Abstract
Our purpose was to validate a reliable method to capture brain activity concomitant with hallucinatory events, which constitute frequent and disabling experiences in schizophrenia. Capturing hallucinations using functional magnetic resonance imaging (fMRI) remains very challenging. We previously developed a method based on a two‐steps strategy including (1) multivariate data‐driven analysis of per‐hallucinatory fMRI recording and (2) selection of the components of interest based on a post‐fMRI interview. However, two tests still need to be conducted to rule out critical pitfalls of conventional fMRI capture methods before this two‐steps strategy can be adopted in hallucination research: replication of these findings on an independent sample and assessment of the reliability of the hallucination‐related patterns at the subject level. To do so, we recruited a sample of 45 schizophrenia patients suffering from frequent hallucinations, 20 schizophrenia patients without hallucinations and 20 matched healthy volunteers; all participants underwent four different experiments. The main findings are (1) high accuracy in reporting unexpected sensory stimuli in an MRI setting; (2) good detection concordance between hypothesis‐driven and data‐driven analysis methods (as used in the two‐steps strategy) when controlled unexpected sensory stimuli are presented; (3) good agreement of the two‐steps method with the online button‐press approach to capture hallucinatory events; (4) high spatial consistency of hallucinatory‐related networks detected using the two‐steps method on two independent samples. By validating the two‐steps method, we advance toward the possible transfer of such technology to new image‐based therapies for hallucinations. Hum Brain Mapp 38:4966–4979, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: independent component analysis, interview, schizophrenia, hallucinations, fMRI, reproducibility, reliability
INTRODUCTION
Validating reliable methods to explore the neural bases of consciousness is a crucial aim in neuroscience. This question has a strong impact on our attempts to correlate brain activation with a given behavioral experience. Here, we would like to illustrate how recent functional magnetic resonance imaging (fMRI) developments allow objective “capture” of the neural correlates of unpredictable and subjective mental events, such as hallucinations. Hallucinations are percepts in the absence of external stimuli (Ey, 1973). In schizophrenia, hallucinations are frequent and may cause long‐term disability (Hor and Taylor, 2010). In adults, auditory‐verbal hallucinations (AVHs) are most frequent (Andreasen and Flaum, 1991), although hallucinations may occur across every sensory modality (David et al., 2011; Llorca et al., 2016). Anatomical and functional disturbances in both primary and association sensory cortices (ASCs) have been proposed to account for AVHs (Allen et al., 2008; Jardri et al., 2011), but the detection of their occurrence while scanning a participant (hallucination capture methods) has long remained very challenging.
In a first subset of capture studies, AVH occurrences were signaled online by asking the participant to press a response button in the MRI scanner (Dierks et al., 1999; Lennox et al., 2000; Silbersweig et al., 1995; Sommer et al., 2008). The subsequent sequence of self‐reports serves as a model for brain activity. Despite the cleverness of this method (later called the “button‐press” method), several drawbacks were noted. First, the cerebral activations linked to motor readiness were shown to disturb the acquisition of resting state signals (Bazán et al., 2015). Second, the reliability of this method was questioned due to the poor insight and executive dysfunctions that may exist in patients with schizophrenia (Tan, 2009). Finally, activity related to AVHs may precede the button press (Diederen et al., 2010) and exhibit complex dynamics (Lefebvre et al., 2016).
A second line of capture studies utilized discontinuous acquisition methods (also called the “random‐sampling” approach), in which many fMRI volumes were acquired at random intervals. Patients were asked for their sensory experiences immediately after each stop (Shergill et al., 2000, 2001). These two strategies (i.e., “button‐press” and “random‐sampling”) both relied on hypothesis‐driven fMRI data analyses in that they were based on patient self‐report of AVHs during scanning. This drawback made these approaches particularly vulnerable to a drop in performance in signaling hallucination occurrences.
A third line of studies used more data‐driven approaches, such as spatial independent component analysis (ICA). Applied to fMRI, this statistical method allows the co‐activated brain regions to be separated without a pre‐defined temporal model of brain activity (Formisano et al., 2004). Even though the first studies combined ICA with online self‐reports (Jardri et al., 2009; van de Ven et al., 2005), this method mainly paved the way to more simple designs for hallucinating patients, since they were only asked to report AVHs after acquisition, using a post‐fMRI interview (Jardri et al., 2009, 2007). Data from this interview were also used to help select the most relevant components among those blindly generated by ICA, that is, spatial functional patterns that best matched the hallucinations' time of occurrence and phenomenology. We named this approach the two‐steps method for hallucination fMRI capture (the “2S” method), for which a proof‐of‐concept study has been published (Jardri et al., 2013).
Although promising, two tests still need to be conducted to rule out critical pitfalls of conventional fMRI capture methods before the “2S” strategy can be adopted in hallucinations research: (1) replication of these findings on an independent sample (reproducibility); and (2) assessment of the consistency of the AVH‐related patterns at the subject level (reliability). In this article, we addressed these issues by recruiting 85 participants in four different experiments. We successively studied the patients' ability to a posteriori report their sensory experiences (1), the concordance between the “2S” and the “button‐press” methods on controlled stimuli (2) and on hallucinations (3), and finally, the consistency of the AVH‐related neural networks identified using the “2S” procedure on independent samples (4).
MATERIALS AND METHODS
Population
We recruited five independent samples of participants who were free from any sensory deficit: 20 schizophrenia patients without hallucinations, 20 healthy subjects, and 3 samples of 5, 20, and 20 schizophrenia patients suffering from frequent AVHs. Patients were assigned to the “no‐hallucination” group if they had not experienced hallucinations in the week prior to participation (Task 1). They were assigned to the “AVH” group if the PANSS P3 item score was ≥ 3, with hallucination experiences frequent enough to occur during an MRI session (Tasks 3 and 4). Please note that in Task 3, five schizophrenia patients were selected for their good self‐report of hallucinatory events (a necessary criterion for using the “button‐press” approach) and that in Task 4, two different subsets of twenty patients each were recruited to control for the possible influence of age and medication on replicability. The main characteristics of these samples are reported in Table 1. All of the patients enrolled in Task 3 only had hallucinations in the auditory modality. For the patients in Task 4, 88% of these experiences occurred in the auditory modality, whereas 35, 12 and 12% were coenesthetic, visual and olfactory, respectively. All the participants were recruited at the University Hospital of Lille, except for those participating in Task 3, which was performed at the University Hospital of Strasbourg.
Table 1.
Characteristics of the enrolled samples (mean ± sd)
| Task | 1 | 2* | 3 | 4a | 4 |
|---|---|---|---|---|---|
| Sample | No. 1 | No. 2 | No. 3 | No. 4 | No. 5 |
| Number of subjects | 20 | 20 | 5 | 20 | 20 |
| Population | Schizophrenia without hallucinations | Healthy subjects | Schizophrenia with hallucinations | First episode psychosis with hallucinations | Schizophrenia with hallucinations |
| Sex Ratio | 17/3 | 15/5 | 3/2 | 17/3 | 14/6 |
| Age (years) | 39.5 ± 10 | 12.9 ± 1.6 | 34.4 ± 9.3 | 13.1 ± 1.8 | 33.7 ± 8.2 |
| Dose of anti‐psychotic treatment (EqOZ) | 20.9 ± 12.7 | 0 | 21.2 ± 10.8 | 0 | 36.2 ± 17.3 |
| PANSS‐P | 13.9 ± 4.2 | NA | 22.8 ± 4.2 | 29.4 ± 5.3 | 22 ± 4.4 |
| PANSS‐P3 | 1 ± 0 | 1 ± 0 | 5.2 ± 0.4 | 5.1 ± 1.3 | 5.3 ± 0.9 |
| Type of acquisition | MR‐simulator | Single shot EPI | Single shot EPI | Single shot EPI | 3D‐PRESTO |
| Acquisition time | 10 min | 10 min | 20 min | 10 min | 10 min |
| Sequence parameters | |||||
| Echo time (ms) | NA | 70 | 43 | 70 | 30 |
| Repetition time (m) | NA | 3,000 | 3,000 | 3,000 | 1,000 |
| Voxel size (mm3) | NA | 4 | 4 | 4 | 3.3 |
| Number of scans | NA | 300 | 400 | 300 | 900 |
| Acquisition per subject | 1 | 1 | 4 | 1 | 1 |
EqOZ, equivalent olanzapine; PANSS, positive and negative syndrome scale; PANSS‐P, positive sub‐score of the PANSS scale; PANSS‐P3, P3 sub‐score of the PANSS scale; NA, not available; EPI, echo‐planar imaging; 3D‐PRESTO, PRinciples of Echo‐Shifting with a Train of Observations; aData from Jardri et al. (2013); There was no overlap between the two samples recruited in Task 4.
Experimental Procedures
Task 1
Task 1 was designed to determine if schizophrenia patients could a posteriori report, with good precision, sensory experiences that occurred in a controlled experimental setting (i.e., using real auditory stimuli, with known characteristics in terms of time of onset, duration, amplitude, etc.). Task 1 was performed in an MRI simulator. We selected patients without AVHs for this first experiment to avoid any confusion in reporting task‐related auditory stimuli vs. endogenous percepts (i.e., AVHs). Patients were asked to lie down at rest without falling asleep and were put in a dark environment. They were only asked to report auditory stimuli a posteriori. For 10 min, the sound of an EPI (e.g. Echo‐Planar Imaging) sequence was delivered without real MRI scan acquisition. In complement, a variable number of unexpected auditory stimuli were randomly presented through the headphones using E‐Prime 1.3 (Psychology Software Tools Inc., Pittsburgh, USA) (normalized amplitude = 75 dB SPL). We used verbal material and selected 0–4 voices/participant (all unknown to the participants), as this is the mean number of AVHs usually reported during an fMRI session (Lefebvre et al., 2016). Stimulus presentations lasted from 6 to 30 s. Patients were interviewed immediately after the experiment about what they heard using a post‐fMRI questionnaire (see the “Analysis” section). The number of voices heard and the moments of occurrence were reported. Voice detection performance was also measured.
Task 2
Task 2 was designed to evaluate the inter‐method reliability of the “2S” method compared with detection of controlled stimuli using hypothesis‐driven analysis. Task 2 was performed in an MRI scanner. Healthy participants were asked to lie down at rest without falling asleep while wearing MR‐compatible headphones that transmitted audible stimuli and attenuated the ambient noise of the scanner. They were only asked to report auditory stimuli a posteriori. During the 10‐min fMRI session, a variable number (n) of words or sentences were presented through the headphones using E‐Prime 1.3 (Psychology Software Tools Inc., Pittsburgh, USA) (normalized amplitude = 75 dB SPL). When compared with Task 1, and because the purpose was no longer to test the quality of reporting of the patients, we chose to enhance power by increasing the total number of stimuli presented from [0–4] to [0–10]. Stimulus presentations lasted from 6 to 30 s. Patients were interviewed immediately after the experiment about what they heard, using a post‐fMRI questionnaire (see the “Analysis” section). The number of heard stimuli and their moments of occurrence were reported. We then compared the “2S” method with a general linear model (GLM) built using the exact time points of stimulus presentation.
Task 3
Task 3 was designed to evaluate the agreement between capture methods (i.e., between the “2S” and the “button‐press” methods) in patients with a good self‐report of their hallucinatory events. Task 3 was performed in an MRI scanner. The patients were asked to lie down at rest without falling asleep during acquisition. Each patient completed four different 20‐min fMRI sessions. During the first three sessions, the patients were instructed to signal the onset of their hallucinations with a response button (right hand) and to release it when the hallucinations stopped, that is, they were explicitly asked to report hallucinations online, which referred to as the “button‐press” condition. In the last session, the “2S” procedure was applied, and the patients were interviewed immediately after this last acquisition about what they heard, using a post‐fMRI questionnaire (see the “Analysis” section). The number of AVHs and their moments of occurrence were reported.
Task 4
Task 4 was designed to test the reproducibility of the “2S” procedure. Task 4 was performed in an MRI scanner. Patients with AVH were asked to lie down at rest without falling asleep during acquisition. They were only asked to report hallucinations a posteriori. Each patient had a 10‐min fMRI session, and the “2S” procedure was applied to identify AVH periods during scanning. Two complementary analyses were conducted. First, we computed the spatial similarity between the AVH‐related functional brain networks obtained at the subject level. Second, the between‐sample consistency in hallucination detection between the current dataset and a previous independent sample (Jardri et al., 2013) as well as with coordinate‐based meta‐analytic findings from 10 different studies (Jardri et al., 2011) (cf. Table 1) was evaluated for the hallucination‐related network (ASCs) (Jardri et al., 2013) and the default mode network (DMN), which is considered a standard, well‐replicated and ubiquitous neural network.
ANALYSES
A Posteriori Voice Detection Performance
This analysis used the data collected in Task 1. To normalize performance across subjects, sensitivity was recorded as 1 if all the voices were detected, and specificity was recorded as 1 if there was no additional recognized sounds. In all other cases, sensitivity and specificity were recorded as 0. We further generated random data for 20 mock participants and matched these data with those of the patients according to the number of voices presented. We generated random detection values using the RAND function (in Matlab R2016a). Each simulated recording was randomly divided into periods with and without voices, and a number of 0 or 1 was randomly assigned to each. Then, as for the patients, if the number was 1 for all of the periods with voices, a sensitivity of 1 was reported. If the number was 0 for all of the periods without voices, the specificity was 1. Accuracy was defined as (true detection + true no detection)/(true detection + false detection + true no detection + false no detection). The patient and simulated data were compared using a two‐sample permutation test with 1,000 iterations (Monte Carlo method) and an α level of 0.05, and these analyses were performed using the ‘perm’ package with R software for statistical computing v3.3.
The Two‐Steps Hallucination fMRI Capture Procedure
This analysis was conducted on data collected in Tasks 2–4. Our capture method is divided into two consecutive steps (Jardri et al., 2013) (cf. Fig. 1a, Supporting Information Fig. S1). This method was developed to capture unpredictable events, such as hallucinations and unexpected stimuli presented to healthy participants. Step 1 is resting‐state fMRI acquisition in participants with or without AVHs. Step 2 occurs immediately after MRI acquisition. Using a standardized post‐fMRI interview, each participant is asked to report all the sensory experiences that occurred during scanning, including the sensory modality and number of events as well as their approximate times of occurrence (a translated version of the interview is available by request to the corresponding author).
Figure 1.
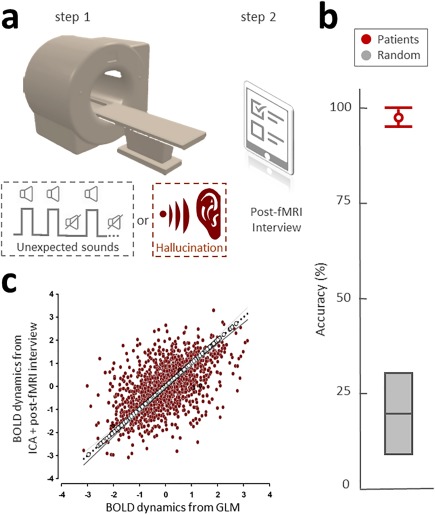
The hallucination capture method. (a) Description of the two‐steps procedure. Step 1 occurs while participants are laying down in the MRI scanner. Two populations were tested with a variation on Step 1: (1) healthy participants, who were exposed to unexpected voices during scanning (grey dotted square); and (2) schizophrenia patients with frequent hallucinations, who were scanned without stimulus presentation because hallucinations constitute internally generated percepts (red dotted square). Step 2 occurs immediately after MRI acquisition. Using a standardized post‐fMRI interview (see “Methods” section), participants were asked to report sensory experiences that occurred during scanning as well as their precise time of occurrence. The collected data were then used to select the most appropriate components resulting from blind multivariate analysis of the fMRI signal (cortex‐based ICA). The results for healthy volunteers (n = 20) are presented in Figure 1c, while those for hallucinators (n = 20) are presented in Figures 3 and 4. (b) Task 1: The ability of schizophrenia patients to report off‐line the number of sensory events and their times of occurrence. Twenty new patients without hallucinations were also tested using the “unexpected voices” procedure in an fMRI simulator. The accuracy of a posteriori sound labeling in the schizophrenia patients was plotted in red (mean 97.5%; 95%IC = 85.3–99.9), while random detection for 0–4 events would be 20% (95%IC = 9.61–36.14, gray). Mean difference = 77.5, P = 0.002. (c) Task 3: Validation of ICA + interview versus gold standard analysis. Two parallel analyses (i.e., ICA + interview and GLM analysis using the sound presentation protocol) were conducted on the same healthy volunteer dataset. Deming regression analysis was used to account for observation errors on both the x‐ and y‐axes (r = 0.57, with a test for slope F 2,98 = 2.1; P < 0.0001) and confirmed the high degree of precision of our capture method, even in the absence of online report. [Color figure can be viewed at http://wileyonlinelibrary.com]
The functional data were pre‐processed using a slice scan time correction, a 3D head motion correction, smoothing using a spatial Gaussian filter (full‐width at half‐maximum = 6.0 mm), a temporal high‐pass filtering with 2 sin/cos, and linear trend removal. The anatomical data were subjected to an intensity inhomogeneity correction algorithm, resampled to a 0.5 mm3 resolution, and normalized in Talairach's stereotactic space (Talairach and Tournoux, 1988). Data from the head tissue, subcortical structures, and cerebellum were then removed with the aim of advanced cortical segmentation processing. This segmentation was performed at the gray/white matter and the gray matter/cerebrospinal fluid boundaries. A boundary‐based registration was finally used to align the functional/anatomical datasets.
Data obtained from Step 1 are first blindly analyzed using cortex‐based ICA analysis (Formisano et al., 2004). For each patient, cb‐ICA (using the spatial decomposition algorithm “FastICA”; Hyvärinen and Oja, 2000) is used to extract (20% of the total volume) independent components (ICs) from the rs‐fMRI signal of the cortical voxels of the matrix, that is, 30 ICs for Tasks 2 and 4 and 40 ICs for Task 3. We referred to a fixed‐point ICA algorithm, i.e., FastICA, which minimizes the mutual information of the components using a robust approximation of the negentropy as a contrast function, and a rapid, iterative (non‐adaptive) algorithm for its maximization. The deflation approach was used to run FastICA, as previously described by Hyvärinen et al. (1999) and Formisano et al. (2004). The resulting ICs corresponded to 3D clusters of voxels with |Z|‐normalized values >2.5. Among these ICs, the most relevant are first selected using the IC‐fingerprint method (De Martino et al., 2007; Jardri et al., 2013). Because ICA does not naturally order the resulting components according to their relevance, we referred to the IC‐fingerprint method, which jointly uses seven spatial and temporal signal properties for IC classification purposes (De Martino et al., 2007). These properties were measured post hoc for each IC to preserve the “data‐driven” characteristic of the analysis. This step allowed us to discard noise‐related ICs (e.g., EPI susceptibility, motion artefacts, high‐frequency noise…), with the aim to only retaining the components related to a neurophysiological source, which were characterized by a high spatial and temporal structure (i.e., degree of clustering and one‐lag serial auto‐correlation, respectively) and by a high entropy, coupled with a maximum power contribution in the low‐frequency range (0.01–0.1 Hz; see also Jardri et al., 2013 and Roquet et al., 2014). This allows one to retain only the components related to a neurophysiological source (blood‐oxygen‐level dependent signal (BOLD)) for the next step. The surviving ICs are then compared with the post‐fMRI interview data, in terms of the number, times of occurrence, and functional networks of interest (e.g., speech‐related for AVHs, etc.). Data pre‐processing, cortex‐based ICA and IC‐fingerprinting were performed using Brain Voyager v20.2.
Inter‐Method Reliability in fMRI Stimulus Detection
This analysis used the data collected in Task 2. Two parallel analyses were applied to the fMRI data in Brain Voyager: (1) the “2S” analysis, as described in the previous section and based on cb‐ICA; (2) a GLM fitted to the experimental protocol generated for each participant using E‐Prime. This GLM was based on controlled stimulus timing, as the time of stimulus presentation was known a priori. Because of the massive univariate nature of GLM analysis applied to fMRI data, the resulting statistical maps were thresholded using a false discovery rate approach (q < 0.01; Genovese et al., 2002). In addition to conventional correlation analysis, which we considered insufficient to confirm agreement of the results of the two analyses for the same dataset, we performed Deming regression (Cornbleet and Gochman, 1979) to account for observation errors on both the x‐ and y‐axes (i.e., on the BOLD dynamics from GLM and cb‐ICA, respectively).
Inter‐Method Reliability in fMRI AVH Capture
This analysis was based on data collected in Task 3 and used Matlab R2012b with the SPM8, statistical non‐parametric mapping (SnPM) and FMRLab v2.3 toolboxes. For the “button‐press” condition, we referred to the GLM approach described in the previous section. The brain activity expected to be related to AVHs was modeled by convolving the box‐car time course of the button‐press from the participants with the canonical hemodynamic response function, that is, a two‐gamma function using SPM standard parameters. This procedure was used to determine the BOLD‐related component with the highest correlation coefficient between its temporal vector and the subject's signaling. As we previously reported that “button‐press” components in the same subjects were highly reproducible, they were averaged for each patient (Foucher, 2013). For the “2S” condition, we referred to the ICA approach described in the “two‐steps hallucination fMRI capture procedure” section.
Although a high spatial correlation coefficient can be considered a measure of inter‐method reliability, here, we used Cohen's kappa coefficient, κ, to assess whether this agreement remains true at the voxel level. The “button‐press” spatial components and “2S” spatial components were successively thresholded at z = 1.5, 2, 2.6, 3, and 3.6 to make binary maps of 0 = [no‐AVH voxel], 1 = [AVH voxels] to measure the κ coefficient. Last, possible systematic differences between the spatial “button‐press” components and “2S” components were assessed using a multi‐subject pseudo‐paired t‐test design with SnPM. A permutation test was adopted due to the limited number of subjects in this task. Significance was set at pseudo‐t > 2 with an extension k > 1 cm3 (125 voxels) within the regions of interest, which were defined as regions that were positively active in either the signaling or resting condition, that is, “button‐press” or “2S” component.
Spatial Consistencies in Hallucination Detection
This analysis used the data collected in Task 4 (Samples nos. 4 and 5). After a first‐level analysis (based on the “2S” capture method) was conducted, a secondary analysis was conducted by submitting individual ICs to a self‐organizing group IC algorithm (sog‐ICA, Esposito et al., 2005). An iterative cluster‐size thresholding procedure based on Monte Carlo simulations (n = 1000) further corrected the resulting random‐effects statistical maps, which were used to evaluate the regional stability of these AVH‐related neural networks.
The between‐subjects' spatial consistency was first tested using multidimensional similarity (MDS) clustering on Sample no. 5 (see Table 1). The MDS algorithm was applied on the sog‐ICA decomposition of per‐hallucinatory fMRI data, and the MDS linear projections were plotted in 2D space (Torgerson, 1952) in Brain Voyager 20.2. To help identify cluster plots of interest, the random‐effects sog‐ICA validation maps were visualized using the same color codes.
Between‐samples spatial consistency was also tested using probabilistic mapping between Samples no. 4 (Jardri et al., 2013) and no. 5 (replication sample). Note that these independent samples were obtained from different scanners using different sequences (single‐shot EPI and 3D‐PRESTO, respectively) and different magnetic fields strength (1.5 and 3 T, respectively) (cf. Table 1). At each spatial location, functional maps were generated to represent the relative number of subjects leading to significant activation patterns within the networks of interest for the initial Sample (no. 4; Jardri et al., 2013; n = 20), the replication Sample (no. 5, n = 20), and coordinate‐based meta‐analytic findings (Jardri et al., 2011).
Linear Regression Analysis between Default Mode and AVH‐Related Signal Time Courses
This analysis used the data collected in Task 4. The AVH‐related ICs were selected using Samples no. 4 (Jardri et al., 2013) and no. 5 (Task 4) according to the “2S” procedure. In parallel, we used the same data sets and selected ICs related to the DMN using a “goodness‐of‐fit” (GoF) procedure. For each participant, the IC with the highest GoF score (i.e., absolute correlation coefficient with a DM template taken from Laird et al., 2009) was assumed to be the DM component. To explore the dynamics of the AVH‐related and DM‐related networks, we normalized their fMRI signals to relative variations with respect to the mean value of the participants' individual time series (Deco et al., 2009). AVH‐ and DM‐related networks signal fluctuations were compared using the Pearson product moment correlation in Samples nos. 4 (Jardri et al., 2013) and 5, respectively.
Ethical Issues/Study ID
All patients gave written informed consent. The study ID for task no. 3 is CPP03/45‐PSY 2003/52S, while that for Tasks 1, 2, and 4 is 2009‐A00842–55. All reported experiments performed by the authors complied with the Helsinki declaration and its amendments.
RESULTS
Are Schizophrenia Patients Able to Report with Precision Sensory Experiences a Posteriori (Task 1)?
We tested the ability of schizophrenia patients to report off‐line unexpected sensory events and their times of occurrence in the scanning context. The accuracy of a posteriori voices labeling in schizophrenia patients was measured at 95% (95%IC = 85.3–99.9), while random detection for 0 to 4 events would be 20% (95%IC = 9.61–36.14). This difference was highly significant (permutation testing, mean difference = 77.5, P = 0.002; Cf. Fig. 1b). Sensitivity was 100% (95%IC = 80.0–100) and specificity was 95% (95%IC = 0.73–99.7), while random detection for 0–4 events would be 20% (95%IC = 6.61–44.3) for both.
Inter‐Method Reliability in Detecting Controlled Auditory Stimuli (Task 2)
A Deming regression analysis was used to account for observation errors on both the x‐ and y‐axes (r = 0.57, with a significant test for slope F 2,98 = 2.1; P < 0.0001), and confirmed the high degree of precision of the “2S” method even in the absence of online report, as shown by the good agreement with the GLM analysis based on controlled stimuli (cf. Fig. 1c).
Inter‐Method Reliability in Detecting Online AVHs (Task 3)
The average spatial correlation coefficient between the “2S” and “button‐press” components was r = 0.68 ± 0.1 (cf. Fig. 2a). The average Cohen's kappa coefficient was 0.50 ± 0.08 and was relatively consistent regardless of the z‐score threshold. Fig. 2b shows the plot of each individual κ according to the z‐score threshold. The SnPM comparison between the “2S” and “button‐press” spontaneous activity maps did not provide any evidence of a significant difference despite the use of a lenient threshold (Cf. Fig. 2c).
Figure 2.
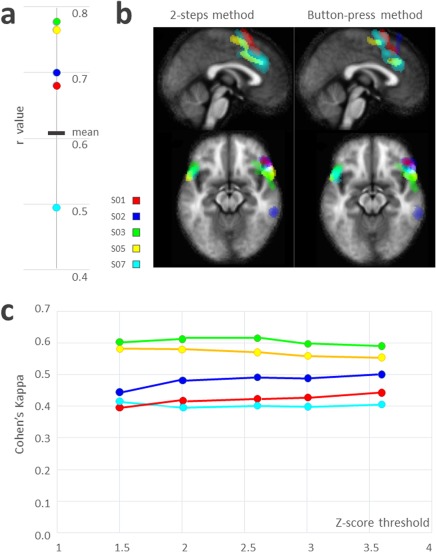
Task 2: Inter‐method reliability in AVH‐related networks. Five schizophrenia patients with refractory hallucinations underwent four different 20‐min sessions of 400 single‐shot EPI fMRI. During the three first sessions, they were instructed to signal the onset of an AVH with a response‐button (right hand) and to release it when the AVH stopped. In the last session, the patients were instructed to lie down with their eyes closed without falling asleep. At the end of this session, they completed a post‐fMRI interview to precisely report the times AVHs occurred during the scan. Components of interest were detected using the “button‐press” method for the three first sessions and then averaged, while they were detected using the “two‐steps” (2S) method for the last session. Each color represents one of the five patients. (a) Correlation between the “2S” and the “button‐press” methods for each participant; (b) Components of interest chosen during AVH experiences for each participant using the “2S” and the “button‐press” methods. (c) Cohen's kappa value, that is, intersession concordance according to different statistical thresholds for SPM analysis. [Color figure can be viewed at http://wileyonlinelibrary.com]
Reproducibility of Neural Networks Identified during AVHs (Task 4)
After MDS projection, four main clusters were identified; these clusters represented the sensorimotor network (Cluster 1), the AVH‐related network (Cluster 2), the salience network (Cluster 3), and the visual rest network (Cluster 4) (cf. Fig. 3a). Random‐effects activation maps resulting from sog‐ICA are presented in a glass brain (cf. Fig. 3b). The AVH‐related network encompasses widespread cortical‐subcortical areas, as listed in Table 2.
Figure 3.
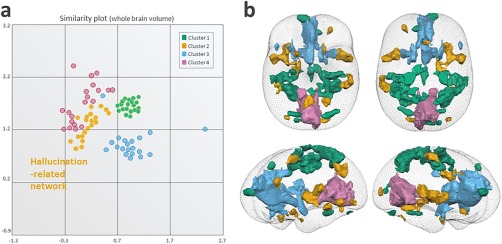
Task 4: Between‐subjects' spatial consistency in AVH detection (n = 20). (a) Cluster plots identified after multi‐dimensional similarity clustering projection. Each circle represents an individual IC taken from the 20 enrolled schizophrenia patients who experienced AVHs while scanning. Four clusters were identified and represented the sensorimotor network (Cluster 1, green), the AVH‐related network (Cluster 2, orange), the salience network (Cluster 3, blue), and the visual rest network (Cluster 4, purple), (b) Random‐effects activation maps resulting from self‐organizing group ICA presented in a glass brain, with colors assigned according to the cluster plot (shown in a). The AVH‐related network is plotted in orange and encompasses the precentral gyrus, culmen, insula, inferior parietal lobule, cingulate gyrus, middle frontal gyrus, superior frontal gyrus, middle occipital gyrus, inferior frontal gyrus, cerebellar tonsil, fusiform gyrus, inferior semi‐lunar lobule, transverse temporal gyrus and limbic lobe. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 2.
Regions involved in the hallucination‐related network after the group‐ICA decomposition of per‐hallucinatory fMRI data
| Identified Clusters | Talairach and Tournoux Coordinates (x,y,z) | Number of voxels |
|---|---|---|
| Right Cerebrum, Sub‐lobar, Insula | 41, 8, 5 | 10,850 |
| Left Cerebellum, Anterior Lobe, Culmen | −2, −59, −9 | 7,104 |
| Left Cerebrum, Sub‐lobar, Insula, | −47, 8, 4 | 7,069 |
| Left Cerebrum, Parietal Lobe, Inferior Parietal Lobule | −55, −30, 36 | 6,078 |
| Right Cerebrum, Limbic Lobe, Cingulate Gyrus, | 2, 23, 31 | 4,152 |
| Right Cerebrum, Frontal Lobe, Sub‐Gyral | 21, −7, 57 | 3,079 |
| Left Cerebrum, Frontal Lobe, Middle Frontal Gyrus | −28, 47, 22 | 1,763 |
| Left Cerebrum, Occipital Lobe, Lingual Gyrus | −19, −85, −4 | 1,335 |
| Right Cerebrum, Occipital Lobe, Middle Occipital Gyrus | 34, −85, 9 | 1,253 |
| Right Cerebellum, Anterior Lobe, Culmen | 46, −37, −28 | 1,021 |
| Right Cerebrum, Limbic Lobe, Uncus | 27, 10, −25 | 943 |
| Left Cerebrum, Frontal Lobe, Inferior Frontal Gyrus | −16, 21, −16 | 923 |
| Left Cerebrum, Occipital Lobe, Cuneus | −16, −91, 14 | 740 |
| Right Cerebrum, Frontal Lobe, Sub‐Gyral | 18, 28, −14 | 700 |
| Left Cerebellum, Posterior Lobe, Cerebellar Tonsi | −36, −55, −39 | 653 |
| Left Cerebrum, Occipital Lobe, Middle Occipital Gyrus | −36, −72, −7 | 574 |
| Right Cerebellum, Posterior Lobe, Inferior Semi‐Lunar Lobule | 7, −76, −39 | 540 |
| Right Cerebrum, Temporal Lobe, Transverse Temporal Gyrus | 54, −18, 11 | 522 |
| Left Cerebrum, Limbic Lobe, Uncus | −18, 5, −22 | 406 |
Data indicate x‐y‐z coordinates in stereotaxic space (TAL) of the weighted center for each identified cluster as well as the total number of voxels.
In a second step, we overlaid the results of the replication sample (2016) with those of the 2013 sample and with coordinate‐based meta‐analytic findings. At the group level, a negative correlation was identified between the BOLD signal of the AVH‐ and DM‐related networks, in both the 2013 sample (r 2 = 0.38, P < 0.0001; taken from Jardri et al., 2013) and the current 2016 replication sample (r 2 = 0.39, P < 0.0001; cf. Fig. 4a). The spatial consistencies in the AVH‐related and DM functional networks across these two independent samples of hallucinators (2013 and 2016) and with coordinate‐based meta‐analytic findings were also computed (cf. Fig. 4b,c). Important overlap was evident within the ASC and the DMN network.
Figure 4.
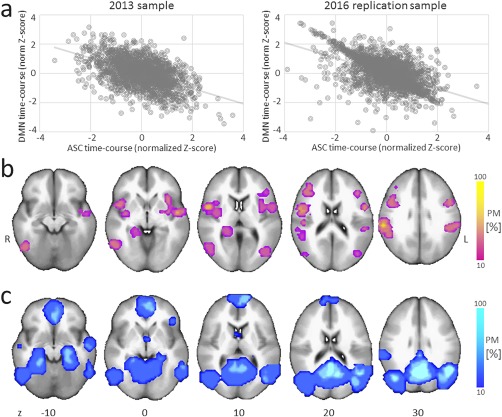
Task 4: Between‐sample consistency in AVH detection. (a) At the group‐level, a negative correlation was observed between the AVH‐related network (within association cortices, ASC) and the DMN BOLD fluctuations, in both Sample no. 4 (taken from Jardri et al., 2013) and the current 2016 replication Sample (no. 5). (b,c) Spatial consistency in AVH‐related and DM‐related functional networks across the two independent samples of hallucinators (nos. 4 and 5). At each spatial location, functional maps represent the relative number of subjects leading to significant activation patterns within the networks of interest for the initial Sample (no. 4, 2013, n = 20) and the replication Sample (no. 5, 2016, n = 20) as well as for coordinate‐based meta‐analytic findings for hallucination capture (b) and for the anti‐correlated DMN (c). PM: probabilistic mapping; R/L indicate the right/left hemispheres. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
A major drawback in fMRI capture methods today remains the absence of a gold standard in detecting hallucinations during scanning. Because hallucinations are complex sensory experiences (David et al., 2011) that are often associated with negative affective states, reporting these symptoms online quickly becomes very challenging in the context of an MRI examination, especially for the most disabled patients. The subjects included in such studies are indeed specifically selected for their ability to report their symptoms online. Thus, to extend AVH capture to the field of clinical applications, developing a method applicable whatever the age and AVH severity appears critical.
Despite its limitations, fMRI capture of hallucinations based on online self‐reports have received some validations in the literature (e.g., Sommer, 2008). Using a button‐press approach, Diederen et al. (2013) notably confirmed the good reproducibility in brain activations obtained through fMRI capture of AVHs after two scans. Using a meta‐analytical approach, the same research group also compared the brain activity measured during auditory stimulus detection with the activity concomitant to AVH (van Lutterveld, 2013a). These authors were able to disentangle specific activation related to AVH from the spatial patterns associated with button‐press signaling. Interestingly, previous works emphasized the pertinence of ICA‐based approaches and their compatibility with button‐press methods. In a study that combined ICA with online self‐reports, van de Ven et al. (2005) demonstrated that a positive correlation exists between the average BOLD time‐course obtained from the positive voxels of the component of interest and the button press reference model. Using a similar approach, Foucher (2013) showed the superiority of ICA over GLM for the analysis of the “button‐press” method of hallucination capture. These encouraging findings pave the way for the assessment of inter‐method reliability, external consistency and quality of sensory experiences reported by patients with hallucinations, as reported here.
Our purpose was thus to validate the “2S” method for fMRI hallucination capture, as initially introduced in a previous paper from our group (Jardri et al., 2013). The use of a post‐fMRI interview proved capable of detecting a large range of modality‐dependent experiences, without needing to put the participant in a dual‐task situation (i.e., experiencing vivid hallucinations and at the same time pressing a response button). Several lines of support for the “2S” approach emerged from the present experiments. In a behavioral task, we first showed that schizophrenia patients were able to report controlled unexpected auditory stimuli with high accuracy in an fMRI environment. Using the same task while scanning healthy participants, we also demonstrated good concordance between a model‐based analysis and the “2S” approach, which combined blind fMRI analysis with a post‐fMRI interview. In a third experiment, we confirmed good agreement between the “2S” and online button‐press approaches to capturing AVHs. Finally, the neural networks (e.g., AVH‐related and DM‐related networks) detected using the “2S” strategy in two independent samples was found to be highly comparable, supporting the good reproducibility of this method.
We showed in Task 1 that despite the presence of an invalidating disorder, patients were fully able to a posteriori report the occurrence of unexpected voices presented during an MR simulation session. The reliability of the patients' report was very high despite very restrictive statistical analysis (if a patient did not recognize one voice out of all of the voices presented, she/he was considered “not able to report”). This result constitutes the first level of validation for the post‐fMRI interview in a population of schizophrenia patients. In the second task, we evaluated the reliability of the “2S” method compared with GLM analysis of controlled stimuli in healthy subjects and confirmed the high degree of precision of the “2S” method in a real fMRI setting, even without online report.
Based on the analysis of repeated scans in patients suffering from hallucinations, we further evidenced the stability of data obtained using the “2S” procedure and the conventional button‐press approach in patients who were able to signal AVHs online. To date, the “button press” method is the most common accepted method, but it has important limitations, as previously listed (mainly due to motor readiness, executive dysfunction in schizophrenia, and the complex neural dynamics of AVHs). Consequently, we could only include five patients with good insight who were able to report their sensory experiences online (Task 3). In contrast, we expect the “2S” method to be applicable to all patients with schizophrenia (we were able to recruit larger samples for Tasks 2 and 4 for instance). The simplicity of the experimental setting of the “2S” method also constitutes an advantage over other capture methods, especially for patients who could have difficulties reporting hallucinations online, such as older participants or children (see e.g., Jardri et al., 2007). Overall, Tasks 1–3 confirmed the feasibility and reliability of the “2S” method despite the use of a post‐fMRI interview. These results are important since a key strength, but also a potential limit of the “2S” method, specifically resides in the a posteriori nature of our interview. This question constitutes a hot but still unresolved topic in consciousness research.
Indeed, two types of methods have been proposed in experiments that test conscious access: (1) report‐based paradigms and (2) no‐report paradigms (Tsuchiya et al., 2015), such as those based on eye‐tracking methods. Crucially, no‐report paradigms could overestimate the occurrence of AVH‐linked neural activation by including activation that occurs just before or after the activation directly related to hallucinations. These activations could be linked to post‐perceptual processes (i.e., cognitive processes) or pre‐perceptual processes (i.e., pre‐neural correlates of AVHs) (Overgaard and Fazekas, 2016). Moreover, the occurrence of AVHs remains strongly subjective and patient dependent, even though we were able to demonstrate good reproducibility in the current study. Currently, we have no reason to prefer subjective variation linked to the operator in no‐report paradigms to the individual variation observed in report‐based paradigms. Furthermore, report‐based paradigms could underestimate AVH occurrences because AVHs are linked to cognitive processes such as attention, working memory, decision making, and action planning. For example, reduced reporting was observed in the context of inattentional amnesia or experience without access (Tsuchiya et al., 2015). Here, our goal was to validate a method for reporting conscious experiences with good reliability and to correlate them with neural activations. By combining the use of a report‐based paradigm (i.e., the interview) and a no‐report paradigm (i.e., blind fMRI analysis), the “2S” fMRI capture method appears fully compatible with recent recommendations on conscious access paradigms to limit issues related to the unpredictable nature of the events of interest (Tsuchiya et al., 2016). We think it would be interesting in the near future to test whether the fMRI‐based approach described in this article could be extended to other spontaneous phasic mental events, such as obsessions and tics.
We also studied the internal and external consistency of the results found using the “2S” procedure by testing the degree of overlap between (1) the cortical areas associated with AVH experiences as reported in the literature (Jardri et al., 2011) and (2) the results obtained in two independent samples of hallucinators (in 2013 and 2016). The overlap was maximal within the ASCs; these areas, including the insula and temporo‐parietal junction, are known to play a core role in AVH experiences (Jardri et al., 2011). Interestingly, in these datasets, a similar degree of overlap was found for well‐replicated intrinsic connectivity networks (Laird et al., 2009), such as the DMN. The negative correlation in BOLD fluctuations between the AVH‐related and DM‐related networks found in Sample no. 4 (Jardri et al., 2013) was replicated in Sample no. 5. By replicating previous findings, these results provide further support for the existence of anti‐correlation between the DMN and sensory cortices during AVH experiences and of a central role of DMN dynamics in these phenomena (Alderson‐Day et al., 2016; Lefebvre et al., 2016). This finding allows us to add the anti‐correlation of DM‐/AVH‐related time courses as a complementary selection criterion for the component of interest in the “2S” method (Lefebvre et al., 2016). These findings reinforce the consistency of the method as applied to fMRI capture of hallucinations. The overlap in the speech‐related network and in the hippocampal complex was up to 90 and 65%, respectively, supporting the previously suggested core role of these areas in hallucinations (Allen et al., 2012; Amad et al., 2014). In contrast, other areas in this network may reflect the phenomenological content of the experiences, which is only shared by a minority of hallucinators (Ffytche et al., 1998; Jardri et al., 2013).
Importantly, the use of different samples of patients recruited from different centers as well as different scanners with various MR field strengths and fMRI sequences constitutes a strength of this paper. Although some of the patients came from the same center (CHU Lille), we avoided overlap between the samples involved in the different tasks. Heterogeneity in terms of age or symptom severity between the tasks further supports the reliability of the “2S” method in various populations, including patients who could have poor reporting ability, such as adolescents experiencing acute psychosis or adults suffering from severe chronic schizophrenia. This approach is further strengthened by the reference to multivariate statistics, such as ICA, which enables better control of false‐positive rates compared with conventional massive univariate approaches (GLM) (Eklund et al., 2016) while also providing access to effect‐size estimates (i.e., fMRI changes during hallucinations at the component level), a criterion recently recommended for good practice in fMRI research (Chen et al., 2017).
From a methodological point of view, ICA presents several advantages in the context of fMRI capture of hallucinations. A first one relies on the use of multivariate statistics. Interestingly, the performance of such algorithms appears to substantially benefit from dimensionality reduction (Formisano et al., 2004) compared with more massive univariate methods. Indeed, we made the choice to perform a cb‐ICA based on the idea that only 20% of the voxels lie within the cortex. Readers should be aware that hallucinations might involve complex cortical‐subcortical interactions (e.g., Hoffman et al., 2011). However, our choice to restrict analyses to the part of the matrix containing cortex stays justified in the context of target definition for neuromodulation tools. Again, the consideration that hallucinations may result from neural dysconnectivity (e.g., Ćurčić‐Blake et al., 2017) favors ICA over more conventional activation‐based approaches, such as GLM. Here, the ICA decomposition of time‐series provides a direct equivalent of functional connectivity components, which is more in line with the process we want to capture.
Finally, we believe that the “2S” method may have crucial therapeutic implications in the near future, notably, in optimizing strategies for repetitive transcranial magnetic stimulation (rTMS) for refractory hallucinations. Although this non‐invasive brain stimulation method has shown moderate, but significant, efficacy in reducing the severity of hallucinations (Demeulemeester et al., 2012), it remains a source of debate (Slotema et al., 2011). Its moderate effect may result from inter‐subject variation in the brain areas associated with AVH, since most rTMS protocols systematically target the left temporo‐parietal junction. Identifying with high reliability the functional networks recruited during AVH in a given individual could pave the way for new subject‐based neuronavigation strategies for rTMS treatment of hallucinations. A randomized controlled trial is currently running to test the superiority of such an fMRI‐guided strategy over conventional rTMS in the treatment of drug‐resistant hallucinations (http://ClinicalTrials.gov Identifier: NCT01373866).
Supporting information
Supporting Information Figure 1.
The authors would like to thank the GDR CNRS‐3557 Institut de Recherche en Psychiatrie, and the Life Imaging Centers from the CHU Lille and the ICube lab, Strasbourg. Experiments in Lille were partly funded by the Programme Hospitalier de Recherche Clinique (PHRC‐N MULTIMODHAL) to R.J. Experiments in Strasbourg were partly funded by the Programme Hospitalier de Recherche Clinique (PHRC‐N CONNECTIVITE) to J.R.F.
REFERENCES
- Alderson‐Day B, Diederen K, Fernyhough C, Ford JM, Horga G, Margulies DS, McCarthy‐Jones S, Northoff G, Shine JM, Turner J, van de Ven V, van Lutterveld R, Waters F, Jardri R (2016): Auditory hallucinations and the brain's resting‐state networks: findings and methodological observations. Schizophr Bull 42:1110–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P, Larøi F, McGuire PK, Aleman A (2008): The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev 32:175–191. [DOI] [PubMed] [Google Scholar]
- Allen P, Modinos G, Hubl D, Shields G, Cachia A, Jardri R, Thomas P, Woodward T, Shotbolt P, Plaze M, Hoffman R (2012): Neuroimaging auditory hallucinations in schizophrenia: from neuroanatomy to neurochemistry and beyond. Schizophr Bull 38:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amad A, Cachia A, Gorwood P, Pins D, Delmaire C, Rolland B, Mondino M, Thomas P, Jardri R (2014): The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Mol Psychiatry 19:184–191. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Flaum M (1991): Schizophrenia: the characteristic symptoms. Schizophr Bull 17:27–49. [DOI] [PubMed] [Google Scholar]
- Bazán PR, Biazoli CE, Sato JR, Amaro E (2015): Motor readiness increases brain connectivity between default‐mode network and motor cortex: impact on sampling resting periods from fMRI event‐related studies. Brain Connect 5:631–640. [DOI] [PubMed] [Google Scholar]
- Chen G, Taylor PA, Shin Y‐W, Reynolds RC, Cox RW (2017): Untangling the relatedness among correlations, Part II: Inter‐subject correlation group analysis through linear mixed‐effects modeling. NeuroImage 147:825–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornbleet PJ, Gochman N (1979): Incorrect least‐squares regression coefficients in method‐comparison analysis. Clin Chem 25:432–438. [PubMed] [Google Scholar]
- Ćurčić‐Blake B, Ford JM, Hubl D, Orlov ND, Sommer IE, Waters F, Allen P, Jardri R, Woodruff PW, David O, Mulert C, Woodward TS, Aleman A (2017): Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog Neurobiol 148:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David CN, Greenstein D, Clasen L, Gochman P, Miller R, Tossell JW, Mattai AA, Gogtay N, Rapoport JL (2011): Childhood onset schizophrenia: high rate of visual hallucinations. J Am Acad Child Adolesc Psychiatry 50:681–686.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Jirsa V, McIntosh AR, Sporns O, Kötter R (2009): Key role of coupling, delay, and noise in resting brain fluctuations. Proc Natl Acad Sci U S A 106:10302–10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martino F, Gentile F, Esposito F, Balsi M, Di Salle F, Goebel R, Formisano E (2007): Classification of fMRI independent components using IC‐fingerprints and support vector machine classifiers. NeuroImage 34:177–194. [DOI] [PubMed] [Google Scholar]
- Demeulemeester M, Amad A, Bubrovszky M, Pins D, Thomas P, Jardri R (2012): What is the real effect of 1‐Hz repetitive transcranial magnetic stimulation on hallucinations? Controlling for publication bias in neuromodulation trials. Biol Psychiatry 71:e15–e16. [DOI] [PubMed] [Google Scholar]
- Diederen KMJ, Charbonnier L, Neggers SFW, van Lutterveld R, Daalman K, Slotema CW, Kahn RS, Sommer IEC (2013): Reproducibility of brain activation during auditory verbal hallucinations. Schizophr Res 146:320–325. [DOI] [PubMed] [Google Scholar]
- Diederen KMJ, Neggers SFW, Daalman K, Blom JD, Goekoop R, Kahn RS, Sommer IEC (2010): Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. Am J Psychiatry 167:427–435. [DOI] [PubMed] [Google Scholar]
- Dierks T, Linden DE, Jandl M, Formisano E, Goebel R, Lanfermann H, Singer W (1999): Activation of Heschl's gyrus during auditory hallucinations. Neuron 22:615–621. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proc Natl Acad Sci U S A 113:7900–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito F, Scarabino T, Hyvarinen A, Himberg J, Formisano E, Comani S, Tedeschi G, Goebel R, Seifritz E, Di Salle F (2005): Independent component analysis of fMRI group studies by self‐organizing clustering. NeuroImage 25:193–205. [DOI] [PubMed] [Google Scholar]
- Ey H (1973) Traité des hallucinations: tome 1. Paris: Masson.
- Ffytche DH, Howard RJ, Brammer MJ, David A, Woodruff P, Williams S (1998): The anatomy of conscious vision: an fMRI study of visual hallucinations. Nat Neurosci 1:738–742. [DOI] [PubMed] [Google Scholar]
- Formisano E, Esposito F, Di Salle F, Goebel R (2004): Cortex‐based independent component analysis of fMRI time series. Magn Reson Imaging 22:1493–1504. [DOI] [PubMed] [Google Scholar]
- Foucher JR (2013) Perspective in brain imaging and computer‐assisted technologies for the treatment of hallucinations In: Jardri R, Cachia A, Thomas P, Pins D, editors. The Neuroscience of Hallucinations. New York, NY: Springer New York; pp 529–547. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15:870–878. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Fernandez T, Pittman B, Hampson M (2011): Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry 69:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hor K, Taylor M (2010): Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol Oxf Engl 24:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen A (1999): Fast and robust fixed‐point algorithms for independent component analysis. IEEE Trans Neural Netw 10:626–634. [DOI] [PubMed] [Google Scholar]
- Hyvärinen A, Oja E (2000): Independent component analysis: algorithms and applications. Neural Netw Off J Int Neural Netw Soc 13:411–430. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pins D, Bubrovszky M, Lucas B, Lethuc V, Delmaire C, Vantyghem V, Despretz P, Thomas P (2009): Neural functional organization of hallucinations in schizophrenia: multisensory dissolution of pathological emergence in consciousness. Conscious Cogn 18:449–457. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pins D, Delmaire C, Goeb J‐L, Thomas P (2007): Activation of bilateral auditory cortex during verbal hallucinations in a child with schizophrenia. Mol Psychiatry 12:319. [DOI] [PubMed] [Google Scholar]
- Jardri R, Pouchet A, Pins D, Thomas P (2011): Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate‐based meta‐analysis. Am J Psychiatry 168:73–81. [DOI] [PubMed] [Google Scholar]
- Jardri R, Thomas P, Delmaire C, Delion P, Pins D (2013): The neurodynamic organization of modality‐dependent hallucinations. Cereb Cortex 1991: 1108–1117. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT (2009): Investigating the functional heterogeneity of the default mode network using coordinate‐based meta‐analytic modeling. J Neurosci 29:14496–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Demeulemeester M, Leroy A, Delmaire C, Lopes R, Pins D, Thomas P, Jardri R (2016): Network dynamics during the different stages of hallucinations in schizophrenia. Hum Brain Mapp 37:2571–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennox BR, Park SB, Medley I, Morris PG, Jones PB (2000): The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res 100:13–20. [DOI] [PubMed] [Google Scholar]
- Llorca PM, Pereira B, Jardri R, Chereau‐Boudet I, Brousse G, Misdrahi D, Fénelon G, Tronche A‐M, Schwan R, Lançon C, Marques A, Ulla M, Derost P, Debilly B, Durif F, de Chazeron I (2016): Hallucinations in schizophrenia and Parkinson's disease: an analysis of sensory modalities involved and the repercussion on patients. Sci Rep 6:38152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard M, Fazekas P (2016): Can No‐Report Paradigms Extract True Correlates of Consciousness?. Trends Cogn Sci 20:241–242. [DOI] [PubMed] [Google Scholar]
- Roquet DR, Pham BT, Foucher JR (2014): Manual selection of spontaneous activity maps derived from independent component analysis: criteria and inter‐rater reliability study. J Neurosci Methods 223:30–34. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK (2000): Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry 57:1033–1038. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Cameron LA, Brammer MJ, Williams SC, Murray RM, McGuire PK (2001): Modality specific neural correlates of auditory and somatic hallucinations. J Neurol Neurosurg Psychiatry 71:688–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L (1995): A functional neuroanatomy of hallucinations in schizophrenia. Nature 378:176–179. [DOI] [PubMed] [Google Scholar]
- Slotema CW, Blom JD, de Weijer AD, Diederen KM, Goekoop R, Looijestijn J, Daalman K, Rijkaart A‐M, Kahn RS, Hoek HW, Sommer IEC (2011): Can low‐frequency repetitive transcranial magnetic stimulation really relieve medication‐resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol Psychiatry 69:450–456. [DOI] [PubMed] [Google Scholar]
- Sommer IEC, Diederen KMJ, Blom J‐D, Willems A, Kushan L, Slotema K, Boks MPM, Daalman K, Hoek HW, Neggers SFW, Kahn RS (2008): Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain J Neurol 131:3169–3177. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988) Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Tan B‐L (2009): Profile of cognitive problems in schizophrenia and implications for vocational functioning. Aust Occup Ther J 56:220–228. [DOI] [PubMed] [Google Scholar]
- Torgerson WS (1952): Multidimensional scaling: I. Theory and method. Psychometrika 17:401–419. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Frässle S, Wilke M, Lamme V (2016): No‐Report and report‐based paradigms jointly unravel the NCC: response to overgaard and fazekas. Trends Cogn Sci 20:242–243. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Wilke M, Frässle S, Lamme VAF (2015): No‐Report paradigms: extracting the true neural correlates of consciousness. Trends Cogn Sci 19:757–770. [DOI] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Röder CH, Prvulovic D, Bittner RA, Dietz MG, Hubl D, Dierks T, Federspiel A, Esposito F, Di Salle F, Jansma B, Goebel R, Linden DEJ (2005): The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. NeuroImage 27:644–655. [DOI] [PubMed] [Google Scholar]
- van Lutterveld R, Diederen KMJ, Koops S, Begemann MJH, Sommer IEC (2013a): The influence of stimulus detection on activation patterns during auditory hallucinations. Schizophr Res 145:27–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Figure 1.


