Abstract
Abstinence self-efficacy, coping skills, and therapeutic alliance are hypothesized mechanisms of behavioral change (MOBCs) in cognitive-behavioral therapy (CBT) for alcohol use disorder (AUD). However, little is known about when these hypothesized MOBCs change during treatment or in relation to the initiation of abstinence from alcohol, which the current study investigated. Patient-reported abstinence self-efficacy, drinking-related coping skills, and therapeutic alliance were measured at every session throughout a 12-session clinical trial that previously showed equivalent drinking reductions in female-specific individual- and group-based CBT for AUD. Participants (N=121 women) were classified into subgroups based on whether and when they first initiated 14 days of continuous abstinence from alcohol during treatment. Interrupted time-series analyses evaluated the magnitude and timing of change in MOBC variables in relation to the initiation of abstinence. All three MOBC measures showed gradual improvements throughout treatment (within-subjects d=0.03 to 0.09 change per week). Participants who initiated abstinence during treatment experienced additional sudden improvements in abstinence self-efficacy (d=0.47) and coping skills (d=0.27), but not therapeutic alliance (d=−0.02), the same week they initiated abstinence. Participants who were already abstinent when treatment started maintained higher abstinence self-efficacy and coping skills, but not therapeutic alliance, throughout treatment compared to participants who never initiated abstinence. Initiating abstinence may help facilitate improvements in abstinence self-efficacy and drinking-related coping skills. Clinicians may help patients anticipate when and how much these variables are expected to improve during treatment and encourage initiation of abstinence to potentially help facilitate improvements in abstinence self-efficacy and coping skills.
Keywords: alcohol use disorder treatment, cognitive-behavioral therapy, mechanism of behavior change, process of change
Introduction
Nearly 13.9% of US adults meet criteria for alcohol use disorder (AUD) within a one-year period (Grant et al., 2015) and nearly 15 million receive AUD treatment annually (Substance Abuse and Mental Health Services Administration, 2016). Cognitive-behavioral therapy (CBT) is consistently identified as one of the most efficacious treatments for AUD (Magill & Ray, 2009), and there is interest in understanding the treatment’s specific mechanisms of behavioral change (MOBCs) to help make it more efficacious, efficient, and personalized (Huebner & Tonigan, 2007; Magill et al., 2015; Morgenstern & Longabaugh, 2000).
CBT models posit that drinking largely is maintained by deficits in coping skills and abstinence self-efficacy (i.e., one’s self-perceived ability to handle high-risk situations without drinking). Improving abstinence self-efficacy and coping skills is seen as a critical treatment goal (Mastroleo & Monti, 2013; Morgenstern & Longabaugh, 2000; Rotgers, 2012), and CBT treatments typically aim to improve these areas through techniques that help patients learn to identify and change drinking-related triggers, thoughts, behaviors, and consequences (Epstein & McCrady, 2009; Rotgers, 2012). Empirical research has supported the hypothesis that abstinence self-efficacy and coping skills are potential MOBCs in CBT (Acosta et al., 2017; Hendricks, Delucchi, & Hall, 2010; Kiluk, Nich, Babuscio, & Carroll, 2010; Roos, Maisto, & Witkiewitz, 2017; Vedel, Emmelkamp, & Schippers, 2008; Witkiewitz, Roos, Tofighi, & Van Horn, 2018) and in non-CBT-based treatments that focus on interpersonal relationships, social network support, or twelve-step program involvement (Litt, Kadden, Cooney, & Kabela, 2003; Litt, Kadden, & Tennen, 2018; Morgenstern, Labouvie, McCrady, Kahler, & Frey, 1997).
Therapeutic alliance also has been described as necessary for facilitating collaborative engagement in CBT (Epstein & McCrady, 2009; Mastroleo & Monti, 2013; Rotgers, 2012), and its association with drinking and drug use outcomes has led to its increased investigation as a potential MOBC (Connors, Carroll, DiClemente, Longabaugh, & Donovan, 1997; Connors et al., 2016; Meier, Barrowclough, & Donmall, 2005; Prince, Connors, Maisto, & Dearing, 2016). In contrast to predicting distal post-treatment outcomes, therapeutic alliance often is a stronger predictor of proximal, within-treatment substance use (Meier et al., 2005). One study of patients receiving 12-week CBT for AUD found that session-level therapeutic alliance during treatment predicted next-week reductions in drinking, but not vice versa (Connors et al., 2016), suggesting that the association between alliance and reductions in drinking may be unidirectional.
The predictive value of these hypothesized MOBCs – abstinence self-efficacy, coping skills, and therapeutic alliance – suggests that clinicians and patients may benefit from targeting these MOBCs in AUD treatment, including, for example, by collaboratively framing these MOBCs as pertinent treatment targets, routinely monitoring these MOBCs to evaluate treatment progress, and utilizing therapeutic interventions and processes that are likely to lead to improvement in these MOBCs. However, there is limited research that can help clinicians and patients more precisely understand the expected the timing and magnitude with which these MOBC variables are likely to change during treatment (Hallgren, Wilson, & Witkiewitz, 2018), including in relation to key events, such as the initiation of abstinence. In contrast, many previous studies have evaluated these MOBC variables at a single time point (e.g., mid-treatment or end-of-treatment) to predict distal treatment outcomes (e.g., drinking over several months or years after treatment), which does not provide information about how, when, or how much these MOBC variables change during treatment.
This knowledge gap limits the clinical impact of MOBC research. For example, even though hypothesized MOBC variables often are viewed as clinically important, there is limited clinical guidance about which treatment processes or patient behaviors facilitate improvement in these variables (Hallgren, Wilson, & Witkiewitz, 2018). Despite having evidence that these MOBCs may be worthwhile treatment targets, clinicians have limited empirically-based information to provide to patients about when, and how much, and in response to what events they may expect these treatment targets to improve.
Previous research has also tended to model drinking as a consequence of changes in MOBCs, with less consideration of how MOBCs are affected by changes in drinking. This may unintentionally lead to overly simplistic theoretical models of AUD treatment, wherein MOBC variables are presumed to impact drinking unidirectionally, without drinking having a reciprocal impact on MOBC variables. Moreover, CBT models explicitly posit that drinking-related triggers, cognitions, behaviors, and consequences are reciprocally determinate (Mastroleo & Monti, 2013), such that the initiation of abstinence from alcohol may be expected to facilitate improvements in MOBC variables that, in turn, help sustain longer-term abstinence. For example, it is plausible that abstaining from alcohol, especially when exposed to cues that would typically trigger a drinking response, could strengthen a person’s ability to use non-drinking coping skills and enhance their self-efficacy for continuing to avoid drinking in similar situations, and those changes in turn could support longer-term abstinence (Epstein & McCrady, 2009). Under the same premise, continued drinking could inhibit one’s opportunity to improve their non-drinking coping skills and abstinence self-efficacy, which in turn could increase their likelihood of longer-term heavy drinking.
In the present study, we aimed to quantify the magnitude and timing of change in repeated within-treatment measures of abstinence self-efficacy, coping skills, and therapeutic alliance and evaluate the timing and magnitude of changes in these MOBC variables throughout the course of treatment and in relation to initiating abstinence from alcohol. This work builds on previous research demonstrating that alcohol craving, another hypothesized MOBC, was reduced significantly in correspondence to the timing of initiating abstinence from alcohol (Hallgren et al., 2016). This effect was observed in two independent samples of men and women receiving CBT-based AUD treatment (Hallgren et al., 2016), and was replicated in a third sample that received pharmacotherapy treatments (prazosin or placebo) with no behavioral intervention (Hallgren, Delker, & Simpson, 2018). All three samples also had significant between-subjects effects wherein patients who entered treatment having already initiated abstinence reported lower levels of craving during treatment than patients who never initiated abstinence during treatment, which was also consistent with the hypothesis that initiating abstinence could facilitate reductions in craving. The latter study (Hallgren, Delker, & Simpson, 2018) also found that initiating abstinence was not associated with sudden reductions in negative affect, although negative affect gradually decreased over time in the weeks after abstinence initiation. In the present study, we aimed to test similar models to evaluate whether patients experienced immediate and/or gradual reductions in abstinence self-efficacy, coping, and therapeutic alliance before and after the initiation of abstinence during CBT for AUD.
We hypothesized that all three MOBC measures would demonstrate significant within- and between-subjects effects that were consistent with the hypothesis that initiating abstinence may help enhance self-efficacy, coping skills, and therapeutic alliance. Specifically, we hypothesized that abstinence initiators would show sudden, within-person improvements in these three MOBC variables when they transitioned from drinking to abstinence, controlling for the gradual week-by-week improvements that we also hypothesized would be present over the course of treatment (both before and after initiating abstinence). We also hypothesized between-group differences, such that participants who initiated abstinence prior to entering treatment would have significantly better MOBC outcomes throughout the course of treatment compared to participants who never initiated abstinence.
Material and Methods
Participants
This study is a secondary analysis of data from a randomized controlled trial comparing group versus individual cognitive-behavioral therapy (CBT) for alcohol use disorder (Epstein et al., 2018). Participants in both treatment conditions were recruited through advertisements, flyers, referral outreach, and media, and received up to 12 sessions of manual-guided, female-specific CBT for AUD. Both treatments were abstinence-based and participants agreed to an explicit treatment goal of abstinence from alcohol. Both treatments included psychoeducation, motivational enhancement, and coping skills training with behavioral rehearsal. Ratings of treatment fidelity and satisfaction were high (see Epstein et al., 2018). Full details about the parent clinical trial are available in Epstein et al. (2018); in brief, participants in both treatment conditions had significant and equivalent reductions in drinking that were maintained over a 12-month post-treatment period and there were no significant differences during treatment in the MOBC variables that are examined in the current paper.
Inclusion criteria included being female, at least 18 years old, having past-year DSM-IV alcohol dependence (American Psychiatric Association, 2000), alcohol use within 60 days prior to telephone screening, no psychotic symptoms in the past six months, no gross cognitive impairment, and no current physiological dependence on any illicit drug. All participants provided written informed consent to participate in the clinical trial. All procedures were conducted in compliance with the Rutgers University Institutional Review Board.
Among the 138 participants who attended at least one treatment session in the parent study, a total of N=121 had enough daily drinking data to assess an abstinence status over 14 or more consecutive days and were included in subsequent analyses. Following identical procedures as previous studies (Hallgren et al., 2016; Hallgren, Delker, & Simpson, 2018), these 121 participants were classified into one of three groups in preparation for longitudinal data analyses based on whether and when they first initiated 14 consecutive days of abstinence during treatment. Abstinence initiators (n=60) drank on at least one of the first 14 days of treatment and then eventually obtained at least 14 consecutive days of abstinence during treatment. Already abstainers (n=24) started the first 14 days treatment with continuous abstinence. Continued drinkers (n=37) drank during the first 14 days of treatment and never obtained 14 consecutive days of abstinence during treatment. This 14-day window for classifying abstinence status was informed by previous research suggesting a low likelihood of 14 or more consecutive days of abstinence between drinking days before alcohol treatment, suggesting that achieving 14 days of continuous abstinence likely reflects a noteworthy and intentional behavioral change (Epstein, Labouvie, McCrady, Swingle, & Wern, 2004; Hallgren, Delker, & Simpson, 2016, 2018). The proportions of participants who were classified as abstinence initiators, already abstainers, or continued drinkers were comparable to previous studies that used identical categorizations (Hallgren et al., 2016; Hallgren, Delker, & Simpson, 2018) and align with research indicating that many individuals significantly reduce their drinking prior to and/or during treatment (Epstein et al., 2005; Stasiewicz et al., 2013).
Drinking statuses within a given 14-day window were considered unknown if data were not available for at least 70% of days within that window; however, participants were classified as drinking if any drinking days were observed within a 14-day window even if <70% of daily data were available. Participants who resumed drinking beyond the first 14 days of abstinence were not reassigned to different subgroups, but of note, the percentage of days abstinent (PDA) was high after the first 14 days of abstinence for both abstinence initiators (M=94.94, SD=7.07) and already abstainers (M=99.32, SD=2.19). Already abstainers reported a mean of 10.25 days of continuous abstinence (SD=11.69) prior to baseline interviews.
Measures
Baseline drinking and AUD criteria.
Participants completed Timeline Followback (TLFB) interviews (Sobell & Sobell, 2003), a reliable and valid method for evaluating daily drinking and drug use (Carey, 1997), to assess drinking over the 90 days prior to the last drinking day before the baseline interview. The percentage of days abstinent (PDA) was calculated using these data for descriptive analyses. The Structured Clinical Interview for DSM-IV Disorders (First, Spitzer, Gibbon, & Williams, 2002) was used at baseline to diagnose alcohol and other substance dependence or abuse. There was a median gap of 25 days between baseline assessment and the first attended treatment session.
Within-treatment daily drinking.
Patients were instructed to keep daily drinking logs throughout treatment and record any drinking, drug use, and drinking urges each day during treatment. Daily drinking logs were then reviewed at each treatment session and used to quantify within-treatment drinking. Missing within-treatment drinking data were filled in using Timeline Followback data collected during post-treatment follow-up interviews, when possible. Daily drinking log and Timeline Followback data have been shown to provide similar, reliable estimates of drinking behavior (McCrady, Epstein, & Hirsch, 1999).
Within-treatment weekly MOBC measures.
Abstinence self-efficacy and coping were assessed at baseline using validated instruments and were then re-assessed prior to each attended treatment session, resulting in up to 12 repeated within-treatment measures per participant. Weekly MOBCs were assessed using abbreviated versions of the baseline instruments to reduce participant burden. Specific items in the abbreviated measures were selected based on factor loadings and internal consistency indices from full measures with baseline data from a prior study of women in AUD treatment (McCrady, Epstein, Hallgren, Cook, & Jensen, 2016; see Epstein et al., 2018 for details).
Abstinence self-efficacy was measured using the Situational Confidence Questionnaire-8 (Breslin, Sobell, Sobell, & Agrawal, 2000), which had high reliability in the current study (alpha=0.86). The measure has been validated in outpatient AUD treatment (Breslin et al., 2000) and shown to yield similar information to the original 100-item Situational Confidence Questionnaire. The eight-item scale was used at baseline and an abbreviated five-item version of the scale was used within-treatment. The measure asks participants to rate their confidence in avoiding drinking across different situations that potentially pose a high risk for drinking (e.g., when experiencing urges or temptations, unpleasant emotions, or social pressures) on a scale of 0% to 100%. Item responses are averaged to produce scores with a possible range of 0 to 100.
Coping strategies were assessed using the Coping Strategies Scale (Litt, Kadden, Cooney, & Kabela, 2003), which had high reliability in the current study (alpha=0.95) and has been validated in outpatient AUD treatment samples (Litt et al., 2003). The full 59-item scale was used at baseline and an abbreviated 30 item-scale was used within treatment. The measure asks participants to rate the frequency with which they used different coping strategies (e.g., “ask people not to offer me drinks” or “just wait and know that the urge to drink will go away”) on a scale of 1 (never) to 4 (frequently) that were averaged, resulting in scores with a possible range of 1 to 4.
For the abstinence self-efficacy and coping skills measures, full-instrument scores were estimated from the shortened instruments, resulting in estimated scores that closely mapped onto the scales of the full instruments. This was done to facilitate more direct comparisons between the brief- and full-instrument scores and to make the brief-instrument scores more interpretable in clinical settings where the full instruments may be used. Specifically, multiple regression prediction models were constructed using baseline data with full-instrument total scores predicted by the subset of items included in the shortened measure, then the resulting regression equations were used to estimate full-instrument total scores from the shortened measures collected at each treatment session. Correlations between model-estimated scores and full-measure scores were high for the baseline data (i.e., the data used to generate the prediction model; r=0.97 and 0.99 for abstinence self-efficacy and coping skills, respectively; normalized root mean square deviations [RMSE]=0.06 and 0.03) and also were high when cross-validated on 3-month outcome data (i.e., new data that were not used to generate the prediction model; r=0.98 and 0.98, normalized RMSE=0.05 and 0.05), suggesting that this approach likely yielded a high degree of accuracy for predicting full-instrument total scores for the weekly within-treatment data.
Therapeutic alliance was measured after each treatment session using the Working Alliance Inventory-Short Form (Tracey & Kokotovic, 1989). The measure includes 12 items rated by patients using a 7-point scale (e.g., “The therapist and I agree about things I will need to do in counseling to help improve my situation”). Each item reflects one of three factors – task agreement, goal agreement, or therapeutic bond – which can be summed to create overall working alliance scores that ranging from 12 to 84. The measure has been shown to have nearly identical reliability and validity indices as the longer Working Alliance Inventory (Busseri & Tyler, 2003) and had high reliability in the current study (alpha=0.85).
Data Analysis
Data analyses aimed to identify systematic changes in within-treatment abstinence self-efficacy, coping skills, and therapeutic alliance before and after initiating abstinence from alcohol. We evaluated these changes separately for each of the three MOBC variables and stratified analyses separately for each of the three drinking subgroups. For abstinence initiators, we evaluated both gradual and sudden changes in each MOBC variable before and after the initiation of abstinence using an interrupted time series approach. Interrupted time-series models are a type of longitudinal model that can address the plausibility of potential causal effects when random assignment to the conditions of interest (e.g., abstinence versus continued drinking) is not possible (Kontopantelis, Doran, Springate, Buchan, & Reeves, 2015). The interrupted time-series approach was used only for patients who initiated abstinence during treatment, for whom pre- and post-abstinence changes in weekly MOBC variables could be evaluated. Already abstainers and continued drinkers did not initiate abstinence during treatment when weekly MOBC measures were available, and the interrupted time-series approach therefore could not be used to evaluate change associated with initiating abstinence for these subgroups. Instead, for these subgroups, we quantified weekly rates of change using growth curve trajectory analyses and compared the two groups in terms of their overall levels and rates of change in MOBC measures during treatment.
We evaluated interrupted time-series and linear trajectory models within a growth curve modeling framework using the R multilevel modeling package lme4 (Bates, Mächler, Bolker, & Walker, 2015). Time variables predicting gradual change were scaled such that 1 unit of time corresponded with one week and were centered such that the time was equal to 0 on the first week that abstinence was initiated (for abstinence initiators) or the first session of treatment (for already abstainers and continued drinkers). An additional time variable for modeling sudden change was coded with a value of 0 for all weeks prior to initiating abstinence and 1 for all weeks after initiating abstinence (only applicable to abstinence initiator subgroup). This resulted in growth curve model coefficients that could be interpreted as reflecting the mean rates of gradual change per week (for gradual change coefficients) and the mean amount of sudden change associated with the transition from drinking to abstinence above and beyond the expected trajectory already accounted for by the pre-abstinence gradual-change trajectory1. The resulting unstandardized model coefficients conveyed effect sizes for gradual and sudden change that mapped onto the original scales of each corresponding measure. A second effect size was computed by dividing each effect by the pooled, between-subjects standard deviation of its respective measure at baseline, resulting in a Cohen’s d-like coefficient2 that reflected the expected gradual change per week or sudden change expressed in standard deviation units.
Change-over-time coefficients were specified as both fixed and random effects, allowing the model to evaluate mean rates of change over time (fixed effect) while allowing the model to consider those rates of change as heterogeneous between individuals (random effects). Models were fit using a Gaussian (normal) link function with restricted maximum likelihood to account for missing data. For abstinence initiators, models included all available data from 4 weeks prior to initiating abstinence to 9 weeks after initiating abstinence, beyond which the majority of participants were not in treatment or providing weekly measures. For already abstainers and continued drinkers, models included all available data from the date of the first treatment session to eleven weeks later for the same reason3.
Results
Baseline Descriptive Statistics
Descriptive statistics for baseline demographics, PDA, and MOBC variables are presented in Table 1. All participants were female and were just under 50 years old on average. Most participants were White non-Hispanic and married/partnered, with just over half employed. The three drinking subgroups did not differ significantly on demographic variables.
Table 1.
Participant Demographics, PDA, and Baseline MOBC Variables
| Full Sample N = 121 |
Abstinence Initiators n = 60 |
Already Abstainers n = 24 |
Continued Drinkers n = 37 |
|||||
|---|---|---|---|---|---|---|---|---|
| M | (SD) | M | (SD) | M | (SD) | M | (SD) | |
| 49.60 | (11.28) | 50.52 | (11.03) | 47.92 | (11.89) | 49.22 | (11.46) | |
| White non-Hispanic, n (%) | 99 | (81.8%) | 47 | (78.3%) | 22 | (91.7%) | 30 | (81.1%) |
| Married/Partnered, n (%) | 74 | (61.2%) | 39 | (65.0%) | 13 | (54.2%) | 22 | (59.5%) |
| Employed, n (%) | 70 | (57.9%) | 36 | (60.0%) | 11 | (45.8%) | 23 | (62.2%) |
| Baseline PDA | 33.07 | (29.51) | 29.80 | (28.48)a | 57.32 | (29.13)ac | 22.65 | (22.57)c |
| Within-Treatment PDA | 75.03 | (25.19) | 82.41 | (13.86)ab | 99.01 | (2.80)ac | 47.49 | (23.28)bc |
| Number of sessions completed | 9.72 | (2.73) | 10.37 | (2.36)b | 9.83 | (2.97) | 8.59 | (2.84)b |
| Abstinence Self-Efficacy | 48.45 | (23.34) | 43.60 | (21.01)a | 63.41 | (20.46)ac | 46.63 | (25.14)c |
| Coping Skills | 2.51 | (0.47) | 2.47 | (0.42)a | 2.89 | (0.49)ac | 2.34 | (0.39)c |
Note. PDA = percentage of days abstinent. Married/Partnered includes being married, living as married, or in a committed relationship. Identical superscript letters indicate significant differences between groups (via t-test or Fisher exact test).
At baseline, participants were abstinent on 33.07% of all days, and PDA increased such that participants were abstinent on 75.03% of all days during treatment. As expected, there were significant differences in baseline and within-treatment PDA among the three drinking subgroups, with already-abstainers having significantly higher baseline and within-treatment PDA than abstinence initiators and continued drinkers who, by definition, were still drinking at the start of treatment. For the full sample, baseline abstinence self-efficacy was rated near the midpoint of the 0–100% confidence scale and coping skills were rated just above the midpoint of the 1–4 scale; ratings for baseline abstinence self-efficacy and coping skills were higher for already abstainers compared to abstinence initiators and continued drinkers (Table 1).
Changes in Within-Treatment MOBCs
Abstinence self-efficacy.
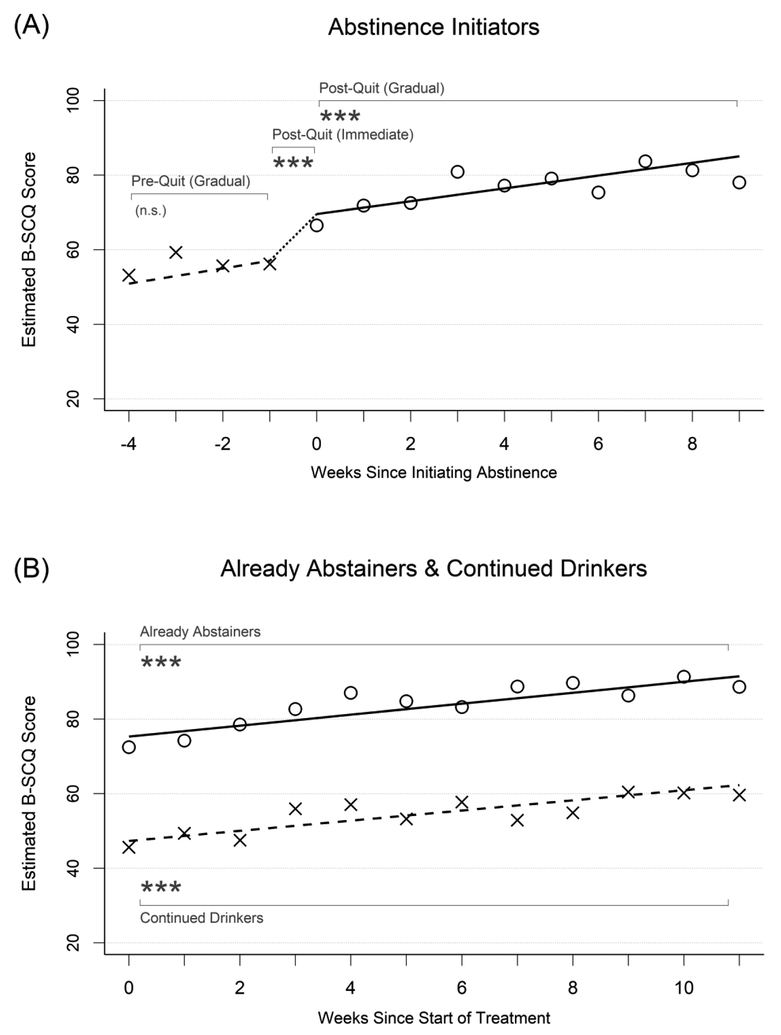
Results from growth-curve models evaluating changes in within-treatment MOBC measures are presented in Table 2, with abstinence self-efficacy results presented under the first major column heading and the three drinking subgroups presented under the major row headings. The values in each row indicate growth curve model parameter estimates for each drinking subgroup, and significant effects for change over time are displayed in bold font. The model-implied growth curves also are displayed as trajectory lines in Figures 1a and 1b with overlaid X and O symbols that show the weekly observed means of abstinence self-efficacy before and after initiating abstinence, respectively.
Table 2.
Changes in Weekly MOBC Measures
| Abstinence Self-Efficacy | Coping Skills | Therapeutic Alliance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est. | (SE) | p | d | Est. | (SE) | p | d | Est. | (SE) | p | d | |
| Abstinence Initiators | ||||||||||||
| Intercept | 59.07 | (2.82) | <.001 | 2.75 | (0.06) | <.001 | 77.90 | (1.11) | <.001 | |||
| Pre-Abstinence Change (Gradual) | 2.04 | (1.02) | .051 | 0.09 | 0.04 | (0.02) | .037 | 0.09 | 0.62 | (0.29) | .037 | 0.07 |
| Post-Abstinence Change (Immediate) | 10.53 | (2.75) | <.001 | 0.47 | 0.12 | (0.05) | .028 | 0.27 | −0.20 | (0.67) | .770 | −0.02 |
| Post-Abstinence Change (Gradual) | 1.72 | (0.39) | <.001 | 0.08 | 0.04 | (0.01) | <.001 | 0.08 | 0.28 | (0.07) | <.001 | 0.03 |
| Already Abstainers | ||||||||||||
| Intercept | 75.34 | (2.09) | <.001 | 3.09 | (0.05) | <.001 | 76.54 | (1.62) | <.001 | |||
| Change over time (Gradual) | 1.47 | (0.36) | <.001 | 0.07 | 0.02 | (0.005) | <.001 | 0.05 | 0.34 | (0.11) | .007 | 0.04 |
| Continued Drinkers | ||||||||||||
| Intercept | 47.35 | (1.80) | <.001 | 2.44 | (0.04) | <.001 | 73.45 | (1.49) | <.001 | |||
| Change over time (Gradual) | 1.36 | (0.35) | <.001 | 0.06 | 0.04 | (0.01) | <.001 | 0.08 | 0.38 | (0.12) | .004 | 0.04 |
Note. Estimates reflect fixed effect coefficients estimated in separate multilevel models. Significant effects for change over time (i.e., non-intercept terms) are in bold font.
Figure 1.
Changes in abstinence self-efficacy in relation to initiating abstinence. X symbols and dashed lines represent the observed weekly mean values and fitted trajectories of change, respectively, prior to initiating abstinence for abstinence initiators (left plots) and for continued drinkers (right plots) – i.e., outcomes during periods of continued drinking. O symbols and solid lines represent the observed weekly mean values and fitted trajectories of change, respectively, after initiating abstinence for abstinence initiators (left plots) and for already abstainers (right plots) – i.e., outcomes after abstinence initiation. Asterisks indicate the significance of within-person change over time. B-SCQ = Brief Situational Confidence Questionnaire.
Abstinence initiators (Figure 1a) had model-implied mean self-efficacy ratings of 50.93 four weeks before initiating abstinence, which rose non-significantly (d=0.09 SD units per week, p=.051) to 57.03 the week prior to initiating abstinence. During the week that these participants initiated abstinence, they experienced additional increases in abstinence self-efficacy that averaged 10.53 points (d=0.47, p<.001) above and beyond the amount accounted for by the gradual change effect, moving to a model-implied mean value of 69.60. Self-efficacy then continued to increase over time after this by a mean of 1.72 points per week (d=0.08, p<.001) to a mean score of 85.06 nine weeks later.
In contrast, already abstainers (Figure 1b) had high levels of abstinence self-efficacy throughout treatment, starting with a mean rating of 75.34 and increasing significantly by a mean of 1.47 points per week (d=0.07, p<.001) to a mean rating of 91.49 eleven weeks later. Continued drinkers (Figure 1b) had lower abstinence self-efficacy, with initial mean ratings of 47.35, which also increased over the course of treatment by a mean of 1.36 points per week (d=0.06, p<.001) to a mean rating of 62.32 eleven weeks later. Overall levels of abstinence self-efficacy differed significantly between these two subgroups throughout the course of treatment (difference=−27.92, SE=4.24, p<.001, d=−1.25), but their rates of change per week were not significantly different (difference=−0.12, SE=0.50, p=.82, d=−.01).
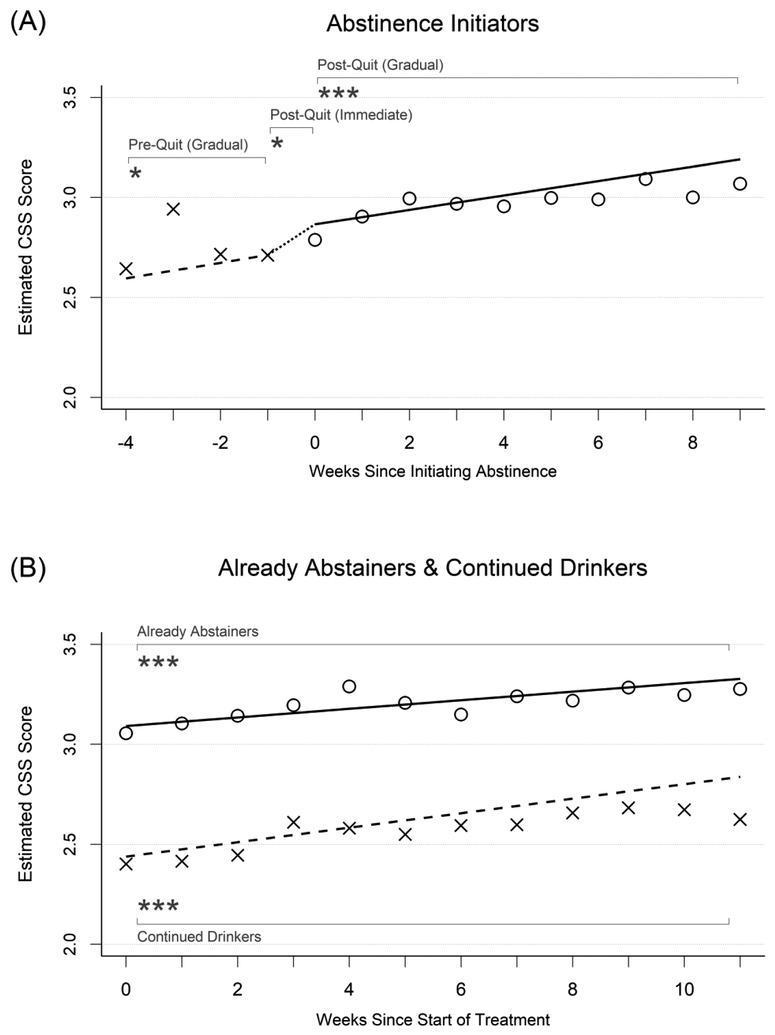
Coping skills.
Growth curve model results for coping skills are presented under the second major column heading in Table 2. Model-implied growth curves and weekly observed means of coping skill measures also are displayed in Figures 2a and 2b. Abstinence initiators (Figure 2a) had model-implied mean coping skill ratings of 2.60 four weeks before initiating abstinence, which rose significantly (p=.037, d=0.09) to 2.71 the week prior to initiating abstinence. During the week that these participants initiated abstinence, they experienced additional increases of coping skills that averaged 0.12 points (p=.028, d=0.27) above and beyond the amount accounted for by the gradual change effect, moving to a model-implied mean value of 2.87 the first week after initiating abstinence. Coping skills then continued to increase significantly over time after this by a mean of 0.04 points (p<.001, d=0.08) per week to a mean score of 3.19 nine weeks later.
Figure 2.
Changes in coping skills in relation to initiating abstinence. See Figure 1 for detailed description.
In contrast, already abstainers (Figure 2b) had high levels of coping skills throughout treatment, starting with a mean rating of 3.09 and increasing by a mean of 0.02 points per week (p<.001, d=0.05) to a mean rating of 3.33 eleven weeks later. Continued drinkers (Figure 2b) had lower coping skills, with initial mean ratings of 2.44, which also increased over the course of treatment by a mean of 0.04 points per week (p<.001, d=0.08) to a mean rating of 2.84 eleven weeks later. Overall levels of coping skills differed significantly between these two subgroups throughout the course of treatment (difference=−0.66, SE=0.10, p<.001, d=−1.53), but their rates of change per week were not significantly different (difference=0.01, SE=0.01, p=.14, d=0.02).
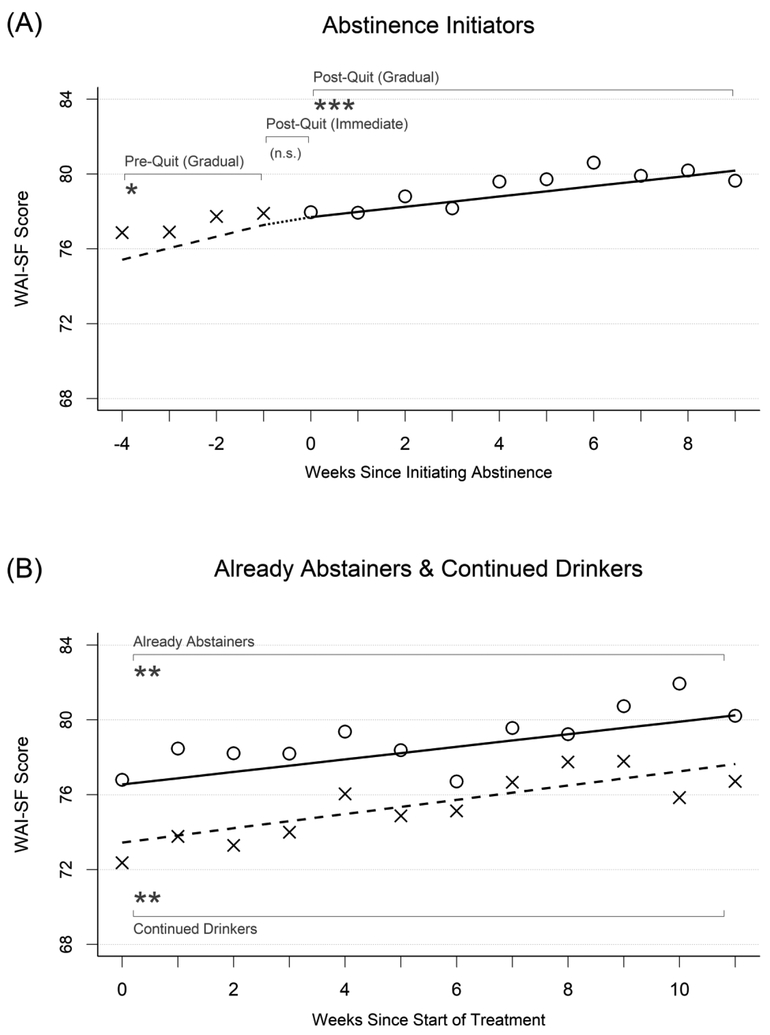
Therapeutic alliance.
Growth curve model results for therapeutic alliance are presented under the third major column heading in Table 2. Model-implied growth curves and weekly mean values of therapeutic alliance also are displayed in Figures 3a and 3b. Abstinence initiators (Figure 3a) started treatment with high therapeutic alliance, with a model-implied mean rating of 75.42 four weeks before initiating abstinence. Therapeutic alliance then increased significantly by 0.62 points per week (p=.037, d=0.07) to 77.28 the week prior to initiating abstinence. During the week that these participants initiated abstinence, they did not experience significant, sudden changes in therapeutic alliance (p=.770, d=−0.02) above and beyond the increase that was already accounted for by the gradual change effect. Therapeutic alliance continued to increase over time after abstinence was initiated by a mean of 0.28 points per week (p < .001, d=0.03) to a mean score of 80.19 nine weeks later.
Figure 3.
Changes in therapeutic alliance in relation to initiating abstinence. See Figure 1 for detailed description.
Already abstainers (Figure 3b) also started treatment with high therapeutic alliance, with a model-implied mean rating of 76.54 the first week of treatment that increased significantly over time by 0.34 points per week (p=.007, d=0.04) to a mean of 80.25 eleven weeks later. Continued drinkers (Figure 3b) also started treatment with high therapeutic alliance, with a model-implied mean rating of 73.45 the first week of treatment which also increased significantly over the course of treatment by 0.38 points per week (p=.004, d=0.04) to mean of 77.64 eleven weeks after that. Although the overall levels of therapeutic alliance differed nominally between already abstainers and continued drinkers, these differences were not significant in terms of the overall levels of therapeutic alliance at the start of treatment (difference=−3.12, SE=2.26, p=.17, d=−0.36), nor in terms of their rates of change over time (difference=0.05, SE=0.17, p=.79, d=0.01).
Discussion
Summary of Findings
Abstinence self-efficacy, coping skills, and therapeutic alliance frequently predict post-treatment substance use outcomes (Kadden & Litt, 2011; Roos & Witkiewitz, 2017). In the present study, we provide further information about the timing and magnitudes of weekly changes in these variables during treatment and evaluate how they change in relation to initiating abstinence from alcohol. Through a series of within- and between-subjects analyses, we found evidence that abstinence self-efficacy and coping skills, but not therapeutic alliance, are likely to show relatively sudden improvements during the week that abstinence is initiated, above and beyond the amount of gradual improvement observed during other weeks of treatment. The magnitudes of these effects were non-trivial; for example, abstinence self-efficacy improved by over 10 points (original scale range = 0 to 100) during the week that abstinence was initiated, which corresponded with an increase of almost half of a standard deviation unit over the baseline scores. Coping skills also had significant, albeit smaller sudden improvements, increasing by 0.12 points (original scale range = 1 to 4) during the week that abstinence was initiated, or approximately one-fourth of a standard deviation of the scores at baseline. Consistent with these within-person effects, we also found between-group effects showing that patients who were already abstinent at the start of treatment had higher abstinence self-efficacy and coping skills throughout the course of treatment compared to people who continue to drink. This study extends previous research showing that alcohol craving (but not negative affect) followed a similar pattern of sudden improvement when patients in AUD treatment initiated abstinence (Hallgren et al., 2016; Hallgren, Delker, & Simpson, 2018). To our knowledge, this is the first study to evaluate the potential impact of initiating abstinence on these hypothesized MOBCs.
Theoretical Implications
The longitudinal models in the present study quantified the rates of change in three hypothesized MOBCs before after, and during the week of initiating abstinence from alcohol, providing a closer examination of how initiating abstinence could potentially impact changes in these MOBCs. Although randomization to abstinence (versus continued drinking) was not possible, which limits our ability to make firm conclusions about causality, our longitudinal models nonetheless can shed light on a potential causal relationship between initiating abstinence and changes in hypothesized MOBCs. Evidence from the present study is consistent with CBT model hypotheses, namely that behavioral changes (e.g., quitting drinking) can drive changes in self-perception (e.g., abstinence self-efficacy) and facilitate opportunities to use and reinforce alcohol-related coping skills (Epstein & McCrady, 2009; Mastroleo & Monti, 2013). Although CBT models posit that situations, cognitions, emotions, behaviors, and consequences are reciprocally related, MOBC research has commonly modeled abstinence self-efficacy and coping skills as unidirectional predictors of drinking. When paired with previous research, the present study provides empirical evidence for potentially bidirectional relationships between two putative MOBCs and drinking, such that changes in drinking may facilitate proximal (e.g., within-treatment) improvements in abstinence self-efficacy and coping, which in turn, based on previous research, are suggested to help facilitate better distal (e.g., post-treatment) drinking outcomes (Kadden & Litt, 2011; Roos & Witkiewitz, 2017). In other words, initiating abstinence may be a mechanism that helps facilitate initial changes in abstinence self-efficacy and coping skills, while abstinence self-efficacy and coping skills may be mechanisms that help facilitate maintained behavioral change (i.e., continued abstinence) over longer periods of time.
Our investigation showed that therapeutic alliance was equally high across all drinking subgroups throughout treatment and provided no evidence that therapeutic alliance was influenced by changes in drinking status. This finding may be reassuring in light of the importance of therapeutic alliance in CBT for AUD (Epstein & McCrady, 2009; Mastroleo & Monti, 2013) and empirical work showing associations between therapeutic alliance and drinking outcomes (Connors et al., 1997, 2016; Meier et al., 2005; Prince et al., 2016). However, our finding also should be interpreted as occurring in the context of a clinical trial that had generally high levels of therapeutic alliance throughout treatment. High alliance at the start of treatment could have introduced ceiling effects that limited our ability to detect additional gains in alliance after initiating abstinence. Compared to frontline treatment settings, treatments in clinical trials are often delivered with notably high fidelity (e.g., high empathy and support for patient autonomy; Hallgren, Dembe, et al., 2018), which may contribute to the high alliance ratings observed here. Participants in the present study also completed an intake session and a lengthy baseline assessment that could have predisposed them to perceive a positive therapeutic alliance at the start of treatment as was observed here. It is possible that different results may have emerged in non-clinical trial contexts where therapeutic alliance and clinician acceptance of continued drinking are often lower and more variable.
Clinical Implications
It is common for patients in AUD treatment to express reluctance about initiating abstinence from alcohol. Among other concerns, patients sometimes express worries that they may lack the pre-requisite self-efficacy or coping skills that they believe would be necessary for initiating abstinence. Clinicians may use the present findings to comfort and encourage patients to initiate abstinence by providing evidence-based information that their confidence and coping skills are likely to improve when they initiate abstinence from alcohol. Clinicians may provide reassurance and encouragement by supporting patients’ views that abstinence self-efficacy and coping skills typically are low while they are still are drinking and that initiating abstinence typically is associated with improvements in abstinence self-efficacy and coping skills in ways that would not occur while they are still drinking.
When patients initiate abstinence, clinicians may inquire about possible improvements in abstinence self-efficacy and coping skills to help patients identify and understand such changes. Routinely monitoring MOBCs during treatment with standardized measures may help patients obtain better awareness of changes that are occurring during their treatment. These data could be graphed and reviewed throughout treatment to facilitate discussions about treatment goals and progress while helping patients better understand how key events (e.g., initiating abstinence) may help facilitate improvements in those targeted variables.
Limitations and Strengths
The present study has several limitations. First, there are limitations regarding our ability to assert causal relationships among the variables studied. For example, we were unable to control whether and when each woman initiated abstinence. Although the interrupted time-series analyses used here were able to help inform the plausibility of hypothesized causal effects, it could not confirm causality with the same degree of certainty as an experimental design.
Second, there are measurement limitations, including other emerging conceptualizations of abstinence self-efficacy and coping skills that focus less on overall quantity of coping skills and more on the flexibility in one’s ability to respond effectively across contextual demands (Roos & Witkiewitz, 2017), which were not utilized in this study. Although we used a validated therapeutic alliance measure, it may have had a ceiling effect in the parent trial that could have contributed to the non-detection of some effects for this measure.
Finally, there are generalizability limitations. The change processes that were examined were evaluated in a well-controlled clinical trial of high-fidelity CBT that included more training and supervision than what often occurs in real-world treatment settings. Although this strengthens the internal validity of the treatment delivery, it also reduces generalizability to real-world AUD treatment settings, which often have substantially lower treatment fidelity and may therefore tap into different MOBCs (Hallgren, Dembe, et al., 2018). The treatment studied here was also explicitly abstinence-oriented, and it is possible that different processes of change would unfold when patients reach different drinking goals, such as reduced drinking or harm reduction, rather than complete abstinence. Finally, the sample was entirely female and predominantly White non-Hispanic, which limits generalizability to men or women of color.
The present study also had several strengths. The use of an entirely female sample is a strength in light of women typically being under-studied in AUD treatment research. Fidelity analyses assured that the CBT program was delivered to patients as intended and as directed by treatment manuals. MOBC data were collected using validated brief measures at every treatment session, supporting finer-grained analyses into the timing of change than is possible when MOBCs are measured less frequently. Our primary statistical models also used a within-subjects design, which yields high statistical power and allows each participant to act as her own control.
Conclusions
As the field increasingly understands which MOBC variables predict drinking outcomes, it will be increasingly important to identify when and how much these MOBC variables change during treatment and how they change as a function of other treatment processes (Hallgren, Wilson, & Witkiewitz, 2018). We have provided information about when and how much three hypothesized MOBC variables change during treatment and in relation to the initiation of abstinence. Our results help provide a more precise understanding about how change processes may unfold over time during outpatient CBT for AUD. More generally, a better empirical understanding of the timing of change can help researchers test CBT model hypotheses, including those that suggest changes in drinking should result in improvements in traditional MOBC measures (e.g., abstinence self-efficacy, coping skills). Such work may also help clinicians provide evidence-based information to patients about the expected courses of change in clinically-important treatment targets.
Highlights.
Patients showed weekly improvements in three hypothesized MOBCs during treatment
Two MOBCs showed sudden improvements in relation to quitting drinking
Results clarify the timing and magnitude of change in clinically important variables
Clinicians can help patients anticipate these improvements when they quit drinking
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: Dr. Hallgren has provided consultation to Pear Therapeutics, a mobile health company. All authors declare no actual or potential conflicts of interest.
Although not included in our final models, we additionally tested for sudden changes in MOBCs from two weeks before initiating abstinence to the week before initiating abstinence and found no significant effects.
There are numerous ways to compute Cohen’s d-like effect size estimates in multilevel models. The method used here generally results in smaller effect size coefficients than alternative approaches (e.g., dividing by the standard deviation of difference scores or naïve conversion from t-test statistics; see Dunlap, Cortina, Vaslow, & Burke, 1996; Westfall, 2016).
Patterns of significance did not change when data from outside of these time periods were used; however correspondence between model-estimated trajectories and raw data trajectories was reduced.
References
- Acosta MC, Possemato K, Maisto SA, Marsch LA, Barrie K, Lantinga L, … & Rosenblum A (2017). Web-delivered CBT reduces heavy drinking in OEF-OIF veterans in primary care with symptomatic substance use and PTSD. Behavior Therapy, 48(2), 262–276. doi: 10.1016/j.beth.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, & American Psychiatric Association. (2000). DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: American Psychiatric Association, 75, 78–85. [Google Scholar]
- Bates D, Mächler M, Bolker BM, & Walker SC (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67(1). doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Breslin FC, Sobell LC, Sobell MB, & Agrawal S (2000). A comparison of a brief and long version of the Situational Confidence Questionnaire. Behaviour Research and Therapy, 38(12), 1211–1220. [DOI] [PubMed] [Google Scholar]
- Busseri MA, & Tyler JD (2003). Interchangeability of the working alliance inventory and working alliance inventory, short form. Psychological Assessment, 15(2), 193. [DOI] [PubMed] [Google Scholar]
- Carey KB (1997). Reliability and validity of the time-line follow-back interview among psychiatric outpatients: A preliminary report. Psychology of Addictive Behaviors, 11(1), 26–33. [Google Scholar]
- Connors GJ, Carroll KM, DiClemente CC, Longabaugh R, & Donovan DM (1997). The therapeutic alliance and its relationship to alcoholism treatment participation and outcome. Journal of Consulting and Clinical Psychology, 65(4), 588. [DOI] [PubMed] [Google Scholar]
- Connors GJ, Maisto SA, Schlauch RC, Dearing RL, Prince MA, & Duerr MR (2016). Therapeutic alliances predict session by session drinking behavior in the treatment of alcohol use disorders. Journal of Consulting and Clinical Psychology, 84(11), 972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap WP, Cortina JM, Vaslow JB, & Burke MJ (1996). Meta-analysis of experiments with matched groups or repeated measures designs. Psychological Methods, 1(2), 170–177. [Google Scholar]
- Epstein EE, Drapkin ML, Yusko DA, Cook SM, McCrady BS, & Jensen NK (2005). Is alcohol assessment therapeutic? Pretreatment change in drinking among alcohol dependent women. Journal of Studies on Alcohol, 66, 369–378. [DOI] [PubMed] [Google Scholar]
- Epstein EE, Labouvie E, McCrady BS, Swingle J, & Wern J (2004). Development and validity of drinking pattern classification: binge, episodic, sporadic, and steady drinkers in treatment for alcohol problems. Addictive Behaviors, 29(9), 1745–1761. [DOI] [PubMed] [Google Scholar]
- Epstein EE, & McCrady BS (2009). Overcoming Alcohol Use Problems: A Cognitive-Behavioral Treatment Program. Oxford University Press: New York. [Google Scholar]
- Epstein EE, McCrady BS, Hallgren KA, Cook S, Jensen N, Graff F, Hildebrandt T, Holzhauer CG, & Litt MD (2018). Individual versus group female-specific cognitive-behavior therapy for alcohol use disorder. Journal of Substance Abuse Treatment, 88, 27–43. 10.1016/j.jsat.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. SCID-I/P. [Google Scholar]
- Hallgren KA, Delker BC, & Simpson TL (2018). Effects of initiating abstinence from alcohol on daily craving and negative affect: Results from a pharmacotherapy clinical trial. Alcoholism: Clinical and Experimental Research, 42(3), 634–645. doi: 10.1111/acer.13591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA, Wilson AD, & Witkiewitz K (2018). Advancing analytic approaches to address key questions in mechanisms of behavior change research. Journal of Studies on Alcohol and Drugs, 79(2), 182–189. doi: 10.15288/jsad.2018.79.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA, Dembey A, Pace BT, Imel ZE, Lee CM, & Atkins DC (2018). Variability in motivational interviewing adherence across sessions, providers, sites, and research contexts. Journal of Substance Abuse Treatment, 84, 30–41. doi: 10.1016/j.jsat.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA, McCrady BS, & Epstein EE (2016). Trajectories of drinking urges and the initiation of abstinence during cognitive-behavioral alcohol treatment. Addiction, 111(5), 854–865. doi: 10.1111/add.13291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks PS, Delucchi KL, & Hall SM (2010). Mechanisms of change in extended cognitive behavioral treatment for tobacco dependence. Drug & Alcohol Dependence, 109(1), 114–119. doi: 10.1016/j.drugalcdep.2009.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner RB, & Tonigan JS (2007). The search for mechanisms of behavior change in evidence‐based behavioral treatments for alcohol use disorders: Overview. Alcoholism: Clinical and Experimental Research, 31(s3). [DOI] [PubMed] [Google Scholar]
- Kadden RM, & Litt MD (2011). The role of self-efficacy in the treatment of substance use disorders. Addictive Behaviors, 36(12), 1120–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiluk BD, Nich C, Babuscio T, & Carroll KM (2010). Quality versus quantity: acquisition of coping skills following computerized cognitive–behavioral therapy for substance use disorders. Addiction, 105(12), 2120–2127. doi: 10.1111/j.1360-0443.2010.03076.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontopantelis E, Doran T, Springate DA, Buchan I, & Reeves D (2015). Regression based quasi-experimental approach when randomisation is not an option: Interrupted time series analysis. BMJ, 350, h2750. doi: 10.1136/bmj.h2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, Cooney NL, & Kabela E (2003). Coping skills and treatment outcomes in cognitive-behavioral and interactional group therapy for alcoholism. Journal of Consulting and Clinical Psychology, 71(1), 118. [DOI] [PubMed] [Google Scholar]
- Litt MD, Kadden RM, & Tennen H (2018). Treatment Response and Non‐Response in CBT and Network Support for Alcohol Disorders: Targeted Mechanisms and Common Factors. Addiction. doi: 10.1111/add.14224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longabaugh R, Donovan DM, Karno MP, McCrady BS, Morgenstern J, & Tonigan JS (2005). Active Ingredients: How and Why Evidence‐Based Alcohol Behavioral Treatment Interventions Work. Alcoholism: Clinical and Experimental Research, 29(2), 235–247. [DOI] [PubMed] [Google Scholar]
- Magill M, Kiluk BD, McCrady BS, Tonigan JS, & Longabaugh R (2015). Active ingredients of treatment and client mechanisms of change in behavioral treatments for alcohol use disorders: Progress 10 years later. Alcoholism: Clinical and Experimental Research, 39(10), 1852–1862. doi: 10.1111/acer.12848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill M, & Ray LA (2009). Cognitive-behavioral treatment with adult alcohol and illicit drug users: a meta-analysis of randomized controlled trials. Journal of Studies on Alcohol and Drugs, 70(4), 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastroleo NR, & Monti PM (2013). Cognitive-behavioral treatment for addictions In McCrady BS & Epstein EE (Eds.), Addictions: A Comprehensive Guidebook (pp. 391–410). New York: Oxford. [Google Scholar]
- McCrady BS, Epstein EE, Hallgren KA, Cook S, Jensen N (2016). Women with alcohol dependence: A randomized trial of couple versus individual plus couple therapy. Psychology of Addictive Behaviors, 30(3), 287–299. doi: 10.1037/adb0000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrady BS, Epstein EE, & Hirsch LS (1999). Maintaining change after conjoint behavioral alcohol treatment for men: Outcomes at 6 months. Addiction, 94(9), 1381–1396. [DOI] [PubMed] [Google Scholar]
- Meier PS, Barrowclough C, & Donmall MC (2005). The role of the therapeutic alliance in the treatment of substance misuse: a critical review of the literature. Addiction, 100(3), 304–316. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, Labouvie E, McCrady BS, Kahler CW, & Frey RM (1997). Affiliation with Alcoholics Anonymous after treatment: A study of its therapeutic effects and mechanisms of action. Journal of Consulting and Clinical Psychology, 65(5), 768. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, & Longabaugh R (2000). Cognitive–behavioral treatment for alcohol dependence: A review of evidence for its hypothesized mechanisms of action. Addiction, 95(10), 1475–1490. [DOI] [PubMed] [Google Scholar]
- Prince MA, Connors GJ, Maisto SA, & Dearing RL (2016). Within treatment therapeutic alliance ratings profiles predict posttreatment frequency of alcohol use. Psychology of Addictive Behaviors, 30(2), 184. doi: 10.1037/adb0000161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CR, Maisto SA, & Witkiewitz K (2017). Coping mediates the effects of cognitive–behavioral therapy for alcohol use disorder among out‐patient clients in Project MATCH when dependence severity is high. Addiction, 112(9), 1547–1557. doi: 10.1111/add.13841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CR, & Witkiewitz K (2017). A contextual model of self-regulation change mechanisms among individuals with addictive disorders. Clinical Psychology Review, 57, 117–128. doi: 10.1016/j.cpr.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotgers F (2012). Cognitive-behavioral theories of substance abuse In Walters ST & Rotgers F (Eds.), Treating Substance Abuse: Theory and Technique (3rd ed.). Guilford: New York. [Google Scholar]
- Stasiewicz PR, Schlauch RC, Bradizza CM, Bole CW, & Coffey SF (2013). Pretreatment changes in drinking: Relationship to treatment outcomes. Psychology of Addictive Behaviors, 27(4), 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (2003). Alcohol consumption measures In Allen JP & Wilson V (Eds.), Assessing Alcohol Problems (2nd ed., pp. 75–99). Rockville, MD: National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA, 2016). Selected drug use, perceptions of great risk, past year substance use disorder and treatment, and past year mental health measures in the United States, by age group. Retrieved from https://www.samhsa.gov/data/sites/default/files/NSDUHsaeSpecificStates2016A/NSDUHsaeSpecificStates2016.htm
- Tracey TJ, & Kokotovic AM (1989). Factor structure of the working alliance inventory. Psychological Assessment, 1(3), 207–210. doi: 10.1037/1040-3590.1.3.207 [DOI] [Google Scholar]
- Vedel E, Emmelkamp PM, & Schippers GM (2008). Individual cognitive-behavioral therapy and behavioral couples therapy in alcohol use disorder: A comparative evaluation in community-based addiction treatment centers. Psychotherapy and Psychosomatics, 77(5), 280–288. doi: 10.1159/000140087 [DOI] [PubMed] [Google Scholar]
- Westfall J (2016). Five different “Cohen’s d” statistics for within-subject designs. http://jakewestfall.org/blog/index.php/2016/03/25/five-different-cohens-d-statistics-for-within-subject-designs/
- Witkiewitz K, Roos CR, Tofighi D, & Van Horn ML (2018). Broad coping repertoire mediates the effect of the combined behavioral intervention on alcohol outcomes in the COMBINE Study: An application of latent class mediation. Journal of Studies on Alcohol and Drugs, 79(2), 199–207. doi: 10.15288/jsad.2018.79.199 [DOI] [PMC free article] [PubMed] [Google Scholar]