‐point separately for FA‐weighted global and local efficiency. The associations between global and local efficiency of the brain with IQ revealed no evidence for quantitative or qualitative genetic sex‐differences.
No differential developmental trajectories for FA‐weighted global efficiency in boys and girls were found (p = 0.11). Locally, however, several regions showed a better fit for the model with different trajectories for boys and girls compared to the model that fitted one trajectory for all participants. This sex‐effect reached FDR‐corrected significance (p < 0.05) for the following 10 regions: left rolandic operculum, left medial orbitofrontal gyrus, left anterior cingulum, left amygdala, bilateral calcarine sulcus, right angular gyrus, right pallidum, right heschl gyrus, and left superior temporal gyrus. Generally, in these 10 regions, boys started with a lower efficiency than girls at age 10, made a steeper increase in efficiency between ages 10 and 13 and end with a higher efficiency than girls at age 13 and 18. For the left superior temporal gyrus, left rolandic operculum, and right heschl gyrus, the fitted age trajectory for boys had a lower level of efficiency over the entire age range compared to girls.
The strong correlations between local efficiency and IQ at age 18 seem to be driven by the girls in our sample: 2 regions reached FDR significance in the boys, whereas 46 regions reached FDR significance in girls of the same age.
4. DISCUSSION
In this longitudinal study, we measured the development of FA‐weighted global and local efficiency of the brain network in relation to intelligence in (young) adolescent twins and their siblings from over 100 families at 3 time‐points. We showed that FA‐weighted efficiency of the structural brain network increased during early adolescence and leveled off during mid adolescence. Moreover, during adolescence, individual differences in intelligence became increasingly reflected in the structural brain network, with widespread correlations between intelligence and FA‐weighted local efficiency at age 18 years. This effect was due to development of network efficiency. Finally, we reported that genes contribute to the brain's network efficiency during development and to its growing association with intelligence, explaining up to 87% of this association by age 18, while unique environmental factors counteract this genetic effect around age 13 years. Thus, during adolescent development, communication in the brain network becomes a reflection of intelligence by the age of 18 years, in part under the influence of genes.
We report for the first time in a longitudinal study that efficiency of the structural brain network increases throughout adolescent development in a nonlinear fashion: an increase in efficiency that is particularly prominent before age 13, and levels off during mid‐adolescence. Moreover, development of local efficiency followed different trajectories, with frontal and subcortical regions having a seemingly small decrease in local efficiency around mid‐adolescence, whereas occipital regions continued to increase in efficiency until adulthood. Earlier, we showed that between 10 and 13 years, there is an increase in FA‐based efficiency of the brain network with most regions showing increases in efficiency over time (Koenis et al., 2015). Here we extended this finding with the results of the third measurement in these twins and their siblings on the verge of adulthood, revealing that the increase in global and local FA‐based efficiency stabilized in late adolescence in most individuals, and decreased in some individuals. Regarding the influence of genes on network efficiency, we found that during adolescence variance in network efficiency could partly be explained by stable genetic influences.
Our finding that FA‐weighted efficiency increased throughout adolescence but levels off between age 13 and 18, is supported by cross‐sectional studies reporting increases in FA with increasing age during adolescence and a pattern of FA development that differs across white matter bundles (Lebel and Beaulieu, 2011; Peters et al., 2014; Schmithorst and Yuan, 2010; Simmonds et al., 2014). More specifically, Simmonds et al. (2014) report for several regions a cubic age pattern of FA with a period of rest during mid‐adolescence. In addition, they find that white matter bundles develop in a hierarchical pattern with bundles involved in basis sensorimotor function to develop earlier than frontal connections. In our study, a decrease in local efficiency during mid adolescence occurs mostly in the (medial) frontal areas. Possibly reflecting later development of these regions since FA still increases until the third decade of life (Lebel et al., 2012; Yap et al., 2013). Efficiency of occipital and parietal regions on the other hand seems reach stable levels in mid‐adolescence.
A recent longitudinal study in adolescents between 15 and 19 years, comparable with our second and third wave, also reported both increases and decreases in FA and streamline count during late adolescence, with preferential maturation in hub‐to‐hub connections (Baker et al., 2015). Our findings are also in alignment with development of white matter volume (Brouwer et al., 2012; Paus, 2010), which is consistent with the small but positive association between white matter volume and FA reported in adult twins (Bohlken et al., 2016a). Also, our FA‐weighted findings align with cross‐sectional reports of streamline‐count weighted efficiency of brain networks showing largely positive correlations with age in childhood and adolescence (Dennis et al., 2013; Hagmann et al., 2010; Zhao et al., 2015) while leveling off or showing a negative correlation in adulthood (Dennis et al., 2013; Gong et al., 2009; Lim, Han, Uhlhaas, & Kaiser, 2015; Zhao et al., 2015). We add to the existing literature that the adolescent FA‐weighted network develops in a non‐linear pattern
The question remains how the brain continues to function in an efficient manner during the years in which both brain and cognition are changing rapidly (Blakemore et al., 2010; Luna et al., 2015). Based on our data, it seems as if the moment the structural brain network has grown into its adult state, we can see the individual levels of general cognitive functioning reflected in the brain's level of network efficiency, while earlier in development there are only some indications of this link. This is probably related to the stable component of IQ, as our findings were similar using mean IQ of all time points. Regions that had a significant correlation between local efficiency and IQ were distributed over the entire brain, suggesting that intelligence is supported by a distributed network (Shaw, 2007; Deary, Penke, & Johnson, 2010). Our results do not seem to support the PFIT theory (Jung & Haier 2007), because they encompass more areas: at age 18, 17 out of the 24 P‐FIT regions are significant, and 59 out of the 66 non‐P‐FIT regions (Chi square test, p = 0.07). However, it is important to keep in mind that the efficiency of a region is a summary statistic on the connections between the neighbors of that region.
Several mechanisms could explain the increase of a (genetic) correlation between white matter network efficiency and IQ in mid adolescence. First, it can be hypothesized that gene expression triggered by education may depend on IQ and thus diverge structural brain network development to more efficient networks in smarter children. For example, rats showed upregulated mRNA expression of brain‐derived neurotrophic factor (BDNF) and basic fibroblast growth factor (bFGF) in the hippocampus a few days after learning a spatial memory task (Gómez‐Pinilla, So, & Kesslak, 1998; Kesslak, So, Choi, Cotman, & Gomez‐Pinilla, 1998). This shows that training or activating the brain could lead to (a cascade of) activation of genes that regulate long‐term plasticity. A second hypothesis is that people with a higher IQ may have different genetic variants that directly or indirectly cause genes related to adolescent brain development to be more efficiently translated. For instance, heritability of FA in the thalamus, the genu and posterior limbs of the internal capsule, and the superior corona radiate was higher in people with high IQ compared to people with lower IQ (Chiang et al., 2011a). In another paper by these same authors, it was shown that the association between FA and object assembly performance was positive in BDNF‐Val carriers but around zero or negative in BDNF‐Met carriers (Chiang et al., 2011b). A third hypothesis is that teenagers may follow their ‘genetic drive’ (based on their IQ) to an environment that will trigger specific developmental changes. For example, genes that are involved in (higher) IQ may also make a person more curious and interested in school‐related work. This may allow for a longer sensitive period in individuals with a higher IQ as reflected by a higher common environmental influence on IQ during adolescence in teenagers with a higher IQ compared to teenagers with a lower IQ (Brant et al., 2013).
That developmental trajectories of white matter connectivity depend on cognitive functioning is supported by the study of Tamnes et al. (2010), which showed that the development of FA between 8 and 30 years is related to the level of verbal intelligence. Other studies support the notion that structural brain associations with IQ are not stable throughout life. Cortical thickness studies showed differential developmental trajectories depending on the IQ (Brans et al., 2010; Brouwer et al., 2014; Karama et al., 2014; Schnack et al., 2015; Shaw et al., 2006). The absence of a correlation between FA‐weighted network efficiency and IQ in late childhood is somewhat inconsistent with a recent study in in children age 6–11 years that reported a positive correlation between block design and FA‐weighted network efficiency, but not with other non‐verbal subtasks (Kim et al., 2016). However, total IQ versus a single IQ‐subtask may be differently related to local brain regions and thus may show a different developmental pattern in relation to brain development.
Global efficiency of the white matter network is already heritable at age 10 years (39%), and remains largely stable throughout adolescence, with estimated heritability of 20% at age 13 years and 43% at age 18 years. This estimate is comparable with the heritability estimates of FA in adult twins (Blokland, de Zubicaray, McMahon, & Wright, 2012; Bohlken et al., 2014; Chiang et al., 2011a; Jahanshad et al., 2013; Kochunov et al., 2015). While significant, its heritability is quite modest when compared to the heritability for white matter volume that is estimated to be over 85% in both adolescence (van Soelen et al., 2013) and adulthood (Bohlken et al., 2014). This leaves ample room for influences of the environment on the network of white matter fiber connectivity in adolescence and adulthood.
Individual differences in genetic makeup became increasingly implicated in the association between IQ and global FA‐weighted efficiency of the structural network: from no significant influence at age 10 years (r ph‐g = 0.02) to a significant influence at age 13 (Wave 2: rph‐g = 0.25) and remaining largely stable at age 18 (Wave 3: r ph‐g = 0.20). By the time adolescents have reached age 18 years, genes explained up to 87% of the association between intelligence and local efficiency of the brain network. Individual environmental factors were found to counteract this development around age 13 years (r ph‐e = −0.10), suggesting that at that time unique environmental differences were important for this association.
Changes in individual IQ over time have been reported earlier (Burgaleta, Johnson, Waber, Colom, & Karama, 2014; Ramsden et al., 2011; Waber et al., 2012). These individual changes in IQ were found to be associated with changes in brain structure (Ramsden et al., 2011) and with changes in efficiency of the brain network in early adolescence (Koenis et al., 2015). In our participants, aged between 13 and 18 years, we did not find that changes in IQ were significantly related to changes in FA‐weighted network efficiency. This could be caused by the more pronounced influences of the genetic background that influences the brain and intelligence during late adolescence. For both IQ (Haworth et al., 2010; van Soelen et al., 2011) and brain structure (Lenroot et al., 2009) genetic influences have been shown to increase with age.
Girls enter puberty earlier than boys (Mul et al., 2001), and their brains also develop at a different pace during adolescence (Gogtay et al., 2004; Gur and Gur, 2016; Raznahan et al., 2010). Although puberty might in addition to age explain variance in brain development (Blanton et al., 2012; Brouwer et al., 2015; Menzies, Goddings, Whitaker, Blakemore, & Viner, 2015; review by Peper, Hulshoff Pol, Crone, & van Honk, 2011), we did not control for differences in pubertal timing. We did find sex related differences in the developmental trajectories of network efficiency in a few regions, with an earlier increase in efficiency in girl than boys, and boys catching up with the girls around age 13. Indeed, several studies report differences between the sexes with regard to development of FA, especially between childhood and early adulthood (Herting, Maxwell, Irvine, & Nagel, 2012; Ladouceur, Peper, Crone, & Dahl, 2012; Lebel et al., 2012; Schmithorst, Holland, & Dardzinski, 2008; Simmonds et al., 2014; Wang et al., 2012; but see Lebel and Beaulieu, 2011; Peters et al., 2014). Despite the difference in the trajectories, no significant differences between the sexes were found within each wave for mean efficiency values in our cohort. In addition, no qualitative or quantitative sex differences were found on the (genetic) association between FA‐weighted local network efficiency and IQ at each wave. However, the brain wide correlation between local efficiency and IQ at age 18 seemed to be more prominent in girls. Sex‐dependent associations in white matter with IQ have been reported earlier, both in children (Luders et al., 2011; Schmithorst, 2009; Wang et al., 2012) and in adults (Gur et al., 1999; Tang et al., 2010, but see Tamnes et al., 2010). Possibly, because girls start their pubertal development earlier than boys do (with regard to both secondary sexual characteristics and brain development) the difference between boys and girls at age 18 we observed, is the result of the timing of developmental processes. A fourth measurement would be able to elucidate this question.
Some limitations need to be taken into consideration when interpreting the findings of this study. First, the network measures are strongly driven by their weights, in our case FA. Although we did not correct for individual differences in mean FA of the total white matter, the results give an indication of the connectivity of the white matter bundles and their strength (measured by FA) of the connections. Especially for local efficiency, a network approach is more informative than measures of local FA. Second, we specifically studied the FA‐weighted networks because our previous study showed a correlation between FA‐weighted network efficiency and IQ, but not streamline weighed network efficiency (Koenis et al., 2015). In addition, we showed that during adolescence, the streamline‐weighted network behaves different from the FA‐weighted network (Koenis et al., 2015). This should be taken into account when comparing the results with other studies. Another limitation is that we have used three different sets of tests from the Wechsler intelligence tasks: all subtests of the WISC at wave 1, 6 WISC subtests at Wave 2, and 4 subtests of the WAIS at Wave 3. However, (genetic) correlations over time were high. Additionally, the results were very similar when using either the three measures of IQ, an average IQ over our three measurements or the—probably most reliable—full‐scale IQ obtained at age 10, suggesting that our findings regarding network development and IQ are driven by changes in the network, rather than changes in IQ measure. The smaller correlations between FSIQ of Wave 1 and local efficiency of Wave 3 could be attributed to cognitive development (cf. Burgaleta et al., 2014; Koenis et al., 2015; Ramsden et al., 2011; Waber et al., 2012). Finally, we fitted a simple tensor model to our data which does not allow for the detection of crossing fibers. More sophisticated methods are available, but given that our data were collected at 1.5 T in children in which some movement may be expected, the data are probably not best suited for robustly estimating more complicating diffusion models.
A last limitation is the use of a relatively low‐resolution atlas since graph parameters of structural networks may differ as a function of scale (Zalesky et al., 2010). Our current study is an extension of our previous study (Koenis et al. 2015). When we started our previous study, the AAL template was the most commonly used atlas for DTI network studies. In addition, Bassett, Brown, Deshpande, Carlson, and Grafton (2011) showed that the AAL atlas produced more within subject reproducible results than the Harvard Oxford atlas and that increased spatial resolution decreased the individual reproducibility of graph metrics. Zhao et al. (2015) found similar lifespan trajectories of streamline weighted efficiency for brain networks based on 90 regions AAL and a 1024 regions AAL template. Concerning the current study, it is possible that results may differ with studies that used a higher resolution parcellation. However, because (a) the effects were clustered in large brain areas and (b) as efficiency measures in this study were strongly driven by FA and correlations between whole brain white matter FA and IQ show the same pattern of an increase in correlation with age, we do not think that a different template would result in different conclusions.
In conclusion, we observed non‐linear development of the structural brain network's efficiency during adolescence that differentiates with intelligence. The correlation between IQ and local FA‐weighted efficiency became more clearly visible when adulthood was reached, and is influenced by genes common to both intelligence and structural network efficiency.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENT
This work was supported by the Netherlands Organization for Scientific Research Grants NWO433‐09‐220 (to HEHP), NWO 51.02.060 (to HEHP), 668.772 (to DIB), NWOMagW480‐04‐004 (to DIB), and NWO/SPI 56–464‐14192 (to DIB), the European Research Council Grant ERC‐230374 (to DIB), and the High Potential Grant from Utrecht University (to HEHP).
Koenis M, Brouwer R, Swagerman S, van Soelen I, Boomsma DI, Hulshoff Pol H. Association between structural brain network efficiency and intelligence increases during adolescence. Hum Brain Mapp. 2018;39:822–836. 10.1002/hbm.23885
Funding information Netherlands Organization for Scientific Research, Grant/Award Numbers: NWO433‐09‐220, NWO 51.02.060, 668.772, NWOMagW480‐04‐004, NWO/SPI 56‐464‐14192; European Research Council, Grant/Award Number: ERC‐230374; High Potential Grant from Utrecht University
This study was carried out at University Medical Center Utrecht and VU University Amsterdam.
REFERENCES
- Achard, S. , & Bullmore, E. (2007). Efficiency and cost of economical brain functional networks. PLoS Computational Biology, 3, 0174–0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson, J. L. R. , & Skare, S. (2002). A model‐based method for retrospective correction of geometric distortions in diffusion‐weighted EPI. NeuroImage, 16, 177–199. [DOI] [PubMed] [Google Scholar]
- Baker, S. T. , Lubman, D. I. , Yucel, M. , Allen, N. B. , Whittle, S. , Fulcher, B. D. , … Fornito, A. (2015). Developmental changes in brain network hub connectivity in late adolescence. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 35, 9078–9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels, M. , Rietveld, M. J. H. , Van Baal, G. C. M. , & Boomsma, D. I. (2002). Genetic and environmental influences on the development of intelligence. Behavior Genetics, 32, 237–249. [DOI] [PubMed] [Google Scholar]
- Basser, P. J. , & Pierpaoli, C. (1996). Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. Journal of Magnetic Resonance. Series B, 111, 209–219. [DOI] [PubMed] [Google Scholar]
- Bassett, D. S. , Brown, J. A. , Deshpande, V. , Carlson, J. M. , & Grafton, S. T. (2011). Conserved and variable architecture of human white matter connectivity. NeuroImage, 54, 1262–1279. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt, C. E. M. , Groen‐Blokhuis, M. , Hottenga, J. J. , Franić, S. , Hudziak, J. J. , Lamb, D. , … Boomsma, D. I. (2013). The Young Netherlands Twin Register (YNTR): Longitudinal twin and family studies in over 70,000 children. Twin Research and Human Genetics, 16, 252–267. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y. (2010). Discovering the false discovery rate. Journal of the Royal Statistical Society: Series B (Statistical Methodology), 72, 405–416. [Google Scholar]
- Blakemore, S. J. , Burnett, S. , & Dahl, R. E. (2010). The role of puberty in the developing adolescent brain. Human Brain Mapping, 31, 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton, R. E. , Cooney, R. E. , Joormann, J. , Eugène, F. , Glover, G. H. , & Gotlib, I. H. (2012). Pubertal stage and brain anatomy in girls. Neuroscience, 217, 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland, G. A. M. , de Zubicaray, G. I. , McMahon, K. L. , & Wright, M. J. (2012). Genetic and environmental influences on neuroimaging phenotypes: A meta‐analytical perspective on twin imaging studies. Twin Research and Human Genetics, 15, 351–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlken, M. M. , Brouwer, R. M. , Mandl, R. C. W. , Van den Heuvel, M. P. , Hedman, A. M. , De Hert, M. , … Hulshoff Pol, H. E. (2016a). Structural brain connectivity as a genetic marker for schizophrenia. JAMA Psychiatry, 73, 11–19. [DOI] [PubMed] [Google Scholar]
- Bohlken, M. M. , Brouwer, R. M. , Mandl, R. C. W. , Hedman, A. M. , van den Heuvel, M. P. , van Haren, N. E. M. , … Hulshoff Pol, H. E. (2016b). Topology of genetic associations between regional gray matter volume and intellectual ability: Evidence for a high capacity network. NeuroImage, 124, 1044–1053. [DOI] [PubMed] [Google Scholar]
- Bohlken, M. M. , Mandl, R. C. W. W. , Brouwer, R. M. , van den Heuvel, M. P. , Hedman, A. M. , Kahn, R. S. , & Hulshoff Pol, H. E. (2014). Heritability of structural brain network topology: A DTI study of 156 twins. Human Brain Mapping, 35, 5295–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker, S. , Neale, M. , Maes, H. , Wilde, M. , Spiegel, M. , Brick, T. , … Fox, J. (2011). OpenMx: An open source extended structural equation modeling framework. Psychometrika, 76, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma, D. , Busjahn, A. , & Peltonen, L. (2002). Classical twin studies and beyond. Nature Reviews. Genetics, 3, 872–882. [DOI] [PubMed] [Google Scholar]
- Brans, R. G. H. , Kahn, R. S. , Schnack, H. G. , van Baal, G. C. M. , Posthuma, D. , van Haren, N. E. M. , … Hulshoff Pol, H. E. (2010). Brain plasticity and intellectual ability are influenced by shared genes. Journal of Neuroscience, 30, 5519–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant, A. M. , Munakata, Y. , Boomsma, D. I. , Defries, J. C. , Haworth, C. M. A. , Keller, M. C. , … Hewitt, J. K. (2013). The nature and nurture of high IQ: An extended sensitive period for intellectual development. Psychological Science, 24, 1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer, R. M. , Mandl, R. C. W. , Schnack, H. G. , Soelen, I. L. C. , van, Baal, G. C. , van, Peper, J. S. , … Hulshoff Pol, H. E. (2012). White matter development in early puberty: A longitudinal volumetric and diffusion tensor imaging twin study. PLoS One, 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer, R. M. , van Soelen, I. L. C. , Swagerman, S. C. , Schnack, H. G. , Ehli, E. A. , Kahn, R. S. , … Boomsma, D. I. (2014). Genetic associations between intelligence and cortical thickness emerge at the start of puberty. Human Brain Mapping, 35, 3760–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer, R. M. , Koenis, M. M. G. , Schnack, H. G. , van Baal, G. C. M , van Soelen, I. L. C. , Boomsma, D. I. , & Hulshoff Pol, H. E. (2015). Longitudinal development of hormone levels and grey matter density in 9 and 12‐year‐old twins. Behavior Genetics, 45, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, T. T. , Kuperman, J. M. , Chung, Y. , Erhart, M. , McCabe, C. , Hagler, D. J. , … Dale, A. M. (2012). Neuroanatomical assessment of biological maturity. Current Biology, 22, 1693–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore, E. , & Sporns, O. (2012). The economy of brain network organization. Nature Reviews. Neuroscience, 13, 336–349. [DOI] [PubMed] [Google Scholar]
- Bullmore, E. T. , & Bassett, D. S. (2011). Brain graphs: Graphical models of the human brain connectome. Annual Review of Clinical Psychology, 7, 113–140. [DOI] [PubMed] [Google Scholar]
- Burgaleta, M. , Johnson, W. , Waber, D. P. , Colom, R. , & Karama, S. (2014). Cognitive ability changes and dynamics of cortical thickness development in healthy children and adolescents. NeuroImage, 84, 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, L.‐C. , Jones, D. K. , & Pierpaoli, C. (2005). RESTORE: Robust estimation of tensors by outlier rejection. Magnetic Resonance in Medicine, 53, 1088–1095. [DOI] [PubMed] [Google Scholar]
- Chiang, M.‐C. , Barysheva, M. , Shattuck, D. W. , Lee, A. D. , Madsen, S. K. , Avedissian, C. , … Thompson, P. M. (2009). Genetics of brain fiber architecture and intellectual performance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29, 2212–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, M.‐C. , McMahon, K. L. , de Zubicaray, G. I. , Martin, N. G. , Hickie, I. , Toga, A. W. , … Thompson, P. M. (2011a). Genetics of white matter development: A DTI study of 705 twins and their siblings aged 12 to 29. NeuroImage, 54, 2308–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, M.‐C. , Barysheva, M. , Toga, A. W. , Medland, S. E. , Hansell, N. K. , James, M. R. , … Thompson, P. M. (2011b). BDNF gene effects on brain circuitry replicated in 455 twins. NeuroImage, 55, 448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, D. L. , Holmes, C. J. , Peters, T. M. , & Evans, A. C. (1995). Automatic 3‐D model‐based neuroanatomical segmentation. Human Brain Mapping, 3, 190–208. [Google Scholar]
- Deary, I. J. , Penke, L. , & Johnson, W. (2010). The neuroscience of human intelligence differences. Nature Reviews. Neuroscience, 11, 201–211. [DOI] [PubMed] [Google Scholar]
- Dennis, E. L. , Jahanshad, N. , McMahon, K. L. , de Zubicaray, G. I. , Martin, N. G. , Hickie, I. B. , … Thompson, P. M. (2013). Development of brain structural connectivity between ages 12 and 30: A 4‐Tesla diffusion imaging study in 439 adolescents and adults. NeuroImage, 64, 161–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme, S. , Hudziak, J. J. , Botteron, K. N. , Albaugh, M. D. , Nguyen, T.‐V. , Karama, S. , & Evans, A. C. Brain Development Cooperative Group (2012). Decreased regional cortical thickness and thinning rate are associated with inattention symptoms in healthy children. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 18–27.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer, D. , & Mackay, T. (1992). Introduction to quantitative genetics. Londen: Prentice Hall. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay, N. , Giedd, J. N. , Lusk, L. , Hayashi, K. M. , Greenstein, D. , Vaituzis, A. C. , … Thompson, P. M. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101, 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Pinilla, F. , So, V. , & Kesslak, J. P. (1998). Spatial learning and physical activity contribute to the induction of fibroblast growth factor: Neural substrates for increased cognition associated with exercise. Neuroscience, 85, 53–61. [DOI] [PubMed] [Google Scholar]
- Gong, G. , Rosa‐Neto, P. , Carbonell, F. , Chen, Z. J. , He, Y. , & Evans, A. C. (2009). Age‐ and gender‐related differences in the cortical anatomical network. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29, 15684–15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur, R. C. , Turetsky, B. I. , Matsui, M. , Yan, M. , Bilker, W. , Hughett, P. , & Gur, R. E. (1999). Sex differences in brain gray and white matter in healthy young adults: Correlations with cognitive performance. Journal of Neuroscience, 19, 4065–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur, R. E. , & Gur, R. C. (2016). Sex differences in brain and behavior in adolescence: Findings from the Philadelphia Neurodevelopmental Cohort. Neuroscience and Biobehavioral Reviews, 70, 159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann, P. , Sporns, O. , Madan, N. , Cammoun, L. , Pienaar, R. , Wedeen, V. J. , … Grant, P. E. (2010). White matter maturation reshapes structural connectivity in the late developing human brain. Proceedings of the National Academy of Sciences of the United States of America, 107, 19067–19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haworth, C. M. A. , Wright, M. J. , Luciano, M. , Martin, N. G. , de Geus, E. J. C. , van Beijsterveldt, C. E. M. , … Plomin, R. (2010). The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular Psychiatry, 15, 1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting, M. M. , Maxwell, E. C. , Irvine, C. , & Nagel, B. J. (2012). The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cerebral Cortex (New York, N.Y.: 1991), 22, 1979–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , & Hulshoff Pol, H. E. (2010). Exploring the brain network: A review on resting‐state fMRI functional connectivity. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 20, 519–534. [DOI] [PubMed] [Google Scholar]
- van den Heuvel, M. P. , Stam, C. J. , Kahn, R. S. , & Hulshoff Pol, H. E. (2009). Efficiency of functional brain networks and intellectual performance. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 29, 7619–7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad, N. , Kochunov, P. V. , Sprooten, E. , Mandl, R. C. , Nichols, T. E. , Almasy, L. , … Glahn, D. C. (2013). Multi‐site genetic analysis of diffusion images and voxelwise heritability analysis: A pilot project of the ENIGMA‐DTI working group. NeuroImage, 81, 455–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, R. E. , & Haier, R. J. (2007). The Parieto‐Frontal Integration Theory (P‐FIT) of intelligence: Converging neuroimaging evidence. The Behavioral and Brain Sciences, 30, 135–187. [DOI] [PubMed] [Google Scholar]
- Karama, S. , Bastin, M. E. , Murray, C. , Royle, N. A. , Penke, L. , Muñoz Maniega, S. , … Deary, I. J. (2014). Childhood cognitive ability accounts for associations between cognitive ability and brain cortical thickness in old age. Molecular Psychiatry, 19, 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesslak, J. P. , So, V. , Choi, J. , Cotman, C. W. , & Gomez‐Pinilla, F. (1998). Learning upregulates brain‐derived neurotrophic factor messenger ribonucleic acid: A mechanism to facilitate encoding and circuit maintenance? Behavioral Neuroscience, 112, 1012–1019. [DOI] [PubMed] [Google Scholar]
- Kim, D.‐J. , Davis, E. P. , Sandman, C. A. , Sporns, O. , O'donnell, B. F. , Buss, C. , & Hetrick, W. P. (2016). Children's intellectual ability is associated with structural network integrity. NeuroImage, 124, 550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov, P. , Jahanshad, N. , Marcus, D. , Winkler, A. , Sprooten, E. , Nichols, T. E. , … Van Essen, D. C. (2015). Heritability of fractional anisotropy in human white matter: A comparison of Human Connectome Project and ENIGMA‐DTI data. NeuroImage, 111, 300–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenis, M. M. G. , Brouwer, R. M. , Van Baal, G. C. M. , Van Soelen, I. L. C. , Peper, J. S. , Van Leeuwen, M. , … Hulshoff Pol, H. E. (2013). Longitudinal study of hormonal and physical development in young twins. Journal of Clinical Endocrinology & Metabolism, 98, 1–10. [DOI] [PubMed] [Google Scholar]
- Koenis, M. M. G. , Brouwer, R. M. , van den Heuvel, M. P. , Mandl, R. C. W. , van Soelen, I. L. C. , Kahn, R. S. , … Hulshoff Pol, H. E. (2015). Development of the brain's structural network efficiency in early adolescence: A longitudinal DTI twin study. Human Brain Mapping, 36, 4938–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur, C. D. , Peper, J. S. , Crone, E. A. , & Dahl, R. E. (2012). White matter development in adolescence: The influence of puberty and implications for affective disorders. Developmental Cognitive Neuroscience, 2, 36–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer, N. , Pedroni, A. , Gianotti, L. R. R. , Hänggi, J. , Knoch, D. , & Jäncke, L. (2012). Functional brain network efficiency predicts intelligence. Human Brain Mapping, 33, 1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel, C. , Gee, M. , Camicioli, R. , Wieler, M. , Martin, W. , & Beaulieu, C. (2012). Diffusion tensor imaging of white matter tract evolution over the lifespan. NeuroImage, 60, 340–352. [DOI] [PubMed] [Google Scholar]
- Lebel, C. , & Beaulieu, C. (2011). Longitudinal development of human brain wiring continues from childhood into adulthood. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31, 10937–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot, R. K. , Schmitt, J. E. , Ordaz, S. J. , Wallace, G. L. , Neale, M. C. , Lerch, J. P. , … Giedd, J. N. (2009). Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human Brain Mapping, 30, 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Liu, Y. , Li, J. , Qin, W. , Li, K. , Yu, C. , & Jiang, T. (2009). Brain anatomical network and intelligence. PLoS Computational Biology, 5, e1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, S. , Han, C. E. , Uhlhaas, P. J. , & Kaiser, M. (2015). Preferential detachment during human brain development: Age‐ and sex‐specific structural connectivity in diffusion tensor imaging (DTI) data. Cerebral Cortex (New York, N.Y.: 1991), 25, 1477–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders, E. , Thompson, P. M. , Narr, K. L. , Zamanyan, A. , Chou, Y.‐Y. , Gutman, B. , … Toga, A. W. (2011). The link between callosal thickness and intelligence in healthy children and adolescents. NeuroImage, 54, 1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna, B. , Marek, S. , Larsen, B. , Tervo‐Clemmens, B. , & Chahal, R. (2015). An integrative model of the maturation of cognitive control. Annual Review of Neuroscience, 38, 151–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes, F. , Collignon, A. , Vandermeulen, D. , Marchal, G. , & Suetens, P. (1997). Multimodality image registration by maximization of mutual information. IEEE Transactions on Medical Imaging, 16, 187–198. [DOI] [PubMed] [Google Scholar]
- Menzies, L. , Goddings, A. L. , Whitaker, K. J. , Blakemore, S. J. , & Viner, R. M. (2015). The effects of puberty on white matter development in boys. Developmental Cognitive Neuroscience, 11, 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, S. , & Van Zijl, P. C. M. (2002). Fiber tracking: Principles and strategies ‐ A technical review. NMR in Biomedicine, 15, 468–480. [DOI] [PubMed] [Google Scholar]
- Mul, D. , Fredriks, A. M. , van Buuren, S. , Oostdijk, W. , Verloove‐Vanhorick, S. P. , & Wit, J. M. (2001). Pubertal development in The Netherlands 1965–1997. Pediatric Research, 50, 479–486. [DOI] [PubMed] [Google Scholar]
- Nederlands Instituut van Psychologen Dienstencentrum (2003). Errata en Normtabellen WISC‐IIINL oktober 2003. Amsterdam: NIP Dienstencentrum. [Google Scholar]
- Park, H.‐J. , & Friston, K. (2013). Structural and functional brain networks: From connections to cognition. Science (New York, N.Y.), 342, 1238411. [DOI] [PubMed] [Google Scholar]
- Paus, T. (2013). How environment and genes shape the adolescent brain. Hormones and Behavior, 64, 195–202. [DOI] [PubMed] [Google Scholar]
- Paus, T. (2010). Growth of white matter in the adolescent brain: Myelin or axon? Brain and Cognition, 72, 26–35. [DOI] [PubMed] [Google Scholar]
- Paus, T. , Keshavan, M. , & Giedd, J. N. (2008). Why do many psychiatric disorders emerge during adolescence? Nature Reviews. Neuroscience, 9, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peper, J. S. , Hulshoff Pol, H. E. , Crone, E. A. , & van Honk, J. (2011). Sex steroids and brain structure in pubertal boys and girls: A mini‐review of neuroimaging studies. Neuroscience, 191, 28–37. [DOI] [PubMed] [Google Scholar]
- Peper, J. S. , Schnack, H. G. , Brouwer, R. M. , Van Baal, G. C. , Pjetri, E. , Székely, E. , … Hulshoff Pol, H. E. (2009). Heritability of regional and global brain structure at the onset of puberty: A magnetic resonance imaging study in 9‐year‐old twin pairs. Human Brain Mapping, 30, 2184–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, B. D. , Ikuta, T. , Derosse, P. , John, M. , Burdick, K. E. , Gruner, P. , … Malhotra, A. K. (2014). Age‐related differences in white matter tract microstructure are associated with cognitive performance from childhood to adulthood. Biological Psychiatry, 75, 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthuma, D. , Beem, A. L. , de Geus, E. J. C. , van Baal, G. C. M. , von Hjelmborg, J. B. , Iachine, I. , & Boomsma, D. I. (2003). Theory and Practice in Quantitative Genetics. Twin Research, 6, 361–376. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Ramsden, S. , Richardson, F. , Josse, G. , Thomas, M. , Ellis, C. , Shakeshaft, C. , … Price, C. (2011). Verbal and non‐verbal intelligence changes in the teenage brain. Nature, 479, 113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan, A. , Lee, Y. , Stidd, R. , Long, R. , Greenstein, D. , Clasen, L. , … Giedd, J. N. (2010). Longitudinally mapping the influence of sex and androgen signaling on the dynamics of human cortical maturation in adolescence. Proceedings of the National Academy of Sciences of the United States of America, 107, 16988–16993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Reus, M. A. , & van den Heuvel, M. P. (2013). Estimating false positives and negatives in brain networks. NeuroImage, 70, 402–409. [DOI] [PubMed] [Google Scholar]
- Rubinov, M. , & Sporns, O. (2010). Complex network measures of brain connectivity: Uses and interpretations. NeuroImage, 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Schmithorst, V. J. (2009). Developmental sex differences in the relation of neuroanatomical connectivity to intelligence. Intelligence, 37, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst, V. J. , Holland, S. K. , & Dardzinski, B. J. (2008). Developmental differences in white matter architecture between boys and girls. Human Brain Mapping, 29, 696–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst, V. J. , Wilkes, M. , Dardzinski, B. J. , & Holland, S. K. (2005). Cognitive functions correlate with white matter architecture in a normal pediatric population: A diffusion tensor HRI study. Human Brain Mapping, 26, 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst, V. J. , & Yuan, W. (2010). White matter development during adolescence as shown by diffusion MRI. Brain and Cognition, 72, 16–25. [DOI] [PubMed] [Google Scholar]
- Schnack, H. G. , Van Haren, N. E. M. , Brouwer, R. M. , Evans, A. , Durston, S. , Boomsma, D. I. , … Hulshoff Pol, H. E. (2015). Changes in thickness and surface area of the human cortex and their relationship with intelligence. Cerebral Cortex, 25, 1608–1617. [DOI] [PubMed] [Google Scholar]
- Shaw, P. (2007). Intelligence and the developing human brain. BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology, 29, 962–973. [DOI] [PubMed] [Google Scholar]
- Shaw, P. , Greenstein, D. , Lerch, J. , Clasen, L. , Lenroot, R. , Gogtay, N. , … Giedd, J. (2006). Intellectual ability and cortical development in children and adolescents. Nature, 440, 676–679. [DOI] [PubMed] [Google Scholar]
- Shaw, P. , Kabani, N. J. , Lerch, J. P. , Eckstrand, K. , Lenroot, R. , Gogtay, N. , … Wise, S. P. (2008). Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28, 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds, D. J. , Hallquist, M. N. , Asato, M. , & Luna, B. (2014). Developmental stages and sex differences of white matter and behavioral development through adolescence: A longitudinal diffusion tensor imaging (DTI) study. NeuroImage, 92, 356–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soelen, I. L. C. , Brouwer, R. M. , Van Baal, G. C. M. , Schnack, H. G. , Peper, J. S. , Collins, D. L. , … Hulshoff Pol, H. E. (2012a). Genetic influences on thinning of the cerebral cortex during development. NeuroImage, 59, 3871–3880. [DOI] [PubMed] [Google Scholar]
- van Soelen, I. L. C. , Brouwer, R. M. , Peper, J. S. , van Leeuwen, M. , Koenis, M. M. G. , van Beijsterveldt, T. C. E. M. , … Boomsma, D. I. (2012b). Brain SCALE: Brain structure and cognition: An adolescent longitudinal twin study into the genetic etiology of individual differences. Twin Research and Human Genetics, 15, 453–467. [DOI] [PubMed] [Google Scholar]
- van Soelen, I. L. C. , Brouwer, R. M. , Van Baal, G. C. M. , Schnack, H. G. , Peper, J. S. , Chen, L. , … Hulshoff Pol, H. E. (2013). Heritability of volumetric brain changes and height in children entering puberty. Human Brain Mapping, 34, 713–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soelen, I. L. C. , Brouwer, R. M. , van Leeuwen, M. , Kahn, R. S. , Hulshoff Pol, H. E. , & Boomsma, D. I. (2011). Heritability of verbal and performance intelligence in a pediatric longitudinal sample. Twin Research and Human Genetics, 14, 119–128. [DOI] [PubMed] [Google Scholar]
- Sowell, E. R. (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience, 24, 8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swagerman, S. C. , de Geus, E. J. , Kan, K. J. , van Bergen, E. , Nieuwboer, H. A. , Koenis, M.M.G. , … Boomsma, D. I. (2016). The Computerized Neurocognitive Battery: Validation, aging effects, and heritability across cognitive domains. Neuropsychology, 30, 53–64. [DOI] [PubMed] [Google Scholar]
- Tamnes, C. K. , Fjell, A. M. , Østby, Y. , Westlye, L. T. , Due‐Tønnessen, P. , Bjørnerud, A. , & Walhovd, K. B. (2011). The brain dynamics of intellectual development: Waxing and waning white and gray matter. Neuropsychologia, 49, 3605–3611. [DOI] [PubMed] [Google Scholar]
- Tamnes, C. K. , Østby, Y. , Walhovd, K. B. , Westlye, L. T. , Due‐Tønnessen, P. , & Fjell, A. M. (2010). Intellectual abilities and white matter microstructure in development: A diffusion tensor imaging study. Human Brain Mapping, 31, 1609–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, C. Y. , Eaves, E. L. , Ng, J. C. , Carpenter, D. M. , Mai, X. , Schroeder, D. H. , … Haier, R. J. (2010). Brain networks for working memory and factors of intelligence assessed in males and females with fMRI and DTI. Intelligence, 38, 293–303. [Google Scholar]
- Toulopoulou, T. , Picchioni, M. , Rijsdijk, F. , Hua‐Hall, M. , Ettinger, U. , Sham, P. , & Murray, R. (2007). Substantial genetic overlap between neurocognition and schizophrenia: Genetic modeling in twin samples. Archives of General Psychiatry, 64, 1348–1355. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer, N. , Landeau, B. , Papathanassiou, D. , Crivello, F. , Etard, O. , Delcroix, N. , … Joliot, M. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single‐subject brain. NeuroImage, 15, 273–289. [DOI] [PubMed] [Google Scholar]
- Waber, D. P. , Forbes, P. W. , Almli, C. R. , & Blood, E. A. (2012). Four‐year longitudinal performance of a population‐based sample of healthy children on a neuropsychological battery: The NIH MRI study of normal brain development. Journal of the International Neuropsychological Society, 18, 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Adamson, C. , Yuan, W. , Altaye, M. , Rajagopal, A. , Byars, A. W. , & Holland, S. K. (2012). Sex differences in white matter development during adolescence: A DTI study. Brain Research, 1478, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler, D. (2011). Wechsler Abbreviated Scale of Intelligence–Second edition (WASI‐II). San Antonio, TX: NCS Pearson. [Google Scholar]
- Wechsler, D. (2004). Wechsler Adult Intelligence Scale – Third edition, Dutch version. Lisse, The Netherlands: Swets & Zeitlinger B.V. [Google Scholar]
- Wechsler, D. , Kort, W. , Compaan, E. L. , Belichrodt, N. , Resing, W. C. M. , Schittekatte, M. , … Verhaeghe, P. (2002). WISC‐IIINL Wechsler Intelligence Scale for Children – Third edition, Ducth version. Amsterdam: Harcourt Test Publishers/Nederlands Insitiuut van Psychologen Dienstencentrum. [Google Scholar]
- Wen, W. , Zhu, W. , He, Y. , Kochan, N. A. , Reppermund, S. , Slavin, M. J. , … Sachdev, P. (2011). Discrete neuroanatomical networks are associated with specific cognitive abilities in old age. Journal of Neuroscience, 31, 1204–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, M. , Wang, J. , & He, Y. (2013). BrainNet Viewer: A network visualization tool for human brain connectomics. PLoS One, 8, e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, Q. J. , Teh, I. , Fusar‐Poli, P. , Sum, M. Y. , Kuswanto, C. , & Sim, K. (2013). Tracking cerebral white matter changes across the lifespan: Insights from diffusion tensor imaging studies. Journal of Neural Transmission, 120, 1369–1395. [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , Fornito, A. , Harding, I. H. , Cocchi, L. , Yücel, M. , Pantelis, C. , & Bullmore, E. T. (2010). Whole‐brain anatomical networks: Does the choice of nodes matter? NeuroImage, 50, 970–983. [DOI] [PubMed] [Google Scholar]
- Zhao, T. , Cao, M. , Niu, H. , Zuo, X.‐N. , Evans, A. , He, Y. , … Shu, N. (2015). Age‐related changes in the topological organization of the white matter structural connectome across the human lifespan. Human Brain Mapping, 36, 3777–3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


