Abstract
In the elderly, brain structural deficits and gait disturbances due to cerebral small vessel disease (CSVD) have been well demonstrated. The relationships among CSVD, brain atrophy, and motor impairment, however, are far from conclusive. Particularly, the effect of CSVD on subcortical nuclear atrophy, motor performance of upper extremities, and associating patterns between brain atrophy and motor impairment remains largely unknown. To address these gaps, this study recruited 770 community‐dwelling subjects (35–82 years of age), including both CSVD and non‐CSVD individuals. For each subject, four motor tests involving upper and lower extremities were completed. High‐resolution structural MRI was applied to extract gray matter (GM) volume, white matter volume, cortical thickness, surface area, and subcortical nuclear (caudate, putamen, pallidum, and thalamus) volumes. The results showed worse motor performance of lower extremities but relatively preserved performance of upper extremities in the CSVD group. Intriguingly, there was a significant association between the worse performance of upper extremities and atrophy of whole‐brain GM and pallidum in the CSVD group but not in the non‐CSVD group. In addition, mediation analysis confirmed a functional CSVD‐to‐“brain atrophy”‐to‐“motor impairment” pathway, that is, a mediating role of thalamic atrophy in the CSVD effect on walking speed in the elderly, indicating that CSVD impairs walking performance through damaging the integrity of the thalamus in aging populations. These findings provide important insight into the functional consequences of CSVD and highlight the importance of evaluating upper extremities functions and exploring their brain mechanisms in CSVD populations during aging.
Keywords: brain atrophy, cerebral small vessel disease (CSVD), elderly, motor performance, thalamus
Abbreviations
- CSVD
cerebral small vessel disease
- FWE
family‐wise error
- GLM
general linear model
- GM
gray matter
- GMV
whole‐brain gray matter volume
- ICV
intracranial volume
- MTG
middle temporal gyrus
- RFT
random field theory
- SD
standard deviation
- WM
white matter
- WMV
whole‐brain white matter volume.
1. INTRODUCTION
Impairment of motor abilities is quite common in the aging population (from approximately 10% between the ages of 60 and 69 years to more than 60% in those over 80 years of age) (Mahlknecht et al., 2013) and can increase the risks of falls, institutionalization, and mortality (van der Holst et al., 2016). A number of studies have demonstrated a contributing role of cerebral small vessel disease (CSVD) in the motor impairment of the elderly. The degree of two major imaging markers of CSVD, lacunar infarct and white matter hyperintensity (WMH), have been associated with gait parameters such as walking velocity and stride length in older people (de Laat et al., 2010; Pinter et al., 2017; Smith et al., 2015).
Notably, the motor impairment associated with CSVD cannot be fully explained by lacunar infarcts and WMH (i.e., the CSVD burdens). To further clarify the pathogenesis of motor impairment in CSVD, a few studies applied cutting‐edge neuroimaging techniques to ascertain associations between brain abnormalities (other than the CSVD burdens) and motor impairment in CSVD individuals. Along this line, the disruption of white matter (WM) integrity in specific normal‐appearing WM regions was found to be partly responsible for gait disturbances (de Laat et al., 2011; Kim et al., 2016) in CSVD, and gray matter atrophy (e.g., cortical thinning) was correlated with gait performance across CSVD individuals (de Laat et al., 2012; Kim et al., 2016; Rosano et al., 2008). However, the causal relationships among CSVD, brain atrophy, and motor impairment in the elderly are far from conclusive.
In the vast majority of these neuroimaging studies, correlational analyses were applied within a CSVD group, and no control group was included. This paradigm led to a lack of information on brain‐motor relationships in a non‐CSVD group, which might be different from those in a CSVD group. CSVD may alter associating patterns between brain structure and motor performance from normal aging conditions. In addition, previous neuroimaging studies have been restricted to gait‐related parameters, mainly characterizing the motor performance of lower extremities. Consequently, the effect of CSVD on the motor performance of upper extremities and its association with brain atrophy are largely unknown. Finally, the subcortical nuclei (e.g., basal ganglia and thalamus), brain structures likely susceptive to CSVD, putatively play crucial roles in motor control functions, and their atrophy has been associated with worse walking performance in older people (Dumurgier et al., 2012; Rosano, Aizenstein, Studenski, & Newman, 2007; Rosano et al., 2008). However, to date, how the abnormalities of subcortical nuclei relate to motor impairment in CSVD has remained largely unexplored.
This study aimed to demonstrate how motor impairment of the elderly is associated with brain atrophy in CSVD. To overcome the issues mentioned above, a large sample from the community containing both CSVD and non‐CSVD individuals was studied. For each subject, the motor function evaluation included four tests involving upper and lower extremities. A set of brain morphometric measures was computed from high‐resolution structural MRI. We hypothesized that (1) substantial brain atrophy induced by CSVD would be observed and that (2) brain‐motor relationships specific to motor performance of the upper or lower extremities may differ between CSVD and non‐CSVD groups.
2. MATERIALS AND METHODS
2.1. Study participants and clinical data collection
All subjects were from the Shunyi cohort study, an ongoing population‐based study (Su et al., 2017). From June 2014 to April 2016, five villages were randomly chosen from the Shunyi district, a suburb area of Beijing. In each village, every household received a letter, inviting all inhabitants aged 35 years and above to participate in this cohort study. The response rate of participation was 79.9%, and a total of 909 subjects (aged 35 years and above) agreed to join the study and completed standard baseline assessments, brain MRI examination, and motor function evaluation. All participants signed an informed consent form. The Medical Review Ethics Committee of Peking Union Medical College Hospital approved the study (reference number: B‐160).
Participants were entered into our analysis if they met the following criteria: (1) no history of stroke, (2) no sign of abnormalities in neurological examination, and (3) had a qualified high‐resolution T1‐weighted MRI image. Accordingly, we excluded 58 participants with a history of stroke, 14 with muscle strength levels lower than grade three or with involuntary movements owing to diseases other than stroke and 67 with poor MRI quality, leaving 770 participants in our final analysis. The baseline characteristics of participants included and not included in the current study were balanced, except that the included participants had a lower proportion of male and current smokers and a higher proportion of those with hyperlipidemia.
Cardiovascular risk factors were defined as follows: hypertension was defined as blood pressure ≥140/90 mmHg; diabetes mellitus (DM) was defined as a fasting plasma glucose level ≥7.0 mmol/L or 2 hr plasma glucose level ≥11.1 mmol/L during an oral glucose tolerance test; hyperlipidemia was defined as total cholesterol >5.2 mmol/L or low‐density lipoprotein >2.58 mmol/L; and current smoker was defined as an individual smoking at least one cigarette per day for more than 6 months before enrollment.
According to the commonly applied criterion (Lawrence, Chung, Morris, Markus, & Barrick, 2014; Takakusaki, 2017), the subjects with severe WMH of a Fazekas scale ≥2, that is, at least two in the Fazekas scale for either deep or periventricular WMH, and at least one lacune were determined to be a part of the CSVD group (68 subjects), and the rest were placed in the control group (702 subjects) (See Table 1).
Table 1.
Characteristics of the study population
| Study population(n = 770) | Control group(n = 702) | CSVD group(n = 68) | Group differences(p value) | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 57.2 (9.3) | 56.3 (9.0) | 66.2 (8.1) | <.001* |
| Female, no. (%) | 501 (65.1) | 474 (67.5) | 27 (39.7) | <.001* |
| BMI, kg/m2 | 26.5 (3.7) | 26.5 (3.6) | 27.3 (3.9) | .082 |
| Current smoker, no. (%) | 165 (21.9) | 143 (20.8) | 22 (32.4) | .028* |
| Hypertension, no. (%) | 387 (50.3) | 333 (47.4) | 54 (79.4) | <.001* |
| Hyperlipidemia, no. (%) | 371 (48.2) | 336 (47.9) | 35 (51.5) | .570 |
| DM, no. (%) | 129 (16.8) | 101 (14.4) | 28 (41.2) | <.001* |
| Lacunes, no. (%) | 117 (15.2) | 49 (7.0) | 68 (100.0) | <.001* |
| Number of lacunes | .35 (1.18) | .11 (.42) | 2.76 (2.76) | <.001* |
| PVWMH | .94 (.81) | .80 (.70) | 2.34 (.54) | <.001* |
| DWMH | .80 (.71) | .70 (.62) | 1.79 (.74) | <.001* |
| Motor performance | ||||
| 3‐m walking, s | 3.56 (.83) | 3.50 (.73) | 3.91 (.95) | .012** |
| Chair‐stand, s | 8.92 (2.11) | 8.70 (1.72) | 9.81 (2.36) | .005** |
| Pronation‐supination, s | 7.53 (1.65) | 7.40 (1.50) | 8.15 (1.61) | .046* |
| Finger‐tapping, s | 5.40 (1.75) | 5.25 (1.50) | 5.75 (1.88) | .788 |
| Global measure | ||||
| ICV, mL | 1,402.9 (123.8) | 1,398.2 (121.3) | 1,439.2 (125.9) | .184 |
| GMV, mL | 584.6 (53.1) | 585.8 (52.1) | 565.8 (49.9) | <.001** |
| WMV, mL | 487.0 (56.1) | 487.1 (54.7) | 477.6 (56.1) | <.001** |
| Mean thickness, mm | 3.1 (.1) | 3.1 (.1) | 3.0 (.2) | <.001** |
| Total area, cm2 | 1,720.4 (139.3) | 1,720.4 (131.1) | 1,769.6 (147.5) | .127 |
| Subcortical nucleus | ||||
| Putamen, mL | 9.92 (1.19) | 9.98 (1.12) | 9.41 (1.39) | <.001** |
| Pallidum, mL | 3.61 (.54) | 3.61 (.43) | 3.48 (.60) | .019* |
| Caudate, mL | 6.45 (.79) | 6.45 (.75) | 6.32 (1.01) | .105 |
| Thalamus, mL | 14.50 (1.35) | 14.56 (1.23) | 13.79 (1.26) | <.001** |
Data are mean (SD) unless other indicated.
Abbreviations: BMI = body mass index; DM = diabetes mellitus; PVWMH, periventricular white matter hyperintensity (Fazekas grade 0 to 4); DWMH, deep white matter hyperintensity (Fazekas grade 0 to 4); ICV = whole‐brain intracranial volume; GMV = whole‐brain gray matter volume; WMV = whole‐brain white matter volume.
Uncorrected p < .05.
Bonferroni corrected p < .05.
2.2. Assessment of motor functions
For each participant, the motor functions of lower and upper extremities were quantitatively evaluated using 3‐m walking, chair‐stand, pronation‐supination, and finger‐tapping tests (Su et al., 2017). For details, please see the Supporting Information.
2.3. MRI acquisition and postprocessing
MRI acquisition was performed from July 2014 to April 2016 using the same 3‐Tesla Siemens Skyra scanner (Siemens; Erlangen, Germany). Three‐dimensional T1‐weighted, T2‐weighted, and fluid‐attenuated inversion recovery (FLAIR) images were acquired (for detailed MRI parameters, please see Supporting Information). WMHs were quantitatively assessed using FLAIR scans, which appeared normal or faintly hyperintense on the T1‐weighted images. Periventricular white matter hyperintensities (PVWMHs) and deep white matter hyperintensities (DWMHs) were scored on axial FLAIR imaging using the Fazekas scale (Fazekas, Chawluk, Alavi, Hurtig, & Zimmerman, 1987). Individuals with severe WMH were defined as those with either PVWMHs or DWMHs rated at least two on the Fazekas scale. Lacunes were defined as focal lesions from 3 to 15 mm in size, which have the same signal characteristics as CSF on all sequences and are situated in the basal ganglia or white matter. Lacunar infarcts were rated on 3D T1‐weighted images, and T2 and FLAIR images were used for confirming lesions. As described above, all participants were then divided into two groups: CSVD or control, using the obtained Fazekas scales and number of lacunes.
Using T1‐weighted images, an automated issue segmentation was applied within the CIVET pipeline (see details in the Supporting Information), yielding three global volumetric measures: the intracranial volume (ICV), whole‐brain gray matter volume (GMV), and whole‐brain white matter volume (WMV), as well as the cortical thickness and surface area at the vertex level (Lerch & Evans, 2005). Next, four subcortical nuclei, that is, the caudate, pallidum, putamen, and thalamus, were accurately extracted using the FIRST algorithm embedded in the FSL (FMRIB Software Library, v5.0) (Patenaude, Smith, Kennedy, & Jenkinson, 2011). For each of these nuclei, the bilateral volumes were summed. We chose these four nuclei because of their well‐known roles in motor functions, and other nuclei (e.g., the hippocampus and amygdala) were not considered to minimize the number of statistical comparisons.
2.4. Statistical analysis
Given our inclusion criteria, there was no missing data in any domain for any subject who was entered into our statistical analysis. The two‐sample t‐test or chi‐square test, when appropriate, was applied to evaluate group differences in demographics.
Step 1. Group effect on motor functions: To assess group differences in motor functions, we used a general linear model (GLM) with the group as the main factor and age and gender as covariates. Corrected p < .05 was considered significant, and the Bonferroni method was applied to correct for the four comparisons as p < .05/4 = .0125.
Step 2. Group effect on brain morphology: To evaluate group differences in global measures and subcortical nuclear (i.e., caudate, pallidum, putamen, and thalamus) volume, similar GLMs were applied, in which the ICV was additionally included as a covariate. The Bonferroni correction was also used as p < .0125. Regarding the group effect on cortical morphology, the same GLMs were applied to cortical thickness or surface area on each vertex of the cerebral cortex on both hemispheres. The random field theory (RFT)‐based method was used to correct for multiple vertex‐wise comparisons, and cortical clusters with a family‐wise error (FWE)‐corrected p < .05 (uncorrected p < .001) were considered significant.
Step 3. Group effect on brain‐motor relations: For each motor function, we further assessed the group effect on brain‐motor relations by testing the “group × brain measure” interaction effect in GLMs, wherein “group,” “brain measure,” and “group × brain measure” were predictor variables and age, gender, and ICV were included as covariates. Here, the “brain measure” was the global measures, subcortical volume, cortical thickness, and surface area. A significant “group × brain measure” interaction effect indicated that the correlation between motor performance and brain measure differed between the CSVD and control groups. For the remaining “brain measures” showing no significant “group × brain measure” interaction, we further tested the “brain measure” effect after removing the “group × brain measure” interaction term in the linear model. Such a significant “brain measure” effect indicated a similar significant correlation between motor performance and brain measures in both groups. Like above, the Bonferroni (p < .05/16 = .0031) and RFT‐based methods were applied when appropriate.
For all statistical analyses described above, outliers were defined as data points beyond three SDs from the mean for each group. For each analysis, all outliers were discarded. The number of outliers for each analysis is summarized in Supporting Information Table S1. All GLM analyses were implemented using SurfStat (Worsley, 2008).
Step 4. Mediation analysis: To determine whether specific brain measures play a mediating role in the CSVD effect on motor functions, we applied a mediation analysis by taking the group factor, brain measures, and motor scores as the predictor, mediator, and outcome, respectively. This type of analysis can be implemented using the Structure Equation Modeling approach, in which all paths are considered simultaneously (Valeri & Vanderweele, 2013). For simplicity, we chose a standard step‐wise convention, including four‐step tests: (1) path c: the group effect on motor performance (see Step 1 above), that is, the total effect of the predictor on the outcome; (2) path a: the group effect on brain measures (see Step 2 above); (3) path b: the correlation between brain measures and motor scores, after controlling for the group factor (see Step 3 above); and (4) the a × b effect, which was referred to as the indirect effect and was indicative of whether the predictor‐outcome relationship was significantly reduced after controlling for the mediator. If all four tests reached the level of significance, the brain measures were considered to significantly mediate the group effect on the motor functions.
In fact, path c, path a, and path b were completed during earlier steps in the analysis (i.e., Step 1, 2, and 3). The remaining a × b indirect effect was evaluated using the PROCESS macro implemented in SPSS (Hayes, 2018). A bootstrap method with 10,000 repetitions was used to estimate the confidence intervals for the indirect effects. An empirical 95% confidence interval that did not include zero indicated significance at the .05 level.
3. RESULTS
3.1. Demographics
The demographic characteristics are summarized in Table 1. A total of 770 participants were included in this study with a mean age of 57.2 years and males accounting for 34.9%. All included subjects were divided into two groups: CSVD (68 subjects) and control (702 subjects) group. Compared with the control group, the CSVD group had a higher age and higher proportion of males, current smokers, and those with histories of hypertension and DM.
3.2. Group differences in motor functions
As shown in Table 1, compared with controls, the CSVD group performed significantly worse in motor performance of lower extremities (3‐m walking, p = .012; chair‐stand, p = .005), after controlling for age and gender. For motor performance of upper extremities, there was a trend of impaired performance in the pronation‐supination test in the CSVD group (p = .046 but did not survive the Bonferroni correction), and no significant group difference was observed in finger‐tapping performance (p = .788).
3.3. Group differences in brain morphology
Age, gender, and ICV were included as covariates when evaluating the group effects on brain morphological measures. Regarding the global brain morphological measures (Table 1), the CSVD group showed a significant overall atrophy of both GM (p < .001) and WM (p < .001) and a thinner cortex on average (mean thickness, p < .001). The total cortical surface area, however, showed no significant group difference (p = .127). Regarding the subcortical nuclei of interest, the putamen and thalamus exhibited a significant atrophy (p < .001 and p < .001, respectively), and the pallidum showed a trend of reduced volume in the CSVD group (p = .019 but did not survive the Bonferroni correction). There was no volumetric difference in the caudate between the two groups (p = .105).
The F value maps for the group effect on vertex‐wise thickness and surface area across the entire cerebral cortex are illustrated in Figure 1. The cortical thickness exhibited a widespread effect of the group factor, with 10 clusters of a significant group difference (FWE‐corrected p < .05). These clusters mainly covered the bilateral frontal lobe, bilateral tempo‐parieto‐occipital joint areas, bilateral superior temporal gyri and temporal pole, and left para‐calcarine areas, and a consistent cortical thinning was observed in the CSVD group compared with cortex thickness in the controls. In contrast, the surface area exhibited a very focal group effect, and only two significant clusters were found, with one around the right angular gyrus and superior frontal gyrus and the other around the left para‐hippocampal gyrus.
Figure 1.
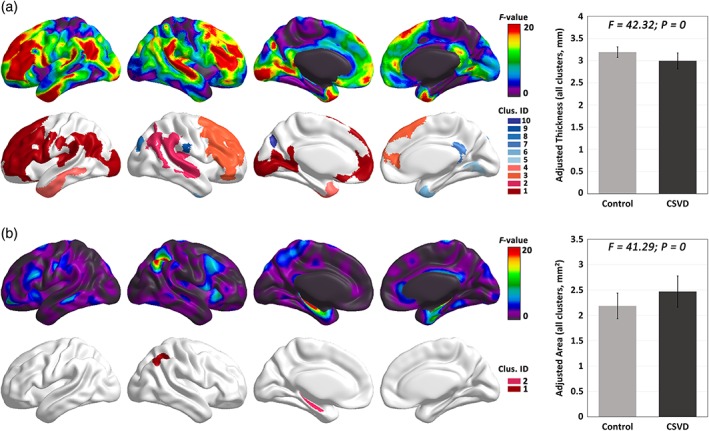
The group effect on cortical thickness and surface area at the vertex level. F‐map of the group effect on cortical thickness (a) and surface area (b). Significant clusters (FWE‐corrected p < .05) are depicted with colors. The mean value across all significant clusters for each group is illustrated. Compared to control, the CSVD group showed an extensive decrease in cortical thickness and an increase in surface area [Color figure can be viewed at http://wileyonlinelibrary.com]
3.4. Interaction of group effects and brain measures
As illustrated in Table 2 and Figure 2a–c, there were significant “GMV × Group” (p < .001) and “Pallidum × Group” interaction effects (p < .001) on “pronation‐supination” performance, and a significant “Pallidum × Group” interaction effect (p = .002) on “finger‐tapping” performance, indicating that GMV‐“pronation‐supination,” pallidum‐“pronation‐supination,” and pallidum‐“finger‐tapping” relations differed between the CSVD and control groups. Specifically, the post hoc analyses further revealed that the relevant brain measures (i.e., GMV or pallidum volume) consistently showed a significant negative correlation with pronation‐supination and finger‐tapping performance only in the CSVD group but not in the control group (Figure 2a–c).
Table 2.
Brain morphometry‐by‐group interaction effects on motor scores
| Pronation‐supination | Finger‐tapping | Chair‐stand | 3‐m walking | |||||
|---|---|---|---|---|---|---|---|---|
| F value | p value | F value | p value | F value | p value | F value | p value | |
| Global measure | ||||||||
| GMV × group | 13.99 | <.001 ** | 3.39 | .07 | 4.63 | .03* | 3.34 | .07 |
| WMV × group | 1.26 | .26 | 1.92 | .17 | 4.80 | .03* | 2.51 | .11 |
| Mean thickness × group | 5.87 | .02* | 3.72 | .05 | .88 | .35 | 1.71 | .19 |
| Total area × group | .19 | .66 | .18 | .67 | .14 | .70 | .77 | .38 |
| Subcortical nucleus | ||||||||
| Putamen × group | .49 | .48 | 2.88 | .09 | .14 | .71 | .43 | .52 |
| Pallidum × group | 15.29 | <.001 ** | 9.60 | .002 ** | 8.14 | .004* | 2.68 | .10 |
| Caudate × group | .70 | .41 | .05 | .83 | .77 | .38 | .86 | .35 |
| Thalamus × group | .35 | .56 | .09 | .76 | .76 | .38 | .41 | .52 |
Abbreviations: GMV = whole‐brain gray matter volume; WMV = whole‐brain white matter volume.
Uncorrected p < .05.
Bonferroni corrected p < .05.
Figure 2.
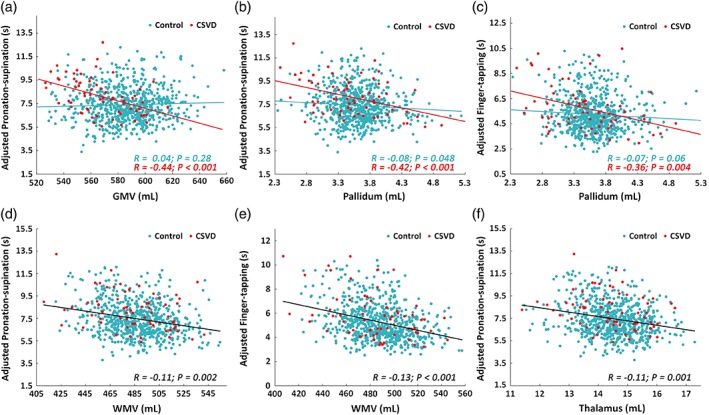
Group differences in brain morphometry‐motor relations. (a–c) scatter plots of significant brain morphometry‐by‐group interaction on motor scores (corrected p < .05). There was a significant negative correlation in the CSVD group (red) but not in the control group (blue). (d–f) scatter plots of significant negative correlations between brain morphometry and motor scores regardless of group (i.e., after controlling for group) [Color figure can be viewed at http://wileyonlinelibrary.com]
The cerebral cortical F‐maps of the “group × thickness/area” interaction effect for all motor scores are illustrated in Supporting Information Figure S1. Only one cortical cluster around the right middle temporal gyrus (MTG) showed a significant “group × thickness” interaction on pronation‐supination performance (FWE‐corrected p < .001). Similarly, the cluster exhibited a significant negative correlation between the thickness of the MTG region and pronation‐supination score in the CSVD group but not in the control group. No significant “group × thickness” interaction cluster was observed for the other motor scores. There were no significant results for the “group × area” interaction effect on all motor scores.
3.5. Group‐independent brain‐motor relations
As illustrated in Table 3 and Figure 2d–f, we found three significant negative brain‐motor correlations after controlling for group, age, gender, and ICV: (1) between WMV and pronation‐supination performance (β = −.02, p = .002); (2) between WMV and finger‐tapping performance (β = −.02, p < .001); (3) between thalamic volume and pronation‐supination performance (β = −.39, p = .001). Across the cerebral surface with nonsignificant “group × thickness/area” interaction, there was one cluster around the cuneus that showed a significant correlation between cortical thickness and chair‐stand performance (See Supporting Information Figure S2). No significant correlation was observed between cortical surface area and any of the motor performances.
Table 3.
Group‐independent correlations between brain morphometry and motor scores
| Pronation‐supination | Finger‐tapping | Chair‐stand | 3‐m walking | |||||
|---|---|---|---|---|---|---|---|---|
| T value | p value | T value | p value | T value | p value | T value | p value | |
| Global measure | ||||||||
| GMV | – | – | −.92 | .36 | .25 | .80 | −1.25 | .21 |
| WMV | −3.04 | .002 ** | −3.63 | <.001 ** | −1.49 | .14 | −.97 | .33 |
| Mean thickness | .00 | .99 | .45 | .65 | −.80 | .42 | −.92 | .36 |
| Total area | 1.36 | .17 | −.67 | .51 | .59 | .55 | .04 | .97 |
| Subcortical nucleus | ||||||||
| Putamen | −.17 | .86 | −1.21 | .23 | −1.70 | .09 | −.62 | .54 |
| Pallidum | – | – | – | – | −.92 | .36 | −2.84 | .01* |
| Caudate | .61 | .54 | .31 | .75 | 1.31 | .19 | 1.34 | .18 |
| Thalamus | −3.20 | .001 ** | −1.93 | .05 | −1.84 | .07 | −2.33 | .02* |
Abbreviations: GMV = whole‐brain gray matter volume; WMV = whole‐brain white matter volume.
Uncorrected p < .05.
3.6. Mediation model from the CSVD to thalamic atrophy and impaired walking performance
Path c, path a, and path b of the mediation model correspond to the above‐described group difference in motor function, group difference in brain measures, and group‐independent brain‐motor relations, respectively. Given the requirement for a significant path c, path a, and path b, there was only one candidate mediation path, in which the group factor, thalamic volume, and walking performance are the predictor, mediator, and outcome, respectively (path c, β = .10, p = .007; path a, β = −.14, p < .001; path b, β = −.11, p = .02). The bootstrap simulation (n = 10,000) further confirmed a significant indirect effect (effect size a × b = .02, 95% confidence interval = [.0084, .0892], p < .05). Therefore, as illustrated in Figure 3, thalamic volume can be considered to significantly mediate the group effect on walking performance. That is, CSVD might partially impair 3‐m walking speed through thalamic atrophy.
Figure 3.
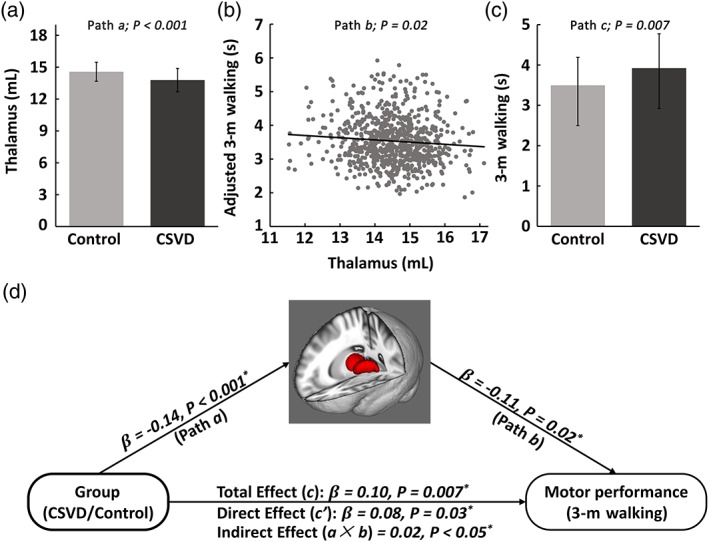
Mediation model of the CSVD‐thalamus‐walking pathway. (a) Path a: Significant CSVD group effect on thalamic volume. (b) Path b: Significant thalamus‐walking correlation after controlling for group. (c) Path c: Significant CSVD group effect on 3‐m walking performance. (d) Schematic diagram of the mediation model of the pathway from CSVD to thalamic atrophy to slower walking speed in the elderly [Color figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
Using a large community‐dwelling cohort data set, this study provided novel insights into the mechanisms of brain damage and “motor deficit” in CSVD by (1) demonstrating CSVD‐induced alterations of specific relationships between brain morphometric measures and motor performance of upper extremities and (2) revealing one specific CSVD‐to‐“brain atrophy”‐to‐“motor deficit” pathway.
With regard to motor performance, the majority of CSVD studies have focused on gait parameters. To address this, our previous CSVD study assessed motor performance for both lower and upper extremities and demonstrated associations of CSVD imaging markers with motor performance of both lower and upper extremities in community‐dwelling populations (Su et al., 2017). Using the same cohort data, our present study further applied comprehensive analyses of brain imaging data, yielding a variety of brain morphometric measures (e.g., brain tissue volume, deep nucleus size, cortical thickness, surface area). The relationships among CSVD, brain structural morphometry, and motor performance were then thoroughly investigated in contrast to our previous study, which simply focused on the relationship between CSVD and motor performance (Su et al., 2017). By dividing the entire population into a CSVD group and non‐CSVD group, the present study found that 3‐m walking and repeated chair‐stand performance were significantly worse in the CSVD group, highly consistent with our previous study that showed an association of WMHs (CSVD marker) with performance in these two lower extremity tasks (Su et al., 2017). These converging results strongly support a central role of WMHs or lacunes in lower motor performance of lower extremities in the elderly (Smith et al., 2015).
The upper extremities did not exhibit a significantly worse performance in the CSVD group, which is compatible with a previous study showing no direct relation between WMHs (CSVD marker) and motor function in upper extremities (Linortner et al., 2012). Intriguingly, however, we observed that the lower motor performance of upper extremities was associated with the atrophy of whole‐brain GM and the pallidum in the CSVD group but not in the non‐CSVD group. These results suggest that severe CVSD burdens can initiate a correlation between GM atrophy and lower motor performance in upper extremities. This effect might relate to specific mechanisms of motor functional reorganization at the neural level and warrants further investigation. These findings highlighted a differential influencing pattern of CSVD on motor performance between the lower and upper extremities, which deserves more attention.
Notably, worse performance in upper extremities was found to be correlated with WM atrophy in both the CSVD and non‐CSVD groups. Accordingly, a few studies have reported that age‐related declines in size and microstructural integrity of the corpus callosum (the biggest white matter tract) were crucial for bimanual control deficits in the elderly (Fling et al., 2011; Fujiyama et al., 2016), indicating the importance of WM integrity in motor performance of upper extremities. The motor performance of upper extremities is a relatively sophisticated skill, likely relying on heavy integration among different brain functions, such as visual, somatosensory, and motor functions (Shumway‐Cook & Woollacott, 2015). Therefore, WM integrity underlying functional integration is crucial to motor abilities of upper extremities, and disruption of WM connections can lead to worse performance in upper motor abilities.
Like previous studies, the present study observed substantial CSVD‐induced atrophy of GM/WM tissues and region‐specific cortical thinning. WM atrophy could be a pathological consequence of subcortical lesions (e.g., WMH and lacunar infarcts in CSVD) that results in WM rarefaction and shrinkage (Appelman et al., 2009; Ikram, Vernooij, Vrooman, Hofman, & Breteler, 2009; Smith et al., 2015). Meanwhile, WM disruption may further lead to secondary degeneration of cerebral GM tissue, which is possibly represented by cortical thinning in the CSVD group (de Laat et al., 2011; Kim et al., 2011; Kim et al., 2016; Tuladhar et al., 2015). Notably, our observed region‐specific cortical thinning in the CSVD group was mainly around the prefrontal and temporal cortex, which is in accordance with previous results (Kim et al., 2016; Tuladhar et al., 2015).
In addition to cortical GM and WM atrophy, the CSVD group also exhibited volumetric loss of deep subcortical nuclei, with a focus on the putamen and thalamus. This observation is compatible with previous studies showing an increased ventricular size and atrophy of the basal ganglia (as a whole) with increasing WM lesion (the major CSVD burden) (Aribisala et al., 2013; Wardlaw et al., 2013). The atrophy of the putamen and thalamus might be a consequence of direct vascular lesion or secondary degeneration. Due to their surrounding small perforating arteries from the middle and posterior cerebral arteries, the deep nuclei are vulnerable to the direct ischemic or hemorrhagic damage associated with CSVD (Gold et al., 2005; Pantoni, 2010). On the other hand, deep subcortical nuclei, especially the thalamus, have rich reciprocal connectivity with various brain regions and therefore are susceptible to secondary hypometabolism and apoptosis due to disturbances in subcortical WM integrity in CSVD (Bjorklund et al., 2014; Houtchens et al., 2007; Petito, Feldmann, Pulsinelli, & Plum, 1987; Pulsinelli, Brierley, & Plum, 1982).
Importantly, there was a correlation between the atrophy of the thalamus and the walking speed across both CSVD and non‐CSVD subjects, indicating a thalamic input on gait in general. As an important relay hub within the motor circuit, specific neurons of the ventrolateral and reticular nucleus in the thalamus are modulated during locomotion and can transmit integrated signals to the motor cortex (Marlinski, Nilaweera, et al., 2012; Marlinski, Sirota, et al., 2012). Furthermore, thalamic degeneration has been proposed to cause severe deficits in motor initiation, control, and learning (Goldberg, Farries, & Fee, 2013). With regard to gait performance, a number of structural and functional imaging studies have demonstrated thalamic involvement in this specific motor function. For example, functional activation in the thalamus was observed during a walking task in healthy subjects but not in individuals with gait disorders, and the degree of thalamic activation was negatively correlated with gait performance scores (Iseki et al., 2010). Moreover, impaired thalamic activity was found to be related to the freezing of gait in Parkinson disease (Mi et al., 2017). Structurally, thalamic atrophy can partially account for the compromised walking speed in multiple sclerosis patients (Motl, Zivadinov, Bergsland, & Benedict, 2016). Additionally, thalamic tracts, such as the anterior thalamic radiation, are associated with gait stabilization in elderly (Bruijn, Van Impe, Duysens, & Swinnen, 2014). Together, these observations suggest an important role of the thalamus in gait performance, supporting the plausibility of our current observation. Our currently identified association between thalamic atrophy and slower gait speed, regardless of CSVD, further implies a robust effect of the thalamus on gait performance in the elderly.
Mediation analyses confirmed that the CSVD‐induced thalamic atrophy mediates the effects of CSVD on slower walking speed in the elderly. That is, CSVD impaired the walking performance of the elderly through damaging the integrity of the thalamus. This pathway is of great value for elucidating functional mechanisms of CSVD because it provides answers to two important questions: (1) what neural structure is damaged by CSVD to impair motor performance? (2) What motor function is impaired by CSVD‐induced thalamic damage in the elderly? However, it should be noted that this pathway is unlikely to be the only pathway by which CSVD influences the brain and motor functions; that is, other CSVD‐to‐brain damage‐to‐motor impairment pathways exist. For example, Kim et al. revealed that cortical thinning or disruption of WM integrity can also mediate the WMH effects on gait disturbances (Kim et al., 2016).
Notably, the mediation analysis in our present study is exploratory in nature. While the Bonferroni correction was used separately for path a (i.e., the CSVD effect on motor functions), path b (i.e., the CSVD effect on brain measures), and path c (i.e., brain‐motor relations) of the mediation model, we did not apply the Bonferroni correction for all paths across all possible mediation models (i.e., all brain measures × all motor tasks). The identified mediating path from CSVD to thalamic atrophy to walking deficit would not survive such a strict correction. In addition, inferring causation from CSVD to thalamic decline to gait impairment should be done with caution due to the cross‐sectional nature of the comparison in this study. A convincing causal inference from mediation models requires temporal precedence from the dependent variable to the mediator to the outcome variable (MacKinnon, Fairchild, & Fritz, 2007). For each individual in our data set, it is rational to assume that the CSVD markers occurred before the MRI scanning, and the individual's motor assessment was then performed shortly after the MRI scan was completed. Therefore, a temporal precedence from CSVD to the acquisition of the brain measurements to motor assessment held for each participant, theoretically supporting a causal mediating path. However, the interval between the onset of CSVD and the acquisition of the brain measurements is uncertain across subjects, and the interval between the acquisition of the brain measurements and motor performance was very short, possibly confounding the causal inference. In the future, longitudinal data with appropriate statistical models (Gollob & Reichardt, 1991; Kraemer, Wilson, Fairburn, & Agras, 2002) is highly warranted to validate this mediating path and its causation (Kraemer, Stice, Kazdin, Offord, & Kupfer, 2001).
Finally, this study has a few limitations. We used a community‐dwelling cohort data set rather than a CSVD cohort or a case‐control data set, resulting in an unbalanced sample size between the CSVD and non‐CSVD groups, reflecting the actual occurrence of CSVD in community‐dwelling populations. Although a contributing role of this sample size unbalance to our findings can be largely ruled out by the illustrated scatter plots, our results would ideally be validated with a balanced dataset in the future. Finally, participants with cognitive disorders such as mild cognitive impairment or mild dementia were not excluded in our analysis, possibly contaminating our results to some degree.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
N.S., X.Y.L., Y.C.Z, and G.G drafted the manuscript and X.Y.L. conducted the statistical analyses. Z.F.F. managed the database. Y.C.Z., L.Y.C., S.Y.Z., and Z.Y.J. contributed to the conception and design of the study and interpretation of the data. G.G. provided expertise on brain imaging analysis. F.T. was the technical expert for motor performance acquisition. All authors gave final approval for the version of the manuscript submitted for publication, and agree to be accountable for the work. Y.C.Z. and G.G. were responsible for the study conception and interpretation of data and had final responsibility for the decision to submit for publication.
Su N, Liang X, Zhai F‐F, et al. The consequence of cerebral small vessel disease: Linking brain atrophy to motor impairment in the elderly. Hum Brain Mapp. 2018;39:4452–4461. 10.1002/hbm.24284
Ning Su, Xinyu Liang contributed equally to this study.
Funding information National Key Research and Development Program of China, Grant/Award Number: 2016YFB1001402, 2016YFC1300500‐5; National High Technology Research & Development Program of China, Grant/Award Number: 2015AA020506; National Natural Science Foundation of China, Grant/Award Number: 81671772, 81173663, 81671173; The 12th Five‐Year National Science and Technology Support Program, Grant/Award Number: 2012BAJ18B04‐3; the Fundamental Research Funds for the Central Universities
Contributor Information
Gaolang Gong, Email: gaolang.gong@bnu.edu.cn.
Yi‐Cheng Zhu, Email: zhuych910@163.com.
REFERENCES
- Appelman, A. P. , Exalto, L. G. , van der Graaf, Y. , Biessels, G. J. , Mali, W. P. , & Geerlings, M. I. (2009). White matter lesions and brain atrophy: More than shared risk factors? A systematic review. Cerebrovascular Diseases, 28(3), 227–242. 10.1159/000226774 [DOI] [PubMed] [Google Scholar]
- Aribisala, B. S. , Valdes Hernandez, M. C. , Royle, N. A. , Morris, Z. , Munoz Maniega, S. , Bastin, M. E. , … Wardlaw, J. M. (2013). Brain atrophy associations with white matter lesions in the ageing brain: The Lothian birth cohort 1936. European Radiology, 23(4), 1084–1092. 10.1007/s00330-012-2677-x [DOI] [PubMed] [Google Scholar]
- Bjorklund, E. , Lindberg, E. , Rundgren, M. , Cronberg, T. , Friberg, H. , & Englund, E. (2014). Ischaemic brain damage after cardiac arrest and induced hypothermia‐‐a systematic description of selective eosinophilic neuronal death. A neuropathologic study of 23 patients. Resuscitation, 85(4), 527–532. 10.1016/j.resuscitation.2013.11.022 [DOI] [PubMed] [Google Scholar]
- Bruijn, S. M. , Van Impe, A. , Duysens, J. , & Swinnen, S. P. (2014). White matter microstructural organization and gait stability in older adults. Frontiers in Aging Neuroscience, 6, 104 10.3389/fnagi.2014.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Laat, K. F. , Reid, A. T. , Grim, D. C. , Evans, A. C. , Kotter, R. , van Norden, A. G. , & de Leeuw, F. E. (2012). Cortical thickness is associated with gait disturbances in cerebral small vessel disease. NeuroImage, 59(2), 1478–1484. 10.1016/j.neuroimage.2011.08.005 [DOI] [PubMed] [Google Scholar]
- de Laat, K. F. , Tuladhar, A. M. , van Norden, A. G. , Norris, D. G. , Zwiers, M. P. , & de Leeuw, F. E. (2011). Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain, 134(Pt 1), 73–83. 10.1093/brain/awq343 [DOI] [PubMed] [Google Scholar]
- de Laat, K. F. , van Norden, A. G. , Gons, R. A. , van Oudheusden, L. J. , van Uden, I. W. , Bloem, B. R. , … de Leeuw, F. E. (2010). Gait in elderly with cerebral small vessel disease. Stroke, 41(8), 1652–1658. 10.1161/STROKEAHA.110.583229 [DOI] [PubMed] [Google Scholar]
- Dumurgier, J. , Crivello, F. , Mazoyer, B. , Ahmed, I. , Tavernier, B. , Grabli, D. , … Elbaz, A. (2012). MRI atrophy of the caudate nucleus and slower walking speed in the elderly. NeuroImage, 60(2), 871–878. 10.1016/j.neuroimage.2012.01.102 [DOI] [PubMed] [Google Scholar]
- Fazekas, F. , Chawluk, J. B. , Alavi, A. , Hurtig, H. I. , & Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. American Journal of Roentgenology, 149(2), 351–356. 10.2214/ajr.149.2.351 [DOI] [PubMed] [Google Scholar]
- Fling, B. W. , Walsh, C. M. , Bangert, A. S. , Reuter‐Lorenz, P. A. , Welsh, R. C. , & Seidler, R. D. (2011). Differential callosal contributions to bimanual control in young and older adults. Journal of Cognitive Neuroscience, 23(9), 2171–2185. 10.1162/jocn.2010.21600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiyama, H. , Van Soom, J. , Rens, G. , Gooijers, J. , Leunissen, I. , Levin, O. , & Swinnen, S. P. (2016). Age‐related changes in frontal network structural and functional connectivity in relation to bimanual movement control. The Journal of Neuroscience, 36(6), 1808–1822. 10.1523/JNEUROSCI.3355-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold, G. , Kovari, E. , Herrmann, F. R. , Canuto, A. , Hof, P. R. , Michel, J. P. , … Giannakopoulos, P. (2005). Cognitive consequences of thalamic, basal ganglia, and deep white matter lacunes in brain aging and dementia. Stroke, 36(6), 1184–1188. 10.1161/01.STR.0000166052.89772.b5 [DOI] [PubMed] [Google Scholar]
- Goldberg, J. H. , Farries, M. A. , & Fee, M. S. (2013). Basal ganglia output to the thalamus: Still a paradox. Trends in Neurosciences, 36(12), 695–705. 10.1016/j.tins.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollob, H. , & Reichardt, C. (1991). Interpreting and estimating indirect effects assuming time lags really matter In Collins L. & Horn J. (Eds.), Best methods for the analysis of change: Recent advances, unanswered questions, future directions (pp. 243–259). Washington, DC: American Psychological Association. [Google Scholar]
- Hayes, A. F. (2018). Introduction to mediation, moderation, and conditional process analysis: A regression‐based approach (2nd ed.). New York: Guilford Press. [Google Scholar]
- Houtchens, M. K. , Benedict, R. H. , Killiany, R. , Sharma, J. , Jaisani, Z. , Singh, B. , … Bakshi, R. (2007). Thalamic atrophy and cognition in multiple sclerosis. Neurology, 69(12), 1213–1223. 10.1212/01.wnl.0000276992.17011.b5 [DOI] [PubMed] [Google Scholar]
- Ikram, M. A. , Vernooij, M. W. , Vrooman, H. A. , Hofman, A. , & Breteler, M. M. (2009). Brain tissue volumes and small vessel disease in relation to the risk of mortality. Neurobiology of Aging, 30(3), 450–456. 10.1016/j.neurobiolaging.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Iseki, K. , Hanakawa, T. , Hashikawa, K. , Tomimoto, H. , Nankaku, M. , Yamauchi, H. , … Fukuyama, H. (2010). Gait disturbance associated with white matter changes: A gait analysis and blood flow study. NeuroImage, 49(2), 1659–1666. 10.1016/j.neuroimage.2009.09.023 [DOI] [PubMed] [Google Scholar]
- Kim, S. H. , Park, J. S. , Ahn, H. J. , Seo, S. W. , Lee, J. M. , Kim, S. T. , … Na, D. L. (2011). Voxel‐based analysis of diffusion tensor imaging in patients with subcortical vascular cognitive impairment: Correlates with cognitive and motor deficits. Journal of Neuroimaging, 21(4), 317–324. 10.1111/j.1552-6569.2010.00527.x [DOI] [PubMed] [Google Scholar]
- Kim, Y. J. , Kwon, H. K. , Lee, J. M. , Cho, H. , Kim, H. J. , Park, H. K. , … Seo, S. W. (2016). Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology, 86(13), 1199–1207. 10.1212/WNL.0000000000002516 [DOI] [PubMed] [Google Scholar]
- Kraemer, H. C. , Stice, E. , Kazdin, A. , Offord, D. , & Kupfer, D. (2001). How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. The American Journal of Psychiatry, 158, 848–856. [DOI] [PubMed] [Google Scholar]
- Kraemer, H. C. , Wilson, G. T. , Fairburn, C. G. , & Agras, W. S. (2002). Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry, 59(10), 877–883. [DOI] [PubMed] [Google Scholar]
- Lawrence, A. J. , Chung, A. W. , Morris, R. G. , Markus, H. S. , & Barrick, T. R. (2014). Structural network efficiency is associated with cognitive impairment in small‐vessel disease. Neurology, 83(4), 304–311. 10.1212/WNL.0000000000000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch, J. P. , & Evans, A. C. (2005). Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage, 24(1), 163–173. 10.1016/j.neuroimage.2004.07.045 [DOI] [PubMed] [Google Scholar]
- Linortner, P. , Fazekas, F. , Schmidt, R. , Ropele, S. , Pendl, B. , Petrovic, K. , … Enzinger, C. (2012). White matter hyperintensities alter functional organization of the motor system. Neurobiology of Aging, 33(1), 197 e191–197 e199, 197.e9. 10.1016/j.neurobiolaging.2010.06.005 [DOI] [PubMed] [Google Scholar]
- MacKinnon, D. P. , Fairchild, A. J. , & Fritz, M. S. (2007). Mediation analysis. Annual Review of Psychology, 58, 593–614. 10.1146/annurev.psych.58.110405.085542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlknecht, P. , Kiechl, S. , Bloem, B. R. , Willeit, J. , Scherfler, C. , Gasperi, A. , … Seppi, K. (2013). Prevalence and burden of gait disorders in elderly men and women aged 60‐97 years: A population‐based study. PLoS One, 8(7), e69627 10.1371/journal.pone.0069627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski, V. , Nilaweera, W. U. , Zelenin, P. V. , Sirota, M. G. , & Beloozerova, I. N. (2012). Signals from the ventrolateral thalamus to the motor cortex during locomotion. Journal of Neurophysiology, 107(1), 455–472. 10.1152/jn.01113.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlinski, V. , Sirota, M. G. , & Beloozerova, I. N. (2012). Differential gating of thalamocortical signals by reticular nucleus of thalamus during locomotion. The Journal of Neuroscience, 32(45), 15823–15836. 10.1523/JNEUROSCI.0782-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi, T. M. , Mei, S. S. , Liang, P. P. , Gao, L. L. , Li, K. C. , Wu, T. , & Chan, P. (2017). Altered resting‐state brain activity in Parkinson's disease patients with freezing of gait. Scientific Reports, 7(1), 16711 10.1038/s41598-017-16922-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motl, R. W. , Zivadinov, R. , Bergsland, N. , & Benedict, R. H. (2016). Thalamus volume and ambulation in multiple sclerosis: A cross‐sectional study. Neurodegenerative Disease Management, 6(1), 23–29. 10.2217/nmt.15.71 [DOI] [PubMed] [Google Scholar]
- Pantoni, L. (2010). Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurology, 9(7), 689–701. 10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- Patenaude, B. , Smith, S. M. , Kennedy, D. N. , & Jenkinson, M. (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage, 56(3), 907–922. 10.1016/j.neuroimage.2011.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petito, C. K. , Feldmann, E. , Pulsinelli, W. A. , & Plum, F. (1987). Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology, 37(8), 1281–1286. [DOI] [PubMed] [Google Scholar]
- Pinter, D. , Ritchie, S. J. , Doubal, F. , Gattringer, T. , Morris, Z. , Bastin, M. E. , … Wardlaw, J. (2017). Impact of small vessel disease in the brain on gait and balance. Scientific Reports, 7, 41637 10.1038/srep41637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsinelli, W. A. , Brierley, J. B. , & Plum, F. (1982). Temporal profile of neuronal damage in a model of transient forebrain ischemia. Annals of Neurology, 11(5), 491–498. 10.1002/ana.410110509 [DOI] [PubMed] [Google Scholar]
- Rosano, C. , Aizenstein, H. , Brach, J. , Longenberger, A. , Studenski, S. , & Newman, A. B. (2008). Special article: Gait measures indicate underlying focal gray matter atrophy in the brain of older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 63(12), 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano, C. , Aizenstein, H. J. , Studenski, S. , & Newman, A. B. (2007). A regions‐of‐interest volumetric analysis of mobility limitations in community‐dwelling older adults. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 62(9), 1048–1055. [DOI] [PubMed] [Google Scholar]
- Shumway‐Cook, A. , & Woollacott, M. H. (2015). Motor control: Translating research into clinical practice. South Holland, Netherlands: Wolters Kluwer. [Google Scholar]
- Smith, E. E. , O'Donnell, M. , Dagenais, G. , Lear, S. A. , Wielgosz, A. , Sharma, M. , … Investigators, P. (2015). Early cerebral small vessel disease and brain volume, cognition, and gait. Annals of Neurology, 77(2), 251–261. 10.1002/ana.24320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su, N. , Zhai, F. F. , Zhou, L. X. , Ni, J. , Yao, M. , Li, M. L. , … Zhu, Y. C. (2017). Cerebral small vessel disease burden is associated with motor performance of lower and upper extremities in community‐dwelling populations. Frontiers in Aging Neuroscience, 9, 313 10.3389/fnagi.2017.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakusaki, K. (2017). Functional Neuroanatomy for posture and gait control. Journal of Movement Disorders, 10(1), 1–17. 10.14802/jmd.16062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar, A. M. , Reid, A. T. , Shumskaya, E. , de Laat, K. F. , van Norden, A. G. , van Dijk, E. J. , … de Leeuw, F. E. (2015). Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke, 46(2), 425–432. 10.1161/STROKEAHA.114.007146 [DOI] [PubMed] [Google Scholar]
- Valeri, L. , & Vanderweele, T. J. (2013). Mediation analysis allowing for exposure‐mediator interactions and causal interpretation: Theoretical assumptions and implementation with SAS and SPSS macros. Psychological Methods, 18(2), 137–150. 10.1037/a0031034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Holst, H. M. , van Uden, I. W. , Tuladhar, A. M. , de Laat, K. F. , van Norden, A. G. , Norris, D. G. , … de Leeuw, F. E. (2016). Factors associated with 8‐year mortality in older patients with cerebral small vessel disease: The Radboud University Nijmegen diffusion tensor and magnetic resonance cohort (RUN DMC) study. JAMA Neurology, 73(4), 402–409. 10.1001/jamaneurol.2015.4560 [DOI] [PubMed] [Google Scholar]
- Wardlaw, J. M. , Smith, E. E. , Biessels, G. J. , Cordonnier, C. , Fazekas, F. , Frayne, R. , … STandards for ReportIng Vascular changes on nEuroimaging (STRIVE v1) . (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurology, 12(8), 822–838. 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley, K. J. (2008). SurfStat: A Matlab toolbox for the statistical analysis of univariate and multivariate surface and volumetric data using linear mixed effects models and random field theory. Retrieved from http://www.math.mcgill.ca/keith/surfstat/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


