CASE HISTORY
Jai was a 14-year-old boy referred to our consultation-liaison team by a pediatric rheumatologist for treatment of functional dystonia in the neck, a subtype of functional neurological symptom disorder (FND).1* Jai lived in a suburban area of a small city with his parents, Mr. and Mrs. P, and his 16-year-old brother, Kumar. Both his parents held academic positions at the university. Mrs. P had a history of generalized anxiety disorder. Kumar was a perfectionist who was academically oriented and achieved well at school, whereas Jai was more engaged in music; he liked to compose songs and to play them on the keyboard. The medical consultations leading up to the diagnosis of FND and referral to our team had been complicated, involving numerous specialists and teams, each with their own frameworks for investigation and assessment.
Presentation
Six weeks prior to presentation to our hospital, Jai suddenly developed left-sided neck pain while getting up from the lawn. On standing, he found that his neck had flexed and twisted to the left side. After two weeks of treatment by the family doctor—one week of analgesics and a second week of analgesics and physiotherapy—Jai’s pain and posture had not improved. He was admitted to his local hospital under the orthopedic team. In the hospital Jai had a computerized tomography (CT) scan of his cervical spine. The results of the scan were difficult to interpret because Jai had not been able to straighten his neck. A subluxation (displacement of bones) of the spine could not be ruled out. Treatment included an opioid analgesic (oxycodone) and neck traction (see Figure 1, Panel A) which gave Jai some relief from the pain. During the weeklong admission, Jai developed weakness in the legs.
Figure 1.
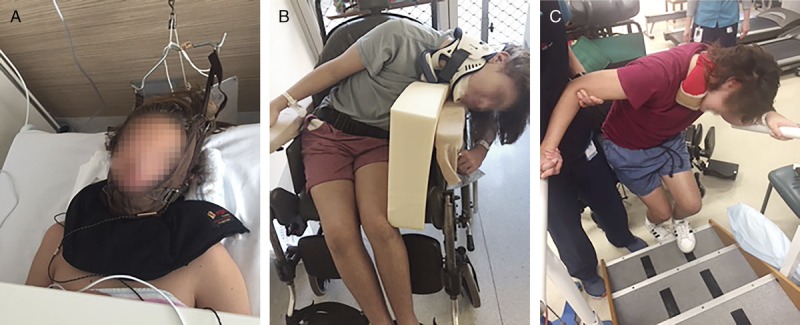
Panel A. Jai treated with traction under an orthopedic team. Panel B. Jai sitting in the wheelchair wearing the Miami J neck collar with his body and neck twisted over the wheelchair hand rests. Panel C. Functional physiotherapy: learning to do stairs toward the latter part of the treatment process.
At discharge, Jai and his parents were told that all test results had been normal and that the neck would eventually correct itself. No explanation was given for the weakness in the legs. Mrs. P reported that she had found the interaction with the orthopedic team uncomfortable and distressing. She felt that they were communicating to her that Jai had been wasting their precious time. The discharge plan was for Jai to continue analgesia, diazepam, and the neck brace (Miami J collar) for six weeks.
Although Jai could not walk and was in a wheelchair when he was discharged, no hospital assistance was provided to help Mrs. P with the task of moving Jai to and into the car. Jai ended up falling just outside the car while attempting to transfer from the wheelchair to the car. In the car he experienced significant pain, could not sit upright, and, with intermittent neck spasms on the left involving the left sternocleidomastoid muscle and part of the left trapezius muscle, became extremely distressed.
Once home, Jai’s physical condition continued to deteriorate. Despite the neck brace, Jai was unable to keep the head in midline, and his body also began to twist to the left (in the same direction as his neck). The intermittent spasms on the left side of Jai’s neck became more frequent and more intense. These spasms were worse when the collar was removed, when Jai tried to sit straight, and when he moved his shoulder. During these spasm episodes, Jai would go white, shake in pain, and grit his teeth. Although he had initially been able to walk short distances in the house with support from his mother, he began to drag his left leg more and more, and began to experience difficulties in coordinating his legs when walking.
By week 5 of his illness (one week prior to presentation to our team), Jai resisted anyone touching his collar, and he avoided any movement to his torso and shoulders because the resulting neck spasms were exceedingly painful. His body flexed more and more to the left, and eventually he dangled in a C-shape over the edge of the wheelchair, making it difficult for anyone to maneuver the wheelchair through doorways without Jai hitting his head (see Figure 1, Panel B). It became increasingly difficult for him to walk because the flexion of his body had worsened and because the movement exacerbated the neck spasms and associated pain. He ended up lying in his bed—in a curved position—for most of the day. As Jai’s physical well-being deteriorated, it became more and more difficult for the family to manage his daily needs without a commode chair and a support chair in shower. Even during sleep, Jai’s body retained the flexed position; he slept in a C-shape. And because of the pain and recurring spasms, he slept for maximum of four hours a night. In week 5 of Jai’s illness, a rheumatologist (who was also a family friend) referred Jai to a pediatric rheumatologist at our tertiary care hospital for further medical evaluation.
At our hospital, Jai was assessed and evaluated by seven different medical teams (see Appendix 1), and he had multiple other investigations (see Appendix 2). Because of Jai’s bent position, the medical teams found the scans—two CT scans and one CT angiography—difficult to interpret. Among the varied medical hypotheses that needed clarification were the following: possible subluxation and fracture of the cervical spine; arthritis of the spine causing possible subluxation; and possible calcification/injury in the vertebral artery, causing dissection. But all of these diagnoses were ruled out by studies using magnetic resonance imaging and angiography (under general anesthesia to enable Jai’s body to relax and straighten, resulting in more accurate interpretations of the images). At this point Jai was evaluated by two neurologists; except for the fixed neck dystonia, Jai’s neurological examination was normal (see Appendix 3). What was noteworthy, however, was that Jai’s trunk musculature revealed no increased tone—no torsional dystonia. The neurologists hypothesized that the C-shape of Jai’s trunk reflected a pain-related reflex response that occurred alongside the dystonia in his neck and that was actually part of the dystonia presentation. The neurologist’s diagnosis was FND, including fixed functional dystonia of the neck and functional motor loss and loss of coordination in the legs (see Text Box 1 for information about functional dystonia). Although chronic pain is technically a separate diagnostic category—a comorbid diagnosis—fixed dystonia of sudden onset is often accompanied by pain. Other types of dystonia tend not to be painful.5
Text Box 1
Functional Dystonia
Functional neurological disorders (FNDs) are among the most common causes of neurological disability and have an incidence of 4–12 per 100,000 population per year and a prevalence of 50 per 100,000 population based on a community registry.2 Dystonia is a movement disorder characterized by sustained or intermittent muscle contractions causing abnormal movements, postures, or both. Functional dystonia, part of the spectrum of FND, is relatively uncommon in the adolescent population. The functional dystonia manifests in paroxysms or as a fixed, persistent muscle contraction that sets the body part in a fixed posture. In contrast to the other functional neurological presentations, the diagnosis of functional dystonia can be especially challenging because of the lack of clinical features (e.g., entrainment of tremor) that distinguish functional from organic dystonias. Sudden onset and the presence of pain are common in functional fixed dystonia and rare in organic dystonia with the exception of cervical dystonia. The key diagnostic findings include the following: sudden onset of dystonia, with the dystonia fixed at onset; presence of pain; and variable resistance to passive manipulation.3 Functional dystonia is usually very disabling and is one of the hardest functional symptoms to treat. One study with children and adults (n = 35) showed improvement in less than a fourth of patients, remission only in 6%, and a worsening of symptoms in a third.4 There is very little literature about treatment. Key recommendations include the following: effectively communicating the diagnosis; using a multidisciplinary treatment approach involving psychological interventions and physical rehabilitation; and setting the primary goals as reestablishing movement as soon as possible and effectively controlling pain. A delay between the symptoms onset and treatment is associated with poor outcomes.
Assessment with the Whole Family
The family assessment involved all members of the immediate family—Mrs. P, Mr. P, and Jai’s brother Kumar—and a multidisciplinary consultation-liaison team (comprising a child psychiatrist, clinical psychologist, and medical resident). Because the medical investigations had been completed only a few days earlier, Jai’s parents communicated their sense of relief that the various medical diagnoses had been excluded. They also told the team, however, that the diagnosis of FND was difficult to accept because Jai came from a good, solid family whose members had experienced, as far as they knew, no significant stressors or traumas. Their sense of being puzzled was acknowledged, and the family and team agreed to proceed with the usual assessment by the psychological medicine team. The interview process used in the initial assessment is summarized in Kozlowka and colleagues (2013).6
Developmental History
Jai’s developmental history, as it emerged from the family assessment, was remarkable for the following:
-
−
Anxiety, including separation anxiety, was evident as early as Jai’s second year of life.
-
−
Dyslexia, diagnosed in fifth grade, resulted in uneven academic performance and significant bullying at school.
-
−
In sixth grade Jai was diagnosed with irritable bowel syndrome, a functional gut disorder.
-
−
In seventh grade Jai became what he described as a “de facto counselor” for school friends who confided in him about self-harm and suicidal intent.
-
−
Jai had told his parents nothing about any of the stressful events at school, though the parents noted that they had observed his stress increase over the years.
Jai’s full developmental history and the case formulation as it emerged from Jai’s story are presented in Appendix 4.
Therapeutic Contract
We offered Jai a three-week admission into the Mind-Body Program, the time available prior to the upcoming, two-week break for school holidays, with the option of a readmission thereafter. For further details of the therapeutic contract, see Appendix 5.7–14
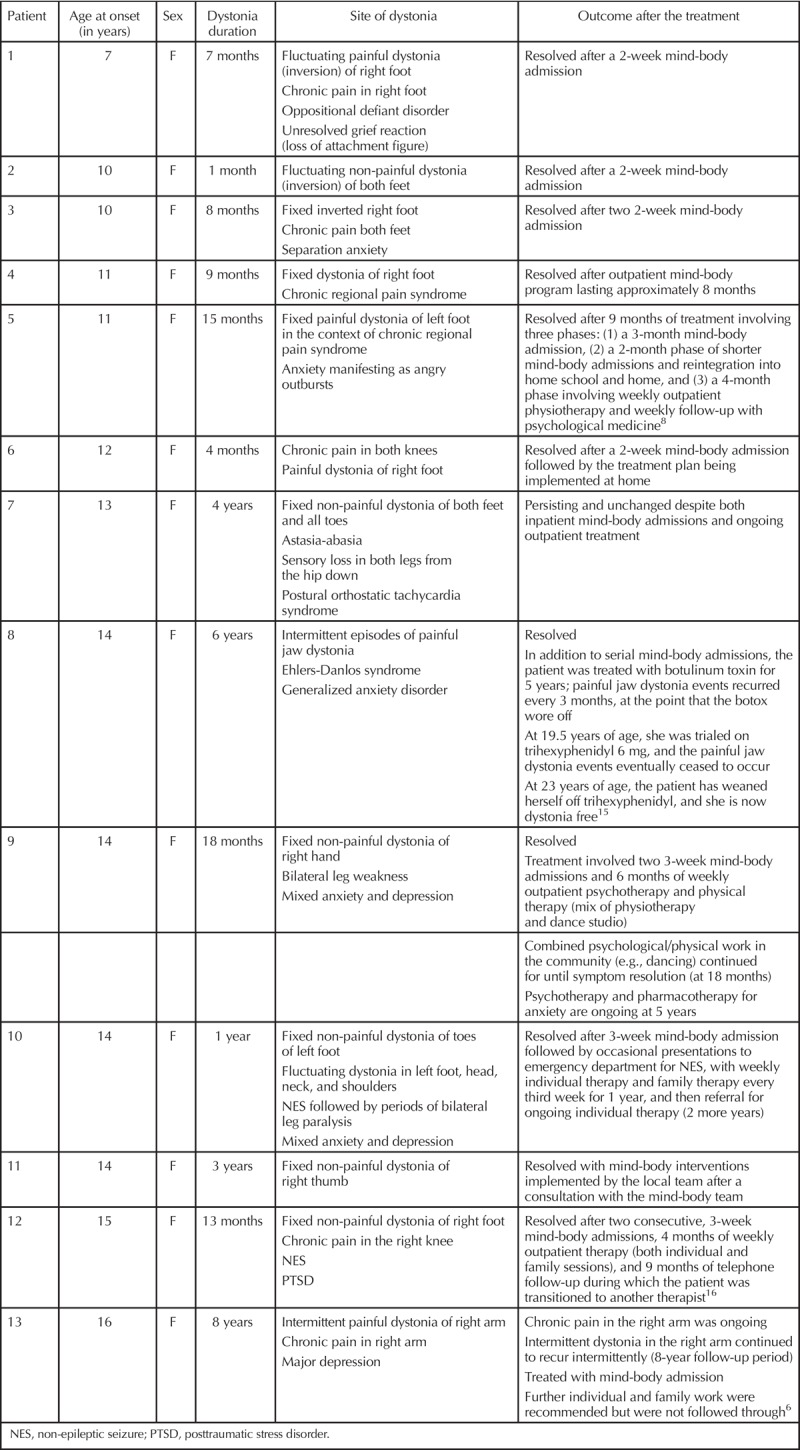
Jai’s parents were made aware that of all FND symptoms, functional dystonia was the most difficult to treat. As a specialist group, the mind-body team has had 15 years’ clinical experience treating children with dystonia (see Table 1). The team’s experience has been mixed: 11 of 13 children (85%) had recovered after one month to six years of treatment. One of 13 children continued to suffer from recurring episodes of dystonia in the right arm during the eight-year follow-up period. One of 13 had fixed dystonia in both feet and ongoing astasia-abasia and stocking distribution sensory loss—with some improvement in the severity of the dystonia and the degree of leg incoordination.
Table 1.
Outcomes of the 13 Patients Treated by the Mind-Body Team for Functional Dystonia in the Previous 15 Years
The team involved in Jai’s care included a child psychiatrist, two clinical psychologists, two residents, a physiotherapist, an occupational therapist, nursing staff on the adolescent medicine ward, and school staff in the adolescent classroom in the hospital school.
The Mind-Body Program
In this section we provide a brief outline of the key therapeutic interventions that made up Jai’s treatment program within the Mind-Body Program’s rehabilitation framework.
Pain management: Withdrawal of opiates
Following a consult with the pain team, we added pregabalin to Jai’s medication regimen to help with chronic pain and opiate withdrawal.† Although Jai reported a reduction in pain with pregabalin, managing his pain after full withdrawal of oxycodone presented a challenge because Jai’s level of pain rendered him unable to participate in physiotherapy sessions and affected his sleep. For details concerning the continued use of oxycodone (through week 14 of the admission), see sections below on sleep interventions and physiotherapy. The pregabalin was itself discontinued once Jai had returned full time to school, a year after the onset of his illness.
Ongoing nursing interventions
The ongoing nursing interventions are described in Appendix 6.
Regulating sleep
As previously mentioned, Jai had difficulties falling asleep when he was admitted to the Mind-Body Program. He would fall asleep between midnight and 2 a.m., and then wake up three or four hours later because of pain resulting from his neck spasms. Unable to manage the pain in his twisted position in bed, he would request nurses to transfer him back to his wheelchair. Jai’s sleep difficulties were also complicated, as was gradually disclosed over a period of weeks, by a severe depression. The components of sleep intervention, which took three months to implement fully, were as follows:
-
–
Sleep hygiene. Jai and the team established a regular bedtime/waking schedule, including a ban on using electronics in late evening. The schedule was displayed on each day’s timetable.
-
–
Pharmacotherapy. Jai was very keen to try any medication that could help him with his sleep. Melatonin (increased from 3 mg to 6 mg to 9 mg) helped somewhat with sleep initiation. Next, clonidine was added to help decrease arousal at bedtime and to help Jai to fall asleep by 11 p.m. Next, because Jai continued to wake with pain and to remain awake in the early hours of the morning, quetiapine was added (starting at 25 mg and gradually increasing up to 75 mg) to help manage arousal, sleep quality, and duration of sleep, and to reduce pain-related agitation in the early morning hours.18 With all these interventions combined, Jai’s sleep normalized (week 3 of the program and week 9 of his illness), and his capacity to participate in the physiotherapy and occupational therapy program increased.
-
–
Hypnosis. A self-hypnosis intervention was added to the nighttime sleep routine. Using it helped Jai get to sleep and to fall back to sleep if he woke in the middle of the night (for more on hypnosis, see later subsection on hypnosis).
Trial of botulinum toxin
Following a consult with the rehabilitation team, botulinum toxin was trialed. The administration of botulinum toxin improved Jai’s capacity to engage in physiotherapy but had no impact on his fixed dystonia. For full description of this intervention, see Appendix 7.
Arousal
Children and adolescents with FND typically present in a state of increased arousal and motor activation.19 Jai’s activated body state was evidenced by a chronic increase in respiratory rate (typically 20–24 breaths per minute in therapy sessions). Slow breathing—which Jai initially found very difficult—was introduced and continued. A range of other mind-body strategies—progressive muscle relaxation and imagery strategies—were introduced to help Jai work on decreasing his arousal and to manage pain. Jai had some difficulty utilizing scripted imagery but found that imagery based on music and positive memories of music was helpful and could assist with pain management.
Depression
By the third hospital week, Jai became more open about his mental state and, in particular, his thoughts of self-harm. Especially at first, however, any efforts to probe further led Jai to immediately shut down.
At home during the two-week holiday break, Jai fell into a depressive funk‡ punctuated by intense, debilitating panic attacks in response to virtually any demand placed upon him. Throughout this period, Jai’s mother and often Jai himself were in regular contact with the treatment team, mostly in an effort to stabilize the situation until Jai could return for treatment after the holiday.
Over the course of Jai’s second hospital stay, which lasted almost four more months, he became increasingly forthcoming in discussions of his mental state. At baseline, he felt deeply ashamed not only of his thoughts of self-harm (which he had mentioned prior to the holiday break) but of his long-standing low mood and intermittent suicidal ideation. To address his ongoing severe depression, we changed the quetiapine to the sedating antidepressant mirtazapine (starting at 7.5 mg and gradually increased to 22.5 mg). Despite significant sleep disruption during this change in medications, Jai’s sleep eventually settled again (at the 22.5 mg dose), and his mood began to improve.
Psychological intervention for depression and anxiety
Jai’s growing capacity to disclose the extent and severity of his depression, including suicidal ideation and compulsions to self-harm, broadened over time to include discussions of his very long-standing anxiety, including (more recently) his severe panic attacks. This act of talking about depression and anxiety, as well as the implementation of a safety plan, was therapeutic in and of itself. With time, and a lot of psychoeducation, Jai’s sense of shame resolved, and he was able to talk about his symptoms in a more matter-of-fact way. With his therapist§ he went on to explore and establish alternative ways to cope with stress, pain, panic attacks, thoughts of self-harm, and periods of wakefulness at night.
To manage Jai’s compulsions to self-harm, distress-tolerance strategies were introduced (e.g., holding ice in hand), as were other sensory strategies (e.g., listening to music) and distraction techniques. Jai was able to utilize these strategies well; thoughts pertaining to self-harming ceased. He also demonstrated an increased ability to communicate when he was distressed—initially with the treating team, but increasingly also with his parents. He began to compose music and used his keyboard to play the music and manage periods of distress.
Psychoeducation regarding panic attacks and avoidance helped Jai accept the graded exposure therapy (in particular, having Jai go outside, which had been a trigger for panic attacks) that was introduced into his schedule. Cognitive-behavioral strategies enabled him to challenge unhelpful thoughts during exposure tasks. Jai was also encouraged to “ride the wave” if he found himself in a panic attack, and to accept that he would not always be able to recognize the early warning signs of his panic attacks and to intervene in time. With continued exposure to triggering situations, Jai’s panic attacks and anxiety reduced. Throughout his admission, however, managing panic attacks continued (by design) to be a challenge, for we added new challenges to his schedule on a weekly basis (e.g., car transfer practice prior to discharge).
In psychology sessions Jai explored his tendency to catastrophize and get stuck in an “I can’t do it” mind-set. As time went on, Jai showed an increased capacity for self-reflection and was able to openly discuss how his thinking patterns sometimes led him to feel “stuck” in terms of functional gains—contributing, in turn, to his depression, anxiety, and low motivation. In sessions with the occupational therapist and physiotherapist, Jai was able to implement, with prompting, cognitive strategies to challenge his counterproductive thinking patterns, but he continued to experience difficulty doing so independently.
Hypnosis
We routinely use hypnosis in our Mind-Body Program. Hypnosis is a top-down regulation strategy that some children are able to use to help in managing physiological arousal, pain, and anxiety.20–23 In Jai’s case, the hypnotherapist quickly realized that Jai could readily enter a trance state. Hypnosis was therefore introduced into all of Jai’s physical sessions—physiotherapy and occupational therapy—to manage his pain and dystonia during those interventions.
Jai would go into trance at the beginning of each physical session. Suggestions were made about Jai being able to experience a state of deep relaxation; about disconnecting from his pain; about the flexibility of his body to move like a reed in the wind or like a tree; and so on. While Jai was in trance, the physiotherapist or occupational therapist would talk to Jai and implement that day’s intervention (see below). Jai was able to use the hypnosis to disconnect from his pain and to allow the therapists to reposition his neck and body into a straighter position.
A key challenge of using hypnosis was the process of coming out of trance. Despite strong, repeated hypnotic suggestions about staying calm, when Jai came out of trance—and when he became aware of his body being in a more upright position—he would perceive this upright position to be twisted and abnormal, and he would panic. In other words, Jai’s brain was processing the C-shape position as straight and the straight position as twisted. Typically, in the early phase of the treatment program, each physical session consequently ended in panic, and usually a full-blown panic attack. Later on, as Jai got used to the discrepancy of what the mirror showed (Jai sitting more straight) and what his brain perceived (Jai as twisted), he began to panic less. With time he began to work on attaining the new position on his own throughout the day.
Jai found the hypnosis less effective when he did it himself (self-hypnosis). At bedtime, using a hypnosis script that his hypnotherapist had recorded on her smartphone and then transferred over to his computer, Jai worked on putting himself in a trance state; this enabled the nursing staff to help Jai position himself in a straighter shape. Over time, Jai was able to establish and maintain a less curled C-shape, then an L-shape, and finally a straight sleep position throughout the night.
Physiotherapy, occupational therapy, and hypnosis
Physiotherapy, occupational therapy, and hypnosis need to be considered together because the three therapists closely collaborated to enable physical therapy sessions. In the first three weeks of the physiotherapy intervention—with no oxycodone available for the pain—Jai could not participate in the sessions at all; movement triggered muscle spasms accompanied by unbearable pain. Given the lack of progress, he was allowed to take oxycodone before physiotherapy sessions, which then enabled him and the therapist to make some small advances such as practicing transfers from wheelchair to bed, practicing standing with the assistance of a forearm frame, and practicing collar-free time, which was necessary for managing pressure sores on the back of Jai’s neck. It was not until hypnosis was used to manage Jai’s pain, however, that the physical work—the physiotherapy—began in earnest. The key components of this intervention included, in order of implementation: weaning of the collar; strengthening exercises; improving functional positions (better sitting position, better sleeping position); gait reeducation; using aids (walking frame, toilet support, shower support chair, walking sticks) for functional independence; and exercise and skill-based training that used games and hydrotherapy to improve mobility and safety, prevent secondary complications, and facilitate independence.
Under hypnosis, Jai was able to disconnect from his pain, to attain a state of pleasant deep relaxation, and to visualize his body as bendable and flexible. In this state he allowed the therapist to work on repositioning him in his wheelchair—bit by bit, over time—into an upright posture. The occupational therapist gradually introduced a sideboard, supported by two pillows and a wedge, to help Jai sit upright. Eventually, Jai was able to start self-adjusting his position himself and to independently insert his postural supports. And as noted in the above subsection on hypnosis, he was, over time and with hypnosis, eventually able to main a straight sleep position on his own through the night. With the help of hypnosis, Jai was also able to transition progressively to increased use of a soft collar instead of the hard Miami J collar, and finally, he was able to keep his neck in a reasonable position without any collar. Three-and-a-half months into the hospital stay—week 20 of his illness—Jai was able to maintain his position without postural supports and was transitioned into a dynamic, self-propelled, manual wheelchair.
Toward the end of his admission, Jai’s goals were entirely focused on his daily-living skills, and his mother became part of the team supporting him. The occupational therapist—working closely with the physiotherapist and hypnotherapist—took on a prominent role at this stage. Examples of the challenges facing Jai include the following:
-
−
In order to go home, Jai needed to be able to travel by car. But at first, the mere idea of getting into a car would trigger a panic attack, and his body and neck would begin to twist painfully. It actually took a month or more of practice for Jai to get into a car and maintain a position that was considered safe.
-
−
He needed to be able to walk up a flight of stairs (see Figure 1, Panel C).
-
−
He did his land-based physiotherapy outside in order to practice walking on rough ground.
-
−
He practiced his toileting and showering independently so that he could continue to implement such daily-living skills at home.
Jai initially experienced mounting pressure in these preparations to return home, but the hypnotherapist continued to have an important role in helping him manage these new tasks. The management of Jai’s fatigue was also a challenge because he found the preparations for home to be exhausting, as was the management of his anxiety and mounting fear (and the family’s fear) about what would happen when he went home.
Hospital school intervention
The hospital school intervention is described in Appendix 8.
Family intervention
For details of the family intervention, see Appendix 9.4
Discharge
When a discharge date was proposed, Jai experienced an increase in anxiety—including a renewed need to wear his Miami J neck collar, even though he had not been using a collar for many weeks. Jai was able to use his psychology sessions to understand that the hospital had become his “safe place”; he was acutely worried about adjusting to being at home again. Jai’s worries were acknowledged, and he was able to work with the team to implement limitations and rules that would come into play in the home setting; he would, as a consequence, be able to adjust to those projected new constraints in advance of going home. As an example, Jai noted that parental restrictions on screen time would make going home difficult, and he came up with a plan of how limits that approximated home could be implemented in hospital. During this time of increased anxiety, his progress in physical therapy became patchy: although he continued to make progress with car transfers, his progress with mobilizing on the frame and crutches stalled.
In the last days of his admission, Jai was in a positive frame of mind. With his psychologist he had formulated some future-oriented plans with regard to visiting the hospital when he was well, including walking into the hospital independently, challenging his physiotherapist to basketball, and taking the occupational therapist for a drive once he got his driver’s license.
At discharge Jai was able to shower himself and use the commode chair; he needed assistance, however, with transferring from the wheelchair to the bathroom, with preparing the bath, and with drying himself. He was able to sit in a car for up to 40 min prior to discharge.
Post-discharge Progress
On discharge (week 30 of Jai’s illness), Jai was referred to local health services—a child and adolescent mental health team and physiotherapy—to continue the multidisciplinary intervention that had begun in the hospital setting. A few weeks after discharge (week 34 of Jai’s illness), in a telephone call with the team, Jai reported that the local physiotherapist was not pushing him enough. The team suggested that Jai had all the physiotherapy experience he needed and that he could push himself to make progress with his walking and with getting himself out of the wheelchair. Five weeks following discharge (week 35 of Jai’s illness), Jai stood upright independently, without any support. His body felt “weird” and “different,” and he felt some panic. In a phone conversation the team reminded Jai that when he had begun to sit up straight, his brain had also tricked him then; it had perceived the C-shape as being straight and the straight position as twisted. The team also reminded Jai that it had taken six weeks to adjust to the change. The team suggested that he slow down his attempts to walk. We suggested that on standing up, he use the grounding imagery of a tree and that he take time to feel his feet on the floor, to help his brain readjust to the new straight standing position (see Figure 2). Within a week (week 36 of Jai’s illness), Jai was walking independently.
Figure 2.
Jai, just recovered from his illness, poses for a photo with the two pediatric residents who were part of the mind-body team that worked with him during his illness.
Jai’s regaining his physical function did not deter the family from following through on the various psychological and physical interventions that had been started in the hospital setting. Jai joined a gym, where he continued his fitness program with the help of an exercise physiologist and a personal trainer; he continued with psychology sessions for managing his anxiety and now resolved depression; and the family pursued ongoing work with a family therapist.**
QUESTIONS TO THE CONSULTANTS
-
−
What are the primary components of treatment for patients with functional dystonia and related functional neurological symptoms?
-
−
What is the role of hypnosis in the treatment of FND and comorbid chronic pain?
-
−
What role does stress play is the underlying neurobiology of FND and comorbid anxiety, depression, and pain symptoms?
David L. Perez, MD, MMSc
This case history is an excellent example of the utility of a biopsychosocial formulation and the use of evidence-based, patient-centered treatments for managing FND. Furthermore, while there are similarities in the management of adult- and adolescent-onset functional dystonia,24 pediatric and adolescent FND offers some distinct treatment challenges, including the need to use age-appropriate treatment strategies and strong consideration of interpersonal dynamics involving the patient, parents, siblings, extended family members, friends, and school environment.
The first and potentially most important step in treating FND is to deliver the diagnosis.25,26 If done correctly, this can aid acceptance of the diagnosis and enhance a patient’s confidence in the treatment plan; less optimal diagnostic discussions may leave the patient and family confused and wondering whether the clinical team is not adequately hearing their concerns. When presenting the diagnosis of FND to patients, emphasis should be given to naming the condition (functional dystonia, a FND subtype) and to communicating that it is real, common, and treatable. It can be discussed with the patient and family that the condition is diagnosed based on the physical examination signs and clinical history (in this case, abrupt onset, fixed posturing, and concurrent onset of pain).27 The opportunity should be given to patients and families to ask questions, and educational material can be provided, including free information from websites such as www.neurosymptoms.org or www.fndhope.org. It is also generally important to highlight that the provider does not feel that the patient is feigning his or her symptoms. Caution should be taken to avoid giving patients the explicit message that symptoms are all “emotional or stress related,” and it should be emphasized that links between affective symptoms, psychosocial stress, and functional neurological symptoms, while important, are often nuanced and indirect.
Once a patient and the immediate family are receptive to the diagnosis, the core components of treatment for functional dystonia and related functional motor symptoms include physical interventions (e.g., physical therapy, occupational therapy),28–30 psychological interventions (including a component of cognitive-behavioral therapy [CBT] to explore the interactions between functional neurological symptoms, thoughts, behaviors, emotions, and psychosocial factors),31,32 and management of comorbid psychiatric and somatic (e.g., pain, fatigue) symptoms that may have prognostic implications.33–35 While the above components are core aspects of treatment, the identification of patient-specific predisposing vulnerabilities, acute precipitants, and perpetuating factors should be elucidated to help individualize treatments with these factors in mind.36 In this case, predisposing vulnerabilities included early-life separation anxiety, dyslexia with academic difficulties, bullying at school, tendency to not verbalize stress and affective symptoms to supports, and the concurrent presence of another functional somatic disorder (irritable bowel syndrome). In the presented case, dystonia and pain symptoms occurred abruptly and spontaneously, though in the literature a minor physical injury has sometimes been described as precipitating onset of some FND presentations.37 Several perpetuating factors were elucidated in the case history, including comorbid pain with opiate use, a severe depression with insomnia that was not initially disclosed, catastrophizing, health anxiety, and altered bodily perceptions that have been described in the literature.38
The mind-body treatment program detailed in this case is a good example of using evidence-based practices with an individualized treatment component tailored to the specifics of the case. The patient’s prominent pain, depression, anxiety, insomnia, poor distress tolerance, negative rumination, and abnormal movements are all considered in the context of an interdisciplinary neuropsychiatric perspective. While the evidence of psychotherapy for FND is strongest for CBT and CBT-informed approaches,39,40 emerging evidence suggests that hypnosis may also be a therapeutic modality to consider in some patients.41 The challenges around discharge planning, including anticipatory anxiety and fluctuations in symptom severity, are also detailed well in this case description. Overall, it is important to note that treatment paradigms for FND, particularly patients with pediatric FND, are very much in evolution and understudied.42,43 Large-scale prospective studies across neurology, psychiatry, and allied disciplines are needed to further investigate and identify the optimal evidence-based treatments for patients with functional dystonia and related motor FND.
Helene Helgeland, MF, PhD
The case of Jai is an excellent example of how hypnosis is integrated in multidisciplinary, integrative treatment of an adolescent with severe FND intertwined with symptoms of pain, physiological arousal, anxiety, and sleep difficulties. In research and the clinic, it is now understood that FND and chronic pain are best conceptualized as resulting from a complex, reciprocal interaction of biopsychosocial factors and that the treatment must be multidisciplinary and tailored to the individual patient.20,44 Most likely, the total effect of such treatment programs has a greater effect than the just the additive effects of the separate treatment components alone.45
An increasing evidence base supports the efficacy and safety of clinical hypnosis in acute, chronic, and procedural pain, depression, anxiety, irritable bowel syndrome, and sleep problems.46–51 Clinical hypnosis may also enhance the efficacy of other therapeutic approaches.45,52,53 As part of an integrative intervention for chronic pain, the value of hypnosis is well documented in adults, and the research evidence for efficacy of pediatric pain management is increasing.45 Audio recordings of hypnotherapy sessions for self-exercise purposes have also been shown to be helpful as a tool to help young patients with functional abdominal pain practice at home.54,55
In the case presented here, the close, interdisciplinary teamwork is a prerequisite for treatment success. In a safe environment surrounded by collaborating, caring clinicians who share the same understanding and use a positive, purposeful language to stimulate mastery, motivation for change, and the expectation of a positive outcome, Jai is willing to engage in hypnosis and self-regulatory processes, which eventually calm him down and reduce his pain and anxiety. Under these circumstances and in hypnosis, he is susceptible to the beneficial suggestions from the therapist. This helps him to let go of his inner expectation and beliefs that it is only by holding his truncus in a fixed, twisted position that he can avoid pain and deterioration. He is subsequently able to engage in physiotherapy and occupational therapy that help him to normalize his movement patterns and to regain his capacity for the activities of daily living.
Hypnotherapy is a relational process that uses “hypnosis in the treatment of a medical or psychological disorder or concern.”56 The case presentation underscores that clinical hypnosis is not just an instrumental procedure but is one that integrates the knowledge and skills of hypnosis into clinical practice with a goal of enhanced patient coping, control, and symptom reduction.57,58 The treatment of Jai illustrates not only that clinical hypnosis is applicable to different symptoms but that it is potentially beneficial in managing other tasks and problems. For example, before discharge from the hospital, Jai uses his skills in practicing new tasks (traveling by car, walking on the ground, toileting) together with the therapist. To complement and reinforce the therapy and to promote skills in self-hypnosis, the treatment of Jai also includes audio recordings of hypnotherapy sessions for self-exercises at bedtime. Finally, but no less important, hypnosis is a ongoing skill set for the patient; people can and do continue to practice self-hypnosis, once learned.59
In one of several existing definitions, hypnosis is defined as a spontaneously or induced “state of consciousness involving focused attention and reduced peripheral awareness characterized by an enhanced capacity for response to suggestion.”56 The suggestions—what you communicate verbally and nonverbally to the patient in hypnosis—are central in hypnotic communication and represent an invitation or a proposal to the patient to experience themselves and the world differently; the process may lead to changes in patient’s sensations, perceptions, emotions, thoughts, and behavior.52,53,60 A person who is able to respond to hypnotic suggestion is often denoted as highly hypnotizable.52,56 To enhance the person’s susceptibility for suggestions (hypnotic responding), the therapist can help the patient to enter hypnosis by using specific suggestion techniques—inductions—such as eye fixation, progressive muscle relaxation, or guided imagery.52
Research on clinical hypnosis is rapidly evolving, and the understanding of hypnosis and above-mentioned concepts are likewise evolving.52,61 Much remains to be elucidated. For example, it remains unclear whether suggestions, to be effective, require induction and a hypnotic state.52,61 Researchers have reported a strong correlation between people’s responses to suggestion both in- and outside hypnosis.61,62 Moreover, it is possible that the effect of induction on hypnotic responding may be due to enhanced patient motivation and expectancies.61
The healing effect of beneficial clinical communication applying basic principles of hypnosis is gaining attention.53,57 Many patients have a narrowed focus of attention when they walk into the doctor’s office, are admitted to the hospital, are in pain, are about to start a painful procedure or treatment, or are receiving crucial information about their scary symptoms.57,58 What the doctor or therapist says and does in the clinical encounter can thus have a substantial impact on patients’ beliefs, evaluation of threats, experience of control, and behavior.53,57
In an elegant investigation, Benedetti and colleagues63 showed that the meaning of pain was changed from negative to positive in healthy subjects through positive verbal information (suggestions) about a forthcoming painful procedure.63 The only difference in the research setup between the two groups of subjects was the use of positive versus negative verbal information. The researchers concluded that, “when the meaning of the pain experience is changed from negative to positive through (positive) verbal suggestions, the opioid and cannabinoid systems are co-activated and these, in turn, increase pain tolerance.”62
The exact mechanisms behind the phenomena of hypnosis and suggestibility are still unclear, and little is known about causality.23,52,64 Hypnosis and suggestions are found to change the function of specific brain networks.52 An individual’s response to hypnosis is highly complex, however, and seems to be best explained by the combined effect of biological, psychological, and social factors (i.e., certain neurophysiological characteristics in the brain, expectancies, trait hypnotizability, motivation, absorptive capacity, rapport, and context).23 An interesting scoping review from 2015 concludes that “different factors may contribute more or less to outcomes in different subsets of individuals or for different conditions.”23
The treatment of Jai illustrates the value of integrative, multidisciplinary, and individualized treatment for this young boy with a severe, complex combination of symptoms and comprehensive functional disability. The use of hypnosis seems to be an independent therapeutic factor itself and a catalyst for the effect of other treatment modalities. It is to be hoped that this case presentation will inspire clinicians to learn more about clinical hypnosis and implement the skills in their clinical practice.
George P. Chrousos, MD, ScD
This is a fascinating case of an adolescent boy with debilitating functional dystonia of the neck, a subtype of functional neurological symptom disorder (FND) associated with medically unexplained neurological symptoms. Skeletal muscle dystonia is due to aberrant, simultaneous activation of agonist-antagonist muscle groups, resulting in spasm and, possibly, pain. This is a top-down problem starting from the motor areas of the brain, which regulate muscle tone and coordinate the proper function of skeletal muscles. FND can vary from mildly symptomatic to a severely symptomatic, even debilitating disease, as observed in this patient.
It is interesting that in the patient’s family there is history of anxiety and that in his early childhood the patient had significant separation anxiety. These point toward an increased genetic/epigenetic background vulnerability to stress in the patient.65,66
The presenting complaint was neck dystonia. Initial evaluation produced no “organic” etiologic explanation. Gradually, however, the patient’s clinical presentation worsened, with development of non-symmetric, one-sided muscle weakness, significantly disturbed body image, impaired sleep (mainly insomnia), anxiety, phobias, panic attacks, depression, and self-harm and suicidal ideation.
The treatment of the patient was lege artis and quite protracted. The process of healing included mind-body methods, including hypnosis and CBT, symptomatic pharmacotherapy, and sleep hygiene therapy. The end result was satisfactory. But the question remains: what was happening in the patient’s brain leading to the development of medically unexplained neurological symptoms, and how did healing work?
Recently, it has become apparent that the so-called medically unexplained symptoms, which actually bring many patients to primary care physicians, are quite common in children, adolescents, and adults.67,68 Medically unexplained symptoms include anxiety and depressive symptomatology, fatigue, and various pains (such as abdominal pain, headaches, cervical and low back pain), as well as dizziness, nausea, and so on. These symptoms were, until recently, called “nonspecific” and “functional,” but they are neither. Now we know that medically unexplained symptoms are actually peripheral manifestations of two very significant survival systems of our organism—the stress and the immune systems. Chronic activation of these systems leads, respectively, to the so-called stress syndrome and sickness syndrome, which may include all the above manifestations. Indeed, adults with medically unexplained symptoms have elevated stress and inflammation biomarkers that are well correlated with chronic effects in body composition, indicative of chronic stress and inflammation—that is, increased adiposity and osteosarcopenia.67
Acute, repeated, and chronic stress are associated with biochemical changes in the brain and periphery of the human body that may result in acute or chronic pathology effected primarily via the classic stress and inflammatory mediators.65,66 Thus, there are acute pathologic processes, such as episodes of asthma, urticaria, eczema, or psychosis, that may be triggered by acute stress, as well as chronic pathologic processes that may be triggered and sustained by repeated or chronic stress. These include anxiety and depressive symptomatology, fatigue, pain, insomnia, obesity, insulin resistance, metabolic syndrome, hypertension, cardiovascular disease, and generally all the so-called chronic noncommunicable diseases that plague modern societies.
In both acute and chronic stress, corticotropin-releasing hormone, vasopressin, α-melanocyte-stimulating hormone (α-MSH), ß-endorphin, and norepinephrine are elevated in the brain, while ACTH, cortisol, norepinephrine, and epinephrine are elevated in the periphery. Corticotropin-releasing hormone is also secreted by peripheral postganglionic nerves that innervate and actively degranulate mast cells, while norepinephrine and epinephrine increase systemic interleukin-6 (IL-6) secretion via ß-adrenergic stimulation. IL-6 is an inflammatory cytokine, which is elevated in response to any type of stress, including that caused by psychosocial stressors, and which causes fatigue and somnolence, as well as other manifestations of the sickness syndrome, including social withdrawal. Substance P, secreted in painful states, α-MSH, and ß-endorphin have a suppressive effect on the hypothalamic-pituitary-adrenal (HPA)–axis component of the stress system, while neuropeptide Y (NPY), co-secreted with catecholamines, has a suppressive effect on its locus coeruleus/norepinephrine component. Interestingly, most adults with a chronic pain or fatigue disorder have low activity of their HPA axis—which might have been the case in the patient.
Why would the brain cause and sustain a painful symptom, such as that of muscle dystonia, that would stimulate the stress response? Possible non–mutually exclusive explanations involve the relief of anxiety and depressive symptomatology through regression to an early stage of life with dependence on adults, redirection of brain pathways away from those associated with psychic pain, and the effects of α-MSH, ß-endorphin, substance P, and neuropeptide Y on the stress system. Alterations in brain connectivity detected by functional MRI might have provided explanations for this patient’s fascinating clinical presentation and response to standard-of-care treatment.
The therapeutic approach that was followed to manage and “cure” the debilitating muscle dystonia, along with the rest of the symptoms and signs of this patient, is the one recommended. One wonders, though, whether more drastic approaches to alter this kind of profound brain dysfunction might have been tried, including the use of electric shock or intravenous ketamine, as employed in severe depression, after which a “rebooting” of the brain might reestablish a healthy brain homeostasis.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
The authors would like to thank Jai and his family for allowing us to publish this case and, through it, to help other patients and their families.
APPENDIX 1. Teams Involved with Jai’s Evaluation and Care
-
−
Pediatric Rheumatology
-
−
Orthopedics
-
−
Neurology
-
−
Pain team
-
−
Neurosurgery
-
−
Rehabilitation Medicine
-
−
Adolescent Medicine
-
−
Psychological Medicine
APPENDIX 2. Summary of Medical Investigations
(All investigations normal except as indicated by bold type)
Blood investigations
-
−
Full blood count
-
−
Erythrocyte sedimentation rate
-
−
C-reactive protein
-
−
Serum electrolytes
-
−
Calcium/magnesium/phosphate
-
−
Renal and liver function tests
-
−
Iron studies, vitamin B12, and folate
-
−
25-hydroxy cholesterol
-
−
ANA
-
−
HLA B27
-
−
Rheumatoid factor
Imaging
-
−
Cervical spine X-ray: Suboptimal visualization due to torticollis and overlapping chin
-
−
Cervical spine CT: No significant subluxation/dislocation and no significant soft tissue abnormality. The left lamina of C1 has a sclerotic border (in the region of the left vertebral artery) suggestive of a possible healed fracture (subsequently this was understood to be a calcification). This appearance was suggestive of a subacute or chronic process, but the appearance could also reflect calcification in a chronic vertebral artery dissection
-
−
CT Angiogram: Focal short segment mural calcification of V3 segment of the left vertebral artery at the level of C1, associated luminal narrowing although there was contrast opacification beyond this point with mild post-stenotic dilatation. The appearance was in keeping with a previous injury of the left vertebral artery at the level of C1
-
−
MRI spine:Normal appearance of the cervical spinal cord, vertebral bodies, and musculature. There was no evidence of vertebral artery stenosis; in particular, the left vertebral artery at C1 appeared to be of normal caliber
-
−
MRI brain: Normal
Electrophysiological studies
-
−
ECG: Normal
APPENDIX 3. Neurology Examination
Cranial nerve examination
-
−
Intact visual field
-
−
Pupils equal and bilaterally symmetrical reacting to light
-
−
Free range of eye movements, with no nystagmus
-
−
Symmetrical face movements
-
−
Symmetrical rise of the palate, midline tongue protrusion
-
−
Shoulder elevation: right side, full range; left side, spasm and elevated in resting position
Tone
-
−
Normal tone in bilateral upper limbs and lower limbs
-
−
Tone assessment difficult in neck muscles due to pain and spasm
-
−
Normal tone in trunk muscles (on palpation)
-
−
No evidence of dystonia in facial muscles
Power
-
−
Shoulder adduction full bilaterally 5/5
-
−
Elbow flexion and extension full bilaterally 5/5
-
−
Wrist dorsiflexion and flexion full bilaterally 5/5
-
−
Finger flexion and extension full bilaterally 5/5
-
−
Hip flexion and extension bilaterally 5/5
-
−
Knee flexion and extension bilaterally 5/5
-
−
Ankle plantar flexion and dorsiflexion bilaterally 5/5
Reflexes
-
−
Triceps jerks 2+ bilaterally
-
−
Biceps jerks 2+ bilaterally
-
−
Knee jerks 2+ bilaterally
-
−
Ankle jerks 2+ bilaterally, no clonus
-
−
Abdominal reflex: normal and symmetrical
-
−
Plantar reflex: down going bilaterally
Sensations: sensation to crude touch – normal over all dermatomes
Cerebellar signs
-
−
Finger to nose testing normal bilaterally with no intention tremor or past pointing
-
−
No dys-diadochokinesia
-
−
Heel to shin test normal
Gait: Unable to walk due to pain, position, and reported leg weakness
APPENDIX 4. Family Assessment Interview
As in the case of many parents whose children have been diagnosed with FND, Jai’s parents came to the initial interview somewhat puzzled. From their perspective, neither Jai nor the family had experienced the stress or trauma that are commonly understood as underlying functional somatic symptoms. The approach we use in the Mindy-Body Program for addressing this situation is to tell the family that we understand their puzzlement but that it needs to be put temporarily on hold while we take a careful development history to see what emerges. The following is Jai’s developmental history at it was disclosed by this process. As intended, this process enabled Jai and his parents to accept the diagnosis and also mobilized their engagement in the treatment program.
DEVELOPMENTAL HISTORY
The early part of Jai’s developmental history was provided by his parents, with Jai and his brother becoming more and more vocal about their recollection of events as the timeline covered events that they could remember.
Jai was born at term as a result of a planned pregnancy that was complicated by intrauterine growth restriction. His birth weight was 1.8 kg. Jai spent four weeks in a special care nursery to establish feeding and for weight gain. Jai met all his developmental milestones appropriately. He had always been a fussy eater, liking only bland foods and preferring to eat foods that he knew. Anxiety—in particular, separation anxiety—emerged in the second year of life and continued to be a recurring problem throughout Jai’s development. In the preschool years, Jai had daily difficulties with separating from his mother to attend preschool, and it took him hours to settle each day. In kindergarten, Jai’s difficulties with separation anxiety continued, and he found the kindergarten work stressful and challenging. Throughout this time Jai also used thumb sucking to regulate, a behavior that continued well into primary school.
In primary school, difficulties with sleep became a problem. Jai slept well when sleeping with his mother, but he found it difficult to sleep on his own. During this period, Jai’s struggles with learning continued, and he reported that he had had to push himself to get out of bed in the morning and go to school. Despite this, he had good friends and enjoyed the social aspects of school. In Year 5, Jaiwas finally diagnosed with dyslexia. He then received some special provisions that made learning a little easier.
When the interview got to Year 6 (a year that Jai repeated in school), Jai took over the telling of the story. He talked about the significant bullying that he had faced in relation to his learning difficulties. He said that he had not told his parents about the bullying because he had not wanted to worry them. He said that on one occasion he had punched a bully in the face and that this intervention had helped to reduce the bullying. Jai denied low mood during that time, though he said that he had been stressed throughout the year.
Jai’s parents listened attentively to his story and were deeply touched by it. Mrs. P confirmed that she had not known about the bullying. She reported, however, that she had noticed that Jai had appeared more stressed. In particular, he had additional difficulties in sleeping and, in the morning, increased anxiety about eating. She recounted how at the end of Year 6, Jai’s anxiety had become so severe that he had resisted getting out of the car at the school gatemost days, and that Mrs. P had had to seek assistance from teachers to get him into the school playground.
Jai agreed with his mother’s account of the story. He shared memories of his mother feeding him food that he had not wanted to eat because he had been worried about vomiting it up or having diarrhea at school. He described how he had suffered from nausea, abdominal pain, and periods of having increased frequency and loose motions. These symptoms had led to a comprehensive medical workup, which was clear, and Jai had been given a diagnosis of irritable bowel syndrome, a functional gut disorder. Kumar, Jai’s brother, remembered how anxious Jai had been to eat as little as possible before school so the other students did not find out that he had problems with his bowels.
Mr. P said that during Year 6, he had become concerned about Jai’s “demotivation”—his withdrawal from friends, negative thinking, and becoming what the father termed a “glass-half-empty person.” He said that Jai had also began to withdraw from him and the family, and to sit in front of the TV or computer in preference to engaging with the family. Jai disagreed with some of his father’s concerns and said that he has always managed to maintain a good friendship group at school. Kumar thought that having an older brother who was academically minded was in itself a stress for Jai. Jai said that his difficulties with stress in school had continued into Year 7, when he described himself as a “de facto counselor” for school friends who confided in him about self-harm and suicidal intent. He described the emotional burden that this role had created in the following ways: “a lot to worry about”; “a lot on my plate”; and “the stress got to 10/10.” Once again, he had not shared his predicaments with his parents and had struggled through the year unsupported. While Jai said that he had managed the stress “pretty well,” he also said that the stress had affected his gut by exacerbating symptoms of nausea, vomiting, and loose stools. He reported that, although his anxiety about going to school had improved, he began to feel tired in the morning, finding it difficult to get up. Jai was also keen to share
another stressor that had occurred in Year 7. He said that his math teacher had publicly shamed him—in front of his class—about his forgetfulness. Around this time, Jai started to listen to rappers who rap about suicide.
Jai presented to our team when he was 14 years of age and in Year 8.
THE CASE FORMULATION THAT EMERGED FROM THE DEVELOPMENTAL STORY
The family assessment session was, in essence, the unfolding of Jai’s story of cumulative stress. As the story emerged, Jai talked more and more, and his brother Kumar added detail to some of Jai’s memories. By the end of the story, both his parents had tears in their eyes, and they felt distressed that they had not known what difficulties their son had been going through. What was also clear to everyone was that Jai’s difficulties had presented in layers—childhood anxiety, autonomic dysregulation involving the gut, a probable depression that had not been picked up and treated, and now FND—each layer adding to the next. Kh
APPENDIX 5. Therapeutic Contract
Because fixed dystonia is very difficult to treat, we offered Jai a three-week admission into the Mind-Body Program (which was the time available prior to the upcoming two-week school holiday break), with the option of a readmission after the school holidays. Our therapeutic program is structured in a particular way,7,8 and Jai and his family agreed to the program’s rules. The program includes a daily structured timetable—daily physiotherapy, daily psychological therapy, and daily attendance at hospital school—and family visiting hours in the evening and weekend. There is also an option of overnight leave one evening during the weekend to facilitate generalization of skills learned in the program to the home setting. Because Jai was a picky eater and because our hospital menu is limited, we agreed that Mrs. P would come in every morning with a smoothie, to make sure that Jai’s food intake remained healthy. Likewise, she could bring in meals at other times of day to minimize intake of the hospital food.
The first therapeutic goal that was agreed upfront—and that was discussed before admission into the Mind-Body Program—was that opioid (oxycodone) and benzodiazepine (diazepam) medications would be withdrawn as soon as possible. This goal was based on the fact that long-term opioid use is known to potentiate chronic pain,9,10 and that the benzodiazepines had not been helpful, were addictive, and were not a treatment of choice for Jai’s long-standing anxiety. We told the family that we would obtain a consult with the pain team to help with the medication withdrawal, and that we would most likely use some pregabalin, a gabapentinoid drug commonly used for pain relief in neuropathic pain and fibromyalgia.*
The second agreed therapeutic goal was to start the treatment intervention with a sleep intervention—trialing a variety of strategies to stabilize Jai’s circadian cycle, so that he got more than four hours of sleep a night. From our clinical experience of over a decade, we as a team had learned that it was difficult to make any therapeutic gains if we failed to regulate the child’s sleep. While regulation of sleep has been our first intervention for many years, the important role of sleep has now also been raised in the FND literature,11 and an emerging literature suggests that disturbed sleep leads to increased pain sensitivity.12 From our own assessment it was clear that Jai’s disturbed sleep fed into his pain cycle—and contributed to his fatigue and anxiety—and that the pain, in turn, made it difficult for him to engage in any physiotherapy and in activities of daily living. In addition, we emphasized that by regulating sleep we were also enabling the restorative properties of sleep to be reinitiated, thereby improving the likelihood of Jai getting well.13,14
In a separate meeting with Jai’s parents, we communicated the difficulty of treating functional dystonia. We wanted them to know that functional dystonia is one of the most difficult FND symptoms to treat. At the same time we highlighted many of the good treatment outcomes obtained in the past ten years by the team (see Table 1). In this context, both the team and the family would need to maintain hope even if the treatment process was very difficult. Because of the challenges in treating Jai and his severe degree of pain and functional impairment, his inpatient treatment took place over a six-month period. This time frame is well outside the typical two-week admission that is sufficient to begin the healing process for most children.
APPENDIX 6. Nursing Interventions
Jai’s nursing care requirements were resource intensive because of his significant functional impairment. Daily nursing interventions included helping Jai with activities of daily living: toileting, showering, transferring from bed to wheelchair, and so on. For example, initially Jai had to use bed pans and bottles for his toileting needs, but as he progressed, with the help of nursing staff, he could be transferred to toilet seat. As another example, nursing staff took over showering duty—negotiating timing and helping with transfer—which took place every second day once Jai was sufficiently mobile. Jai had been avoiding showers because of his fear of pain secondary to taking off the neck brace. Jai saw the collar as a safety object and continued to wear it even after it had broken and was no longer providing any actual support. When Jai developed pre-pressure sores at the points of contact—so that he needed second day dressings and adequate air time—the nursing staff provided him with ice to hold in his hand and music to listen to, while the skin on his neck had the required air time.
During the times that Jai’s mood was low, the nursing staff regularly checked his mood and compliance with the safety plan. They also helped with implementing sleep hygiene measures (see sleep section in main text) and with positioning Jai in a more normal position in bed when he went into self-hypnosis. The nursing staff implemented incidental physiotherapy in ward: prompting Jai about the position of his feet on the footplate of the wheelchair; supervising and assisting Jai when he first started to mobilize in his room (initially Jai needed two assistants to help him mobilize out of the bed); and supervising Jai as he walked to hospital school (initially on a forearm frame and later with crutches).
Throughout the six months that Jai was in hospital, the nursing staff motivated Jai with their jokes, while at the same time maintaining a firm and matter-of-fact therapuetic frame, thereby ensuring that what needed to be done was done. The nursing staff also worked closely with Jai’s mother, who was the parent who spent the most time at the hospital.
APPENDIX 7. Administration of Botulinum Toxin
It was very difficult to make significant gains in physiotherapy in the first three weeks of the Mind-Body Program because any neck movement (including taking off the neck brace) would result in painful neck spasms (see physiotherapy section in main text). Jai would blanch in pain, shake, grit his teeth, and wait for the episode to subside. He resisted any movement to avoid another spasm. Because of this impasse, the team consulted with a Rehabilitation Team specialist—who had expertise in the use of botulinum toxin in other types of dystonia—and it was decided to trial of botulinum toxin in the left neck muscles (left sternocleidomastoid and upper fibers of left trapezius). The hope was that the botulinum toxin would function as a “circuit breaker,” with the intended benefit of progressing with physiotherapy. This intervention proved to be an important contribution to treatment because it decreased both the frequency of Jai’s debilitating neck spasms and the pain associated with them. In conjunction with the daily use of an opiate prior to his physiotherapy sessions, the administration of botulinum toxin enabled Jai to make tangible gains in those sessions, which had previously been impossible. Unfortunately, however, the botulinum toxin had no impact on the fixed dystonia of Jai’s neck—and accompanying baseline pain—or with the C-shape of his body. At the three-month mark, when botulinum toxin could have been re-administered, Jai was making progress, and further botulinum toxin was not indicated.
APPENDIX 8. The Hospital School Intervention
Jai attended hospital school daily and was able to maintain the regular attendance at the hospital school despite significant pain and fatigue. Even in the C-shape position, he was able to use a laptop at school. As his function improved, so did Jai’s ability to engage in school work. He became a very positive influence in the dynamics of the classroom. In particular, he became a source of encouragement to other children who were suffering and disabled from other types of functional disorders.
APPENDIX 9. Teams Involved with Jai’s Evaluation and Care
The family intervention involved three overlapping components. First, the family attended weekly family meetings with the therapists from the mind-body team—psychiatrist, psychologists, pediatric resident, and other team members as needed—where progress was reviewed and treatment goals for the next week were set. A key function of these meetings was to contain the family’s anxiety regarding Jai’s prognosis. While the parental anxiety about progress and prognosis was valid—the outcome literature about functional dystonia is not promising4 (see Text Box 1)—the anxiety also had the potential to overwhelm the treatment team consisting of the family and clinicians. In this context, the psychiatrist who ran the family sessions maintained a mindful focus on the here and now, what each member of the team had to focus on and deliver— including the management of his or her own anxiety—in the next week’s program. Concerns that went beyond this time frame were explicitly put aside, to be dealt with in the future. This stance allowed the team—Jai, his family, and the clinical team—to remain focused, hopeful, united, and working collaboratively on shared goals.
The second family interventionwas implemented by a different family therapist and took place alongside the program. It involved three family sessions and two individual sessions with Jai’s mother. The key issues addressed included an exploration by the family as to how family dynamics may have contributed to Jai’s presentation and what needed to change to maximize the probability of a good outcome when Jai returned home. Each family member articulated his or her perception of how each had contributed to Jai’s presentation. Themes that the family explored together included the following: fear of conflict causing upset and strong emotions; rigid adherence to time and schedules; and inconsistency in the parental team in managing some of Jai’s behaviors. In addition, Jai and Mrs. P were able to identify their close relationship and the positive and negative effects that the relationship had on how they responded to each other’s emotional discomfort. Time was also spent in strengthening the parenting team. This intervention included recognition of the individual strengths that each parent had to offer, and the impact of Jai’s FND diagnosis and treatment process on the marital couple.
The third family intervention involved Mrs. P attending a parenting program called “Tuning into Teens.” Mrs. P found this especially helpful with regard to limit setting and goal setting.
Footnotes
In the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders, the expression functional neurological symptom disorder has replaced conversion disorder.1
Gabapentin has been shown to help with opioid withdrawal in chronic pain.17 An agreement to discontinue opioids is a precondition of entering our Mind-Body Program.
Merriam-Webster’s online dictionary defines funk (noun, sense 1) as “a state of paralyzing fear” or as “a depressed state of mind”—which, together, capture Jai’s mental state over the vacation period. See https://www.merriam-webster.com/dictionary/funk.
One of the clinical psychologists took on the role of Jai’s primary therapist, whereas the other implemented the hypnosis intervention across the various components of the program.
The case history was prepared by Yogesh Khachane, Kasia Kozlowska, Blanche Savage, Georgia McClure, Gretel Butler, Nicola Gray, Andrea Worth, and Samantha Mihailovich.
Gabapentinoids (gabapentin and pregabalin) are GABA analogues that act on the gabapentin receptor.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: APA, 2013. [Google Scholar]
- 2.Carson A, Lehn A. Epidemiology. Handb Clin Neurol 2016;139:47–60. [DOI] [PubMed] [Google Scholar]
- 3.Espay AJ, Aybek S, Carson A, et al. Current concepts in diagnosis and treatment of functional neurological disorders. JAMA Neurol 2018;75:1132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim NM, Martino D, van de Warrenburg BP, et al. The prognosis of fixed dystonia: a follow-up study. Parkinsonism Relat Disord 2009;15:592–7. [DOI] [PubMed] [Google Scholar]
- 5.Ganos C, Edwards MJ, Bhatia KP. The phenomenology of functional (psychogenic) dystonia. Mov Disord Clin Pract 2014;1:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kozlowska K, English M, Savage B. Connecting body and mind: the first interview with somatizing patients and their families. Clin Child Psychol Psychiatry 2013;18:223–45. [DOI] [PubMed] [Google Scholar]
- 7.Kozlowska K, English M, Savage B, Chudleigh C. Multimodal rehabilitation: a mind-body, family-based intervention for children and adolescents impaired by medically unexplained symptoms. Part 1: The program. Am J Fam Ther 2012;40:399–419. [Google Scholar]
- 8.Kozlowska K, English M, Savage B, et al. Multimodal rehabilitation: a mind-body, family-based intervention for children and adolescents impaired by medically unexplained symptoms. Part 2: Case studies and outcomes. Am J Fam Ther 2013;41:212–31. [Google Scholar]
- 9.Liang X, Liu R, Chen C, Ji F, Li T. Opioid system modulates the immune function: a review. Transl Perioper Pain Med 2016;1:5–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM. Pain and poppies: the good, the bad, and the ugly of opioid analgesics. J Neurosci 2015;35:13879–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham CD, Kyle SD. A preliminary investigation of sleep quality in functional neurological disorders: poor sleep appears common, and is associated with functional impairment. J Neurol Sci 2017;378:163–6. [DOI] [PubMed] [Google Scholar]
- 12.Alexandre C, Latremoliere A, Ferreira A, et al. Decreased alertness due to sleep loss increases pain sensitivity in mice. Nat Med 2017;23:768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Vivo L, Bellesi M, Marshall W, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 2017;355:507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedergaard M. Neuroscience. Garbage truck of the brain. Science 2013;340:1529–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branson JA, Kozlowska K, Kaczynski KJ, Roesler TA. Managing chronic pain in a young adolescent girl with Ehlers-Danlos syndrome. Harv Rev Psychiatry 2011;19:259–70. [DOI] [PubMed] [Google Scholar]
- 16.Chudleigh C, Kozlowska K, Kothur K, et al. Managing non-epileptic seizures and psychogenic dystonia in an adolescent girl with preterm brain injury. Harv Rev Psychiatry 2013;21:163–74. [DOI] [PubMed] [Google Scholar]
- 17.Stoicea N, Russell D, Weidner G, et al. Opioid-induced hyperalgesia in chronic pain patients and the mitigating effects of gabapentin. Front Pharmacol 2015;6:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz TL, Goradia V. Managing insomnia: an overview of insomnia and pharmacologic treatment strategies in use and on the horizon. Drugs Context 2013;2013:212257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kozlowska K. A stress-system model for functional neurological symptoms. J Neurol Sci 2017;383:151–2. [DOI] [PubMed] [Google Scholar]
- 20.Tsui P, Deptula A, Yuan DY. Conversion disorder, functional neurological symptom disorder, and chronic pain: comorbidity, assessment, and treatment. Curr Pain Headache Rep 2017;21:29. [DOI] [PubMed] [Google Scholar]
- 21.Jiang H, White MP, Greicius MD, Waelde LC, Spiegel D. Brain activity and functional connectivity associated with hypnosis. Cereb Cortex 2017;27:4083–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terhune DB, Cleeremans A, Raz A, Lynn SJ. Hypnosis and top-down regulation of consciousness. Neurosci Biobehav Rev 2017;81:59–74. [DOI] [PubMed] [Google Scholar]
- 23.Landry M, Lifshitz M, Raz A. Brain correlates of hypnosis: a systematic review and meta-analytic exploration. Neurosci Biobehav Rev 2017;81:75–98. [DOI] [PubMed] [Google Scholar]
- 24.Stephen CD, Sharma N, Callahan J, Carson AJ, Perez DL. A case of functional dystonia with associated functional neurological symptoms: diagnostic and therapeutic challenges. Harv Rev Psychiatry 2017;25:241–51. [DOI] [PubMed] [Google Scholar]
- 25.Carson A, Lehn A, Ludwig L, Stone J. Explaining functional disorders in the neurology clinic: a photo story. Practical neurology 2016;16:56–61. [DOI] [PubMed] [Google Scholar]
- 26.McKee K, Glass S, Adams C, et al. The inpatient assessment and management of motor functional neurological disorders: an interdisciplinary perspective. Psychosomatics 2018;59:358–68. [DOI] [PubMed] [Google Scholar]
- 27.Schmerler DA, Espay AJ. Functional dystonia. Handb Clin Neurol 2016;139:235–45. [DOI] [PubMed] [Google Scholar]
- 28.Nielsen G, Stone J, Matthews A, et al. Physiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry 2015;86:1113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardiner P, MacGregor L, Carson A, Stone J. Occupational therapy for functional neurological disorders: a scoping review and agenda for research. CNS Spectr 2017:1–8. [DOI] [PubMed] [Google Scholar]
- 30.Ranford J, Perez DL, MacLean J. Additional occupational therapy considerations for functional neurological disorders: a potential role for sensory processing. CNS Spectr 2018;23:194–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharpe M, Walker J, Williams C, et al. Guided self-help for functional (psychogenic) symptoms: a randomized controlled efficacy trial. Neurology 2011;77:564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaFrance WC, Jr., Baird GL, Barry JJ, et al. Multicenter pilot treatment trial for psychogenic nonepileptic seizures: a randomized clinical trial. JAMA psychiatry 2014;71:997–1005. [DOI] [PubMed] [Google Scholar]
- 33.Gelauff J, Stone J, Edwards M, Carson A. The prognosis of functional (psychogenic) motor symptoms: a systematic review. J Neurol Neurosurg Psychiatry 2014;85:220–6. [DOI] [PubMed] [Google Scholar]
- 34.Glass SP, Matin N, Williams B, et al. Neuropsychiatric factors linked to adherence and short-term outcome in a U.S. functional neurological disorders clinic: a retrospective cohort study. J Neuropsychiatry Clin Neurosci 2018;30:152–9. [DOI] [PubMed] [Google Scholar]
- 35.Jalilianhasanpour R, Ospina JP, Williams B, et al. Secure attachment and depression predict 6-month outcome in motor functional neurological disorders: a prospective pilot study. Psychosomatics 2018. [DOI] [PubMed] [Google Scholar]
- 36.Adams C, Anderson J, Madva EN, LaFrance WC, Jr., Perez DL. You’ve made the diagnosis of functional neurological disorder: now what? Pract Neurol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parees I, Kojovic M, Pires C, et al. Physical precipitating factors in functional movement disorders. J Neurol Sci 2014;338:174–7. [DOI] [PubMed] [Google Scholar]
- 38.Stone J, Gelauff J, Carson A. A “twist in the tale”: altered perception of ankle position in psychogenic dystonia. Mov Disord 2012;27:585–6. [DOI] [PubMed] [Google Scholar]
- 39.LaFrance WC, Jr., Friedman JH. Cognitive behavioral therapy for psychogenic movement disorder. Mov Disord 2009;24:1856–7. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein LH, Chalder T, Chigwedere C, et al. Cognitive-behavioral therapy for psychogenic nonepileptic seizures: a pilot RCT. Neurology 2010;74:1986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deeley Q. Hypnosis as therapy for functional neurologic disorders. Handb Clin Neurol 2016;139:585–95. [DOI] [PubMed] [Google Scholar]
- 42.Kozlowska K, Chudleigh C, Cruz C, et al. Psychogenic non-epileptic seizures in children and adolescents: Part II – Explanations to families, treatment, and group outcomes. Clin Child Psychol Psychiatry 2018;23:160–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grattan-Smith PJ, Dale RC. Pediatric functional neurologic symptoms. Handb Clin Neurol 2016;139:489–98. [DOI] [PubMed] [Google Scholar]
- 44.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull 2007;133:581–624. [DOI] [PubMed] [Google Scholar]
- 45.Wren AA, Ross AC, D’Souza G, et al. Multidisciplinary pain management for pediatric patients with acute and chronic pain: a foundational treatment approach when prescribing opioids. Children (Basel) 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adachi T, Fujino H, Nakae A, Mashimo T, Sasaki J. A meta-analysis of hypnosis for chronic pain problems: a comparison between hypnosis, standard care, and other psychological interventions. Int J Clin Exp Hypn 2014;62:1–28. [DOI] [PubMed] [Google Scholar]
- 47.Thompson T, Terhune DB, Oram C, et al. The effectiveness of hypnosis for pain relief: a systematic review and meta-analysis of 85 controlled experimental trials. Neurosci Biobehav Rev 2019;99:298–310. [DOI] [PubMed] [Google Scholar]
- 48.Uman LS, Chambers CT, McGrath PJ, Kisely S. A systematic review of randomized controlled trials examining psychological interventions for needle-related procedural pain and distress in children and adolescents: an abbreviated Cochrane review. J Pediatr Psychol 2008;33:842–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palsson OS. Hypnosis treatment of gastrointestinal disorders: a comprehensive review of the empirical evidence. Am J Clin Hypn 2015;58:134–58. [DOI] [PubMed] [Google Scholar]
- 50.Shih M, Yang YH, Koo M. A meta-analysis of hypnosis in the treatment of depressive symptoms: a brief communication. Int J Clin Exp Hypn 2009;57:431–42. [DOI] [PubMed] [Google Scholar]
- 51.Chamine I, Atchley R, Oken BS. Hypnosis intervention effects on sleep outcomes: a systematic review. J Clin Sleep Med 2018;14:271–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jensen MP, Jamieson GA, Lutz A, et al. New directions in hypnosis research: strategies for advancing the cognitive and clinical neuroscience of hypnosis. Neurosci Conscious 2017;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hauser W, Hagl M, Schmierer A, Hansen E. The efficacy, safety and applications of medical hypnosis. Dtsch Arztebl Int 2016;113:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutten JM, Reitsma JB, Vlieger AM, Benninga MA. Gut-directed hypnotherapy for functional abdominal pain or irritable bowel syndrome in children: a systematic review. Arch Dis Child 2013;98:252–7. [DOI] [PubMed] [Google Scholar]
- 55.van Tilburg MA, Chitkara DK, Palsson OS, et al. Audio-recorded guided imagery treatment reduces functional abdominal pain in children: a pilot study. Pediatrics 2009;124:e890–7. [DOI] [PubMed] [Google Scholar]
- 56.Elkins GR, Barabasz AF, Council JR, Spiegel D. Advancing research and practice: the revised APA Division 30 definition of hypnosis. Int J Clin Exp Hypn 2015;63:1–9. [DOI] [PubMed] [Google Scholar]
- 57.Pendergrast RA. Incorporating hypnosis into pediatric clinical encounters. Children (Basel) 2017;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barabasz AF, Barabasz M. The new APA definition of hypnosis: spontaneous hypnosis MIA. Am J Clin Hypn 2015;57:459–63. [DOI] [PubMed] [Google Scholar]
- 59.Kohen DP. Long-term follow-up of self-hypnosis training for recurrent headaches: what the children say. Int J Clin Exp Hypn 2010;58:417–32. [DOI] [PubMed] [Google Scholar]
- 60.Dell PF. What is the essence of hypnosis? Int J Clin Exp Hypn 2017;65:162–8. [DOI] [PubMed] [Google Scholar]
- 61.Lynn SJ, Laurence JR, Kirsch I. Hypnosis, suggestion, and suggestibility: an integrative model. Am J Clin Hypn 2015;57:314–29. [DOI] [PubMed] [Google Scholar]
- 62.Braffman W, Kirsch I. Imaginative suggestibility and hypnotizability: an empirical analysis. J Pers Soc Psychol 1999;77:578–87. [DOI] [PubMed] [Google Scholar]
- 63.Benedetti F, Thoen W, Blanchard C, Vighetti S, Arduino C. Pain as a reward: changing the meaning of pain from negative to positive co-activates opioid and cannabinoid systems. Pain 2013;154:361–7. [DOI] [PubMed] [Google Scholar]
- 64.Jensen MP, Adachi T, Tome-Pires C, Lee J, Osman ZJ, Miro J. Mechanisms of hypnosis: toward the development of a biopsychosocial model. Int J Clin Exp Hypn 2015;63:34–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol 2009;5:374–81. [DOI] [PubMed] [Google Scholar]
- 66.Agorastos A, Pervanidou P, Chrousos GP, Baker DG. Developmental trajectories of early life stress and trauma: a narrative review on neurobiological aspects beyond stress system dysregulation. Front Psychiatry 2019;10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsigos C, Stefanaki C, Lambrou GI, Boschiero D, Chrousos GP. Stress and inflammatory biomarkers and symptoms are associated with bioimpedance measures. Eur J Clin Invest 2015;45:126–34. [DOI] [PubMed] [Google Scholar]
- 68.Stefanaki C, Peppa M, Boschiero D, Chrousos GP. Healthy overweight/obese youth: early osteosarcopenic obesity features. Eur J Clin Invest 2016;46:767–78. [DOI] [PubMed] [Google Scholar]


