Supplemental Digital Content is available in the text
Homing of mesenchymal stem cells (MSCs) from the endplate into the intervertebral disc (IVD) has been described as alternative intradiscal application route. Our findings indicate that the homed MSCs enhance the Tie2-positive disc progenitor cell population, prevent disc cell death, and induce a proliferative response in the IVD cells.
Keywords: organ culture, MSC migration, MSC homing, disc cell survival, disc cell proliferation, IVD regeneration, Tie2, CD202, disc progenitor cells, human mesenchymal stem cells
Study Design.
Experimental study with human mesenchymal stem cells (MSCs) and intervertebral disc (IVD) tissue samples.
Objective.
This study aimed to characterize the effect of MSC homing on the Tie2-positive IVD progenitor cell population, IVD cell survival, and proliferation.
Summary of Background Data.
Homing of human MSCs has been described as potential alternative to MSC injection, aiming to enhance the regenerative capacity of the IVD. IVD cells expressing Tie2 (also known as CD202b or Angiopoietin-1 receptor TEK tyrosine kinase) represent a progenitor cell population with discogenic differentiation potential. However, the fraction of Tie2-positive progenitor cells decreases with aging and degree of IVD degeneration, resulting in a potential loss of the IVD's regenerative capacity.
Methods.
Human MSCs, isolated from vertebral bone marrow aspirates, were labeled and seeded onto the endplate of bovine IVDs and human IVD tissue. Following MSC migration for 5 days, IVD cells were isolated by tissue digestion. The fractions of Tie2-positive, dead, apoptotic, and proliferative IVD cells were evaluated by flow cytometry and compared to untreated IVDs. For human IVDs, 3 groups were investigated: nondegenerated (organ donors), IVDs of patients suffering from spinal trauma, and degenerative IVD tissue samples.
Results.
MSC homing enhanced the fraction of Tie2-positive IVD cells in bovine and human IVD samples. Furthermore, a proliferative response and lower fraction of dead cells were observed after MSC homing in both bovine and human IVD tissues.
Conclusion.
Our findings indicate that MSC homing enhances the survival and regenerative capability of IVD cells, which may be mediated by intercellular communication. MSC homing could represent a potential treatment strategy to prevent the onset of the degenerative cascade in IVDs at risk such as IVDs adjacent to a fused segment or IVDs after herniation.
Level of Evidence: N/A
Recruitment or homing of mesenchymal stem cells (MSCs) is a physiological mechanism to maintain tissue homeostasis and promote tissue repair. MSC homing has been reported to play a role in the endogenous regeneration of different skeletal tissues, including bone1 and cartilage.2 Thereby MSCs are mobilized from their niches in response to injury, immune or physical–chemical signals and are stimulated to migrate to the effector site. In vivo, this process is tightly controlled by gradients of signaling molecules, oxygen, and mechanical cues.3 In the intervertebral disc (IVD), recruitment of bone marrow cells toward the degenerative IVD was demonstrated in a mouse tail model in vivo.4 Nevertheless, the findings from the IVD degeneration model suggested that the pool of available cells or their recruitment efficiency may need to be enhanced by exogenous means to achieve a significant regenerative effect.
Migration of exogenously delivered bone marrow-derived MSCs through the endplate into the IVD has been described as an alternative approach for intradiscal cell application in several whole IVD organ culture models.5–8 Hereby, MSC migration through the IVD tissue was enhanced in IVDs cultured under degeneration-inducing conditions.5 The homed MSCs were shown to promote matrix remodeling, whereby more sustained effects are expected by cell homing compared to injection of potentially unphysiological sources and numbers of cells.7,9,10 Moreover, injection of cells through the annulus fibrosus (AF) has been suggested to foster a degenerative cascade, and it is still uncertain whether high numbers of injected cells will survive in the harsh environment of the degenerative IVD.11–13
Evidence from other tissue types indicates that besides cell–cell interactions, an important regenerative effect of recruited or delivered MSCs consists in the stimulation of the resident tissue cells by paracrine factors.14In vitro co-culture experiments of MSCs and degenerative nucleus pulposus (NP) cells revealed that MSCs could enhance the gene expression of extracellular matrix proteins and reverse the expression of proinflammatory cytokines in the degenerated NP cells.15–17 In this respect, different growth factors have been reported to support IVD cell survival and enhance matrix production.7,18,19 Homing of MSCs might therefore represent an alternative strategy to deliver growth factors and other biologics into the IVD.
Tie2 (angiopoietin-1 receptor)-positive IVD progenitor cells have been reported to hold a multilineage differentiation capacity and their presence is suggested to reflect the IVD's regenerative capacity.20,21 In the present study, we hypothesized that homing of MSCs would exert a potential protective effect by enhancing the Tie2-positive disc progenitor cell population and thus the IVD's regenerative capacity. Bovine whole organ culture models and human IVD tissues were used to test our hypothesis.
MATERIALS AND METHODS
Human MSC Isolation and Expansion
Vertebral bone marrow aspirates were obtained with written consent from patients undergoing spine surgery (Figure 1A). MSCs were isolated by Ficoll gradient centrifugation and adherence to tissue culture plastic as previously described.22 Cells were expanded in alpha-minimum essential medium (αMEM, Gibco) containing 100 U/mL penicillin, 100 μg/mL streptomycin, 10% fetal bovine serum (FBS, Pan Biotech) and 5 ng/mL basic fibroblast growth factor (Fitzgerald Industries). Early passage (P1-P2) MSCs from nine different donors were used in this study (Supplementary Fig. 1AB, http://links.lww.com/BRS/B443).
Figure 1.
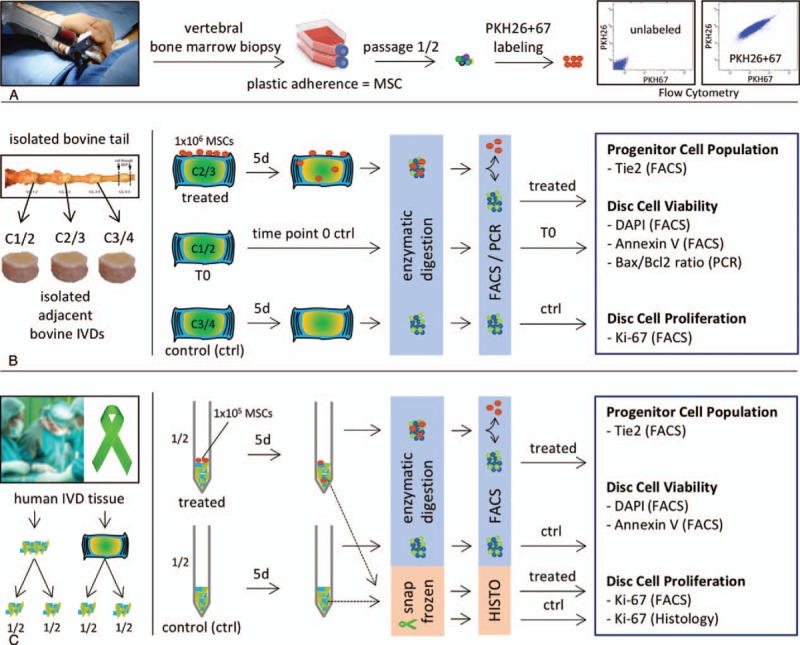
(A) Isolation of MSCs from vertebral bone marrow aspirate by plastic adherence. MSCs were double labeled with PKH26 and PKH67 and labeling was confirmed by flow cytometry. (B) IVDs with endplates were isolated from bovine tails. Adjacent IVDs were randomly assigned to: time point zero ctrl (T0), day 5 untreated control (ctrl) and day 5 treated disc by MSC homing (treated). After 5 days, the IVDs were digested overnight and the cells were analyzed by flow cytometry. MSCs were excluded by gating, and disc cells were either processed for gene expression analysis (PCR) or analyzed for expression of Tie2, DAPI, Annexin V, or Ki-67 (FACS). (C) Human IVD tissue was isolated during surgery or from organ donors. Tissue from one donor was divided into halves. One half was treated by MSC homing (treated), the second half was used as untreated control (ctrl). After 5 days, a tissue piece from both groups was embedded in cryocompound and snap frozen for histological analysis (Ki-67). From the remaining tissue, cells were isolated by overnight digestion and analyzed by flow cytometry. MSCs were excluded and disc cells were analyzed for expression of Tie2, DAPI, Annexin V or Ki-67 (FACS). IVD indicates intervertebral disc; MSCs, mesenchymal stem cells; PCR, polymerase chain reaction.
Bovine Organ Culture Model
An established IVD organ culture model of MSC migration through the endplate was used as previously described (Figure 1B).5–8 IVDs were harvested from bovine tails (n = 27, 6–8 months old) obtained from the local abattoir within 2 hours of death. Discs were excised with a band saw (Exakt Apparatebau) and rinsed in phosphate buffered saline (PBS) containing 10% penicillin–streptomycin.23 IVDs with endplates were cultured in Dulbecco's Modified Eagle Medium (DMEM) containing 1 g/L glucose, supplemented with 25 mmol/L Hepes (Gibco), 2% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1% insulin-transferrin-selenium (ITS+, Corning) and 50 μg/mL Primocin (InvivoGen, San Diego, CA).
MSCs were labeled with fluorescent membrane dyes (PKH26 and PKH67 Fluorescent Cell Linker Kit; Sigma-Aldrich). A 30-μL suspension containing 1 × 106 MSCs in DMEM was added onto the IVD endplate.5 Following cell attachment (20 minutes), medium was added to cover the IVD. Adjacent discs were used as time zero (T0) and untreated controls, respectively. After 5 days of migration, endplates were removed, and the disc tissue was digested with 0.2% pronase (Sigma-Aldrich) for 1 hour followed by collagenase II (Gibco) for 12 hours (NP: 50 U/mL; AF: 100 U/mL). Percentages of Tie2-positive, dead (4′,6-diamidino-2-phenylindole/DAPI-positive), apoptotic (Annexin V positive) and proliferative (Ki-67 positive) disc cells were evaluated by flow cytometry (fluorescence activated cell sorting (FACS); Table 1). The following gating strategy was applied: 1st gate, doublets exclusion (FSC-H/ FSC-A); 2nd gate, exclusion of dead cells (DAPI; only for Tie2, Ki-67); 3rd gate, exclusion of PKH26-positive/PKH67-positive MSCs. Gates were defined with unstained samples. Analysis of initial experiments included data at day 5 and the day 0 control (n = 4). To confirm the findings observed at day 5, experiments for day 5 were repeated, allowing for a higher sensitivity of the used statistical tests (n = 13, including data from initial experiments).
TABLE 1.
List of Antibodies Used for Flow Cytometry Analysis and IHC
| Name | Cat. number | Company | Clone | Color | Dilution |
| Bovine Tie2 | bs-1300R | Bioss | 450–500/1124 | Alexa F488 | 1st: 1 in 50 2nd: 1 in 200 |
| Human Tie2 | 334210 | BioLegend | Ab33 | Alexa F647 | 1 in 20 |
| Annexin V | V13242 | Thermo Fischer | Alexa F488 | 1 in 20 | |
| DAPI | D9542 | Sigma | — | Alexa F594 | 1 in 5000 |
| eFluor 780 | eBioscience | — | Alexa F750 | 1 in 1000 | |
| Ki-67—flow cytometry | 350504 | BioLegend | — | Alexa F610 | 1 in 20 |
| Ki-67—IHC | NB110-89719 | Novus | — | Alexa F546 | 1 in 100 |
IHC indicates immunohistochemistry.
For gene expression analysis, isolated cells were separated by flow cytometry in PKH26/67-positive (MSCs) and -negative (disc) cells. Total RNA was isolated with TRI reagent (Molecular Research Center, Cincinnati, OH). For reverse transcription, SuperScript Vilo cDNA Synthesis kit (Invitrogen, Thermo Fisher Scientific) was used. Expression levels of genes of interest (Table 2) were measured by real-time PCR (QuantStudio 6 Flex, Applied Biosystems). For relative quantification, the dCt method was used with GAPDH serving as endogenous control.24
TABLE 2.
List of Bovine Genes Analyzed by Real-time RT-PCR
| Abb. | Name | Cat. Number (Applied Biosystems) or Sequence |
| Bax | Bcl-2-associated X protein | Bt01016551_g1 |
| Bcl2 | B-cell lymphoma 2 | Bt04298952_m1 |
| Casp3 | Apoptosis-related cysteine peptidase | Bt03250956_g1 |
| Tie2-TEK | Angiopoietin-1 receptor | Bt03212059_m1 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | Fwd. primer seq: 5’-GGC TGC TTT TAA TTC TGG CAA A-3’ Rev. primer seq: 5’-AAT CAT ACT GGA ACA TGT AGA CCA TGT A-3’ Probe seq: 5’-TGG ACA TCG TCG CCA TCA ATG ACC-3’ |
RT-PCR indicates real-time polymerase chain reaction.
MSC Treatment in Spatial Separation
A 0.22-μm porous membrane was placed on the endplate of the bovine IVDs (Figure 2A). PKH26/67-labeled MSCs were seeded onto the membrane (1 × 106 cells). An adjacent IVD was used as untreated control. The experiment was carried out as described above.
Figure 2.
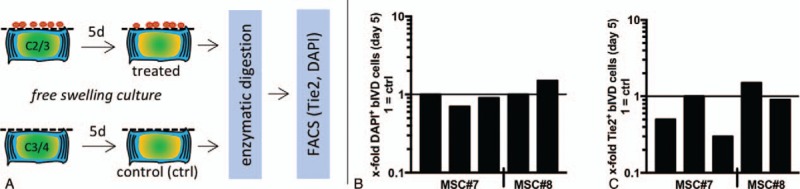
(A) bovine IVD (bIVD) treatment by MSCs in spatial separation (0.22 μm porous membrane). (B-C) No changes in Tie2 expression (0.8- ± 0.5-fold) and fraction of dead cells (1.0- ± 0.3-fold) relative to donor corresponding ctrl disc was found at day 5 (n = 5 bovine tails, 2 MSC donors). IVD indicates intervertebral disc; MSCs, mesenchymal stem cells.
Human Organ Culture Model
IVD tissue from patients suffering from spinal trauma (referred to as traumatic) and degenerative IVD tissue from patients undergoing spinal fusion (referred to as degenerative) were obtained with written consent from patients undergoing spine surgery at the University Hospital Bern and were processed within 6 hours (Figure 1C). Nondegenerated (referred to as healthy) lumbar spines were harvested by the McGill Scoliosis & Spinal Research Group from organ donors after donor and familial consent via a collaboration with Transplant Quebec. All procedures on nondegenerated IVDs were approved by the institutional review board of McGill University (IRB# A04-M53–08B) for the project titled “Human Intervertebral Discs used for Culture and Extracellular Matrix.” Spines were processed within 4 hours post-mortem. After elimination of ligaments and soft tissues, whole discs were extracted by cutting parallel to and near the endplates. Discs were further processed using a high-speed drill (Foredom, Bethel, CT), equipped with a surgical fluted ball burr (Conmed Linvatec, Largo, FL), to remove the remaining bone and expose the cartilaginous endplate.25 The discs were then washed three times with antibiotic solution,26 placed in sterile containers with culture medium (DMEM with l-glutamine and 15 mmol/L HEPES, supplemented with 5% fetal bovine serum, 50 μg/mL gentamycin, 50 μg/mL Primocin, 50 μg/mL l-ascorbate) and shipped on ice to the research facility. Discs arrived at the research facility within 36 to 48 hours after they were isolated from organ donors. All collected tissue was placed in red-cell lysis buffer for 5 minutes. Following, the tissue was rinsed in PBS containing 1% penicillin–streptomycin. Based on the wet weight, the tissue was then divided in two equal portions, placed in 15-mL tubes and centrifuged for 5 minutes at 500g. A 30-μL suspension of 1 × 105 PKH26/67-labeled MSCs was added onto one tissue portion. The other portion was used as untreated control. Following 20 minutes of attachment, 5-mL medium was added. After 5 days of migration, tissue was digested. Percentages of Tie2-positive, dead (DAPI positive), apoptotic (Annexin V positive), and proliferative (Ki-67 positive) disc cells were evaluated by flow cytometry using the gating strategy described above.
Fraction of Ki-67-positive Disc Cells by Immunohistochemistry
After 5 days of culture, pieces of MSC-treated and untreated healthy human IVD tissues were collected, snap-frozen, and embedded in cryocompound. Mouse spleen was used as a positive control tissue. Ten micrometer sections were cut (Microm HM560), fixed in 70% methanol, and stained overnight with primary antibody against Ki-67 (Novus NB110–89719) followed by staining with secondary antibody (AlexaFluor 546, 55312A). Sections without primary antibody were used as negative control. Sections were mounted with ProLong™ Gold Antifade Mountant with DAPI (Thermo Fischer) and imaged (Olympus BX63F, camera: DP74). The fraction of Ki-67-positive disc cells was determined using the ImageJ cell counting plugin.
MSC Migration in Intact Human IVD
MSCs were labeled with fluorescent membrane dyes as described above. Following, 3 × 106 labeled MSCs were seeded onto the endplate of an intact human IVD in a volume of 90 μL. After 20 minutes of incubation to allow cell attachment, culture medium was added until the IVD was completely covered. The IVD was cultured in free-swelling conditions with medium changes every second day. After 5 days of culture, the IVD was rinsed with PBS, fixed in 4% buffered formalin for 1 week, and cut sagittally with a Padgett blade using a custom-made holding device.6 The IVD sections were visualized on a confocal microscope (LSM800; Zeiss, Jena, Germany) in epifluorescence at 5× and 20× magnification (Supplementary Fig. 2, http://links.lww.com/BRS/B443).
Statistical Analysis
Statistical analyses were conducted in Prism 7 and R Studio v1.1.456 (multiple regression) with P ≤ 0.05 being considered statistically different. Paired nonparametric t tests were conducted (Wilcoxon matched-pairs signed rank test). The lm function was used to fit a multiregression model using Tie2 as dependent and Bcl2, Bax, and Caspase 3 as independent variables. Results are displayed as box plots; error bars indicate 95% confidence interval. Results of the Bax/Bcl2 ratio are presented as single donors connected by lines. For clarity, mean ± standard deviation is provided in the text of the results section.
RESULTS
MSCs from nine patients (age 63 ± 10.5y, supplementary Fig. 1A, http://links.lww.com/BRS/B443), bovine IVDs from 18 tails, and human IVDs from nine patients were included (age 60 ± 19.4 years, supplementary Fig. 1B, http://links.lww.com/BRS/B443). The fractions of Tie2-positive disc progenitor cells, dead (DAPI-positive), apoptotic (Annexin V-positive), and proliferating (Ki-67-positive) disc cells were evaluated in bovine and human IVDs following MSC homing (Figure 1A–C).
Bovine Organ Culture
No change in the fraction of Tie2-positive cells was found between the day 0 (T0, 3.8% ± 3.4%) and the control discs at day 5 (3.8% ± 2.1%, P > 0.99) (Figure 3A, B). A significantly higher fraction of Tie2-positive disc cells (3.0% ± 3.0%) was observed at day 5 after MSC treatment compared to the untreated control (1.6% ± 1.9%, P = 0.04) (Figure 3C, D). This indicated that the increase in Tie2-positive IVD cells was because of MSC homing and not spontaneous changes following 5 days of culture.
Figure 3.
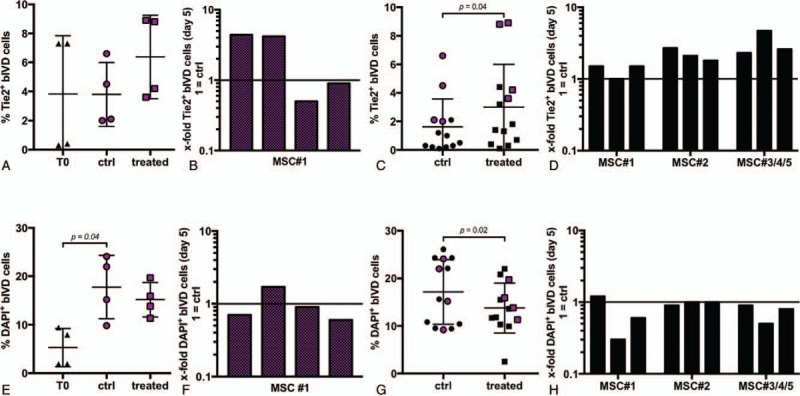
(A) Fraction of Tie2+ bovine IVD (bIVD) cells at day 0 (T0: 3.8% ± 3.4%) and day 5 (ctrl, treated) (n = 4 bovine tails, hMSC#1). (B) Individual fold change (relative to donor corresponding ctrl) of data points represented in Fig. 3A. (C) MSC homing significantly enhanced the Tie2+ progenitor cell population at day 5 (ctrl: 1.6% ± 1.9%, treated: 3.0% ± 3.0%) (n = 13 bovine tails, 5 MSC donors). Red dots refer to data presented in Fig 3A, B. (D) Individual fold change (relative to donor corresponding ctrl) of data points represented in Fig. 3C. (E) Fraction of dead bIVD cells at day 0 (T0: 5.3% ± 3.9%) and day 5 (ctrl, treated) (n = 4 bovine tails, hMSC#1). (F) Individual fold change (relative to donor corresponding ctrl) of data points represented in Fig. 3E. (G) MSC homing significantly reduced the fraction of dead bIVD cells at day 5 (ctrl: 17.2% ± 7.3%, treated: 13.8% ± 6.0%) (n = 13 bovine tails, 5 MSC donors). (H) Individual fold change (relative to donor corresponding ctrl) of data points represented in Fig. 3G. IVD indicates intervertebral disc; MSCs, mesenchymal stem cells.
A significant increase in dead disc cells was found after 5 days (17.8% ± 6.5%) compared to the T0 control (5.3% ± 3.9%, P = 0.04) indicating that culture in free swelling conditions represented an unphysiological environment (Figure 3E, F). After 5 days, MSC homing significantly prevented cell death (13.8% ± 6.0%) compared to the untreated control (17.2% ± 7.3%, P = 0.02) (Figure 3G, H).
MSCs delivered under spatial separation from the IVD (Figure 2A) did neither prevent cell death (control: 12.0% ± 1.8%, treated: 12.4% ± 3.8%, Figure 2B) nor enhance the Tie2-positive cell population (control: 0.3% ± 0.2%, treated: 0.2% ± 0.2%, Figure 2C). This indicated that the positive effects of MSCs on IVD cell survival and the Tie2-positive IVD cell fraction were not evident when MSC migration into the IVD was impeded.
Furthermore, a significant increase in apoptotic disc cells was observed after 5 days in culture (13.2% ± 2.7%) compared to the T0 control (2.9% ± 0.4%, P = 0.04) (Figure 4A). MSC homing slightly, although not significantly, prevented apoptosis at day 5 (10.5% ± 4.2%) relative to the untreated IVDs (12.7% ± 3.3%, P = 0.07) (Figure 4B). A trend of a lower Bax/Bcl2 gene expression ratio was also observed in five of seven bovine IVDs treated by MSC homing (Figure 4C). Interestingly, multiple regression revealed a positive correlation between the expression of Tie2 and Bcl2 (P = 0.042, R2 = 0.96) in the IVDs treated by MSC migration (Figure 4D).
Figure 4.
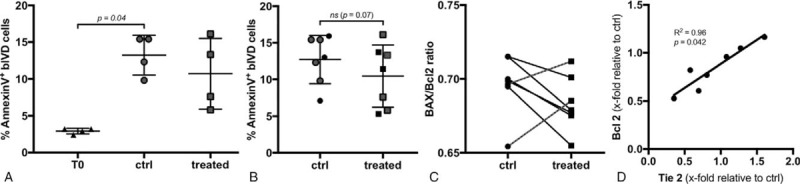
(A) Fraction of apoptotic bovine IVD (bIVD) cells at day 0 (T0: 2.9 ± 0.4%) and day 5 (ctrl, treated) (n = 4 bovine tails, hMSC#1). (B) A slight not significant difference in the fraction of apoptotic cells was found at day 5 (ctrl: 12.7% ± 3.3%, treated: 10.5% ± 4.2%) (n = 5 bovine tails, 2 MSC donors). (C) The Bax/Bcl2 gene expression ratio was decreased in five and increased in two of seven MSC-treated IVDs. (D) The gene expression of Tie2 was positively correlated with the expression of Bcl2 (P = 0.042, R2 = 0.96) (n = 7 bovine tails, hMSC#6). IVD indicates intervertebral disc; MSCs, mesenchymal stem cells.
Significantly higher fractions of proliferating disc cells were observed after 5 days of MSC treatment (3.8% ± 0.7%) compared to T0 (0.9% ± 0.1%, P = 0.04) (Figure 5A) and compared to the control disc at day 5 (4.3% ± 0.9% vs. 2.5 ± 1.6%, P = 0.013) (Figure 5B,C).
Figure 5.
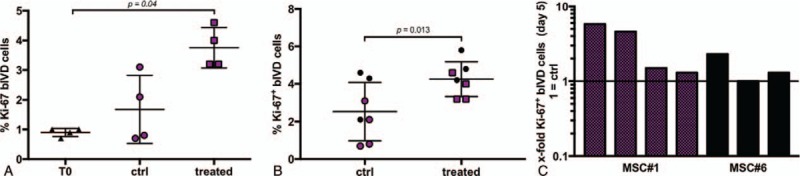
(A) Fraction of proliferating bovine IVD (bIVD) cells significantly increased between day 0 (T0: 0.9% ± 0.1%) and day 5 after MSC homing (3.8% ± 0.7%, P = 0.04) (n = 4 bovine tails, hMSC#1). (B) MSC homing induced a significantly higher proliferative response at day 5(ctrl: 2.5% ± 1.6%, treated: 4.3% ± 0.9%, P = 0.013) (n = 7 bovine tails, 2 MSC donors). (C) Individual fold change (relative to donor corresponding ctrl) of data points represented in Fig. 5B. IVD indicates intervertebral disc; MSCs, mesenchymal stem cells.
Human Organ Culture
To verify MSC homing into a human IVD in organ culture, a cell migration assay was performed. The experiment confirmed that MSCs migrated through the endplate into the intact human IVD. Labeled cells were observed in all regions of the IVD, namely the NP, and inner and outer AF (Supplementary Fig. 2, http://links.lww.com/BRS/B443).
MSC homing enhanced the fraction of Tie2-positive disc cells in seven of 10 human IVDs, although this change was not significant (2.6- ± 2.1-fold, P = 0.16; healthy 3.8- ± 2.9-fold, trauma 2.2- ± 1.9-fold, degenerative 2.0- ± 1.9-fold) (Figure 6A–C). The fraction of dead cells was nonsignificantly lower following MSC homing in seven of 10 IVDs (0.9- ± 0.4-fold, P = 0.15; healthy 0.8- ± 0.2-fold, trauma 0.7- ± 0.6-fold, degenerative 1.1- ± 0.6-fold). Similar to the findings in the bovine organ culture, no change in fraction of apoptotic disc cells was observed after homing of MSCs (7.2% ± 4.2%) compared to the untreated control (7.9% ± 5.1%; P = 0.56) (Figure 7).
Figure 6.
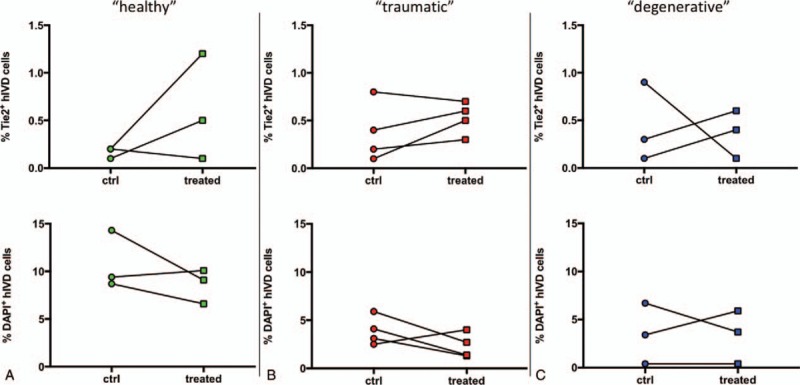
An enhanced Tie2+ disc progenitor cell population and a lower fraction of DAPI+ disc cells were found in seven of 10 analyzed human IVDs (hIVD) after MSC treatment, although changes were not significant (n = 10, MSC#7,8,9). (A) Healthy (green) Tie2: 3.8- ± 2.9-fold; DAPI: 0.8- ± 0.2-fold. (B) Trauma (red) Tie2: 2.2- ± 1.9-fold; DAPI: 0.7- ± 0.6-fold. (C) Degenerative (blue) Tie2: 2.0- ± 1.9-fold; DAPI: 1.1- ± 0.6-fold.
Figure 7.
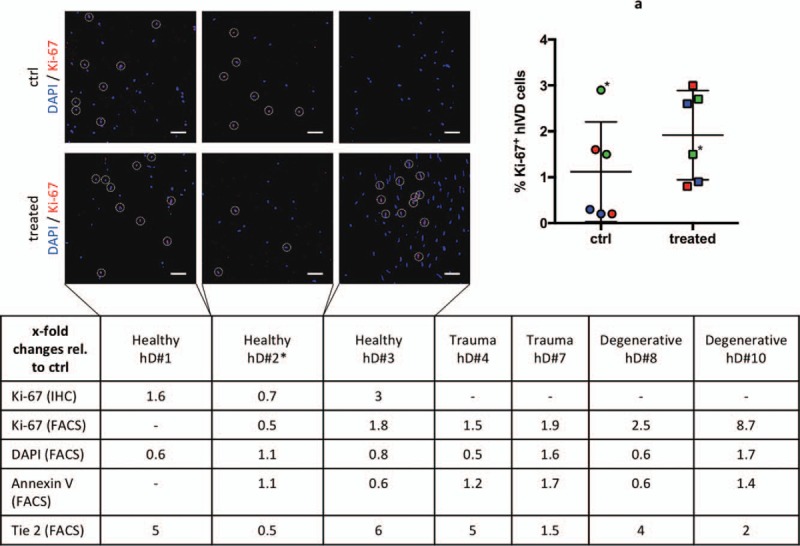
(A) MSC homing nonsignificantly enhanced the fraction of Ki-67+ disc cells (healthy (green) n = 2: 1.2- ± 0.6-fold; trauma (red) n = 2: 1.7- ± 0.2-fold; degenerative (blue) n = 2: 5.6- ± 3.1-fold) in five of six human IVDs. Table: lists fold changes (relative to ctrl disc) of Ki-67, DAPI, Annexin V and Tie2 expression of individual human IVDs. Owing to limited amounts of human tissue, some analyses could not be performed for all donors (−). IHC staining for Ki-67 confirmed results by flow cytometry (healthy n = 3: 1.2- ± 0.6-fold) (scale bar = 50 μm). A higher fraction of proliferating disc cells (indicated by circles) was found in all human IVDs with an enhanced fraction of the Tie2+ disc progenitor cell population. IHC indicates immunohistochemistry.
MSC homing nonsignificantly enhanced the fraction of proliferating disc cells in five of six human IVDs (2.8- ± 2-fold; P = 0.17; healthy 1.2- ± 0.6-fold, trauma 1.7- ± 0.2-fold, degenerative 5.6- ± 3.1-fold) (Figure 7A). Interestingly, a higher fraction of proliferating cells was found in all human IVDs that demonstrated an enhanced fraction of the Tie2-positive cells after MSC homing. The FACS results were supported by IHC staining for Ki-67, which revealed more stained cells following MSC treatment in all responding donors (single data points in Figure 7).
DISCUSSION
Several earlier organ culture studies have demonstrated migration of exogenously applied MSCs through the endplate into the IVD.5–8 Although bovine IVDs have been used for those studies, results of the present work showed that MSCs could migrate through the endplate into an intact human IVD (Supplementary Fig. 2, http://links.lww.com/BRS/B443).
This study explored the effect of MSC homing on the Tie2-positive disc progenitor cell population, disc cell survival, and proliferation. A bovine whole organ culture model was used to investigate the effect of MSC homing after 5 days. Furthermore, the effect of MSC homing was investigated in human nondegenerated IVDs from organ donors, IVDs obtained from patients suffering from spinal trauma, and degenerative human IVD tissue samples.
Our results demonstrate an enhanced Tie2-positive disc progenitor cell population upon MSC homing into bovine and nonsignificantly in human IVDs. This finding is highly relevant, as the Tie2-positive disc cell population has been described to hold a discogenic differentiation capacity.21,27 Moreover, the fraction of Tie2-positive disc cells reflects the IVDs’ regenerative capacity, as Tie2-positive cells diminish with age and degree of IVD degeneration.20 New cell-based treatment strategies aim to isolate such progenitor cell subpopulations from the overall pool of IVD cells and reinject them after expansion in vitro.21 Interestingly, homing of MSCs did enhance the Tie2-positive disc cell population in human discs from patients following spine trauma and in nondegenerated human discs. In line with the findings of Li et al,28 we were able to detect Tie2-positive disc cells in the degenerative human IVDs; however, the change upon MSC homing was less pronounced than in the nondegenerative and spine trauma cases. Interestingly, when impeding MSCs migration into the bovine IVD, we could not observe such increased levels of the Tie2-positive disc cell subpopulation (Figure 2C). This indicates that MSCs may need to be actively migrating through the tissue and/or located near the IVD cells to exert a stimulatory effect. In addition, diffusion of the factors released from the MSCs through the endplate might be limited in the culture system used in this study.
A lower fraction of dead (DAPI-positive) cells was observed, in bovine and nonsignificantly in human IVDs, when homing occurred. To be able to migrate, MSCs naturally secrete proteolytic enzymes such as metalloproteinases.29 This could potentially harm the resident disc cells. In this respect, our results did not indicate any higher fraction of apoptotic disc cells following MSC migration. An increase in apoptotic cells was, however, observed in bovine IVDs at day 5 of culture compared to freshly isolated IVDs (day 0). This may be related to the nonphysiological ex vivo culture and potential nutrient deprivation under free swelling conditions.30 MSC treatment led to a slight but insignificant reduction of apoptotic cells, suggesting a marginal effect of MSCs on IVD cell apoptosis in the present study. Interestingly, a positive correlation was observed between the upregulation of Tie2 and Bcl2 transcripts. Bcl2 represents an antagonist of the apoptosis cascade, where it counteracts the apoptosis-promoting factor Bax; hence, increased Bcl2 expression may contribute to the improved survival phenotype of IVD cells upon MSC homing.31
A higher fraction of (Ki-67-positive) disc cells undergoing proliferation was observed in bovine and nonsignificantly in human IVDs following homing of MSCs. This is in line with the in vitro findings reported by Shim et al17 who described an enhanced proliferative response in degenerative disc cells following co-culture with MSCs. Pratsinis et al32 reported a proliferative response of IVD cells stimulated by PDGF, bFGF, and IGF-I. With respect to the paracrine stimulation by MSCs, Pereira et al7 observed an induction of growth factors following homing of MSCs in a whole organ culture model. These findings suggest that the homed MSCs may promote proliferation of the resident disc cells by paracrine stimulation.
Organ culture models represent the most complex ex vivo platforms, compared to 2D or 3D cell culture systems, and offer an opportunity to investigate the effect of MSC homing as a potential regenerative strategy. However, like clinical data sets, they are associated with a high interdonor variability, which makes the data analysis challenging. Several strategies were used to reduce or control the donor variance. Each experiment was repeated multiple times to compensate for potential interexperimental errors. By increasing the number of data points, the sensitivity of the statistical tests was improved. Hereby, only paired tests were used, without pooling the datasets, and single data points were described to avoid misinterpretation. In terms of experimental design, different methods were used to monitor cell survival with direct (DAPI by FACS) and indirect (Tie2 and Annexin V by FACS, Bax, and Bcl2 by gene expression) measurements.
Clinical Relevance
Our data indicate that MSC homing might have the highest impact on IVDs with moderate or beginning degenerative changes. In this respect, IVDs adjacent to a fused segment (16% risk of adjacent disc degeneration33,34) and IVDs with an AF injury (disc herniation as sign of early degeneration35) represent clinical cases wherein MSC homing could prevent or reverse the onset of the degenerative cascade. Laminectomy and spinal fusion are the most frequently performed surgeries in the United States, which highlights the unmet need for a preventive treatment strategy.36 Transpedicular injection of MSCs into the vertebral body, close to the endplate of the adjacent IVD or above the IVD treated by laminectomy, might therefore offer a strategy to increase the limited natural pool of intervertebral MSCs.37 Nevertheless, further studies are required to investigate the feasibility of this approach.
Limitations
The generalization of our results may be affected by some limitations. First, the Tie2-positive disc progenitor cell population was originally described within the NP. However, our study did not distinguish between NP and AF cells, as homing of MSC has been shown to occur into both tissues.6 Second, all MSC donors used in this study were obtained from bone marrow aspirates of trauma patients; 90% of the MSC donors were male patients, and 80% of the IVD donors were male, which discounts sex as a biological variable for the purpose of these experiments.
CONCLUSION
In conclusion, the stimulation of resident IVD cells by homed MSCs resulted in an enhanced population of Tie2-positive disc progenitor cells, reduced IVD cell death, and induced a proliferative response. Our findings highlight the importance of direct or indirect intercellular communication as a fundamental process to prevent or reverse ongoing degenerative processes.
Key Points
MSC homing from the endplate into the IVD represents a strategy for intradiscal cell application.
MSC homing enhanced the Tie2-positive disc progenitor cell population and prevented IVD cell death.
A proliferative response was induced following MSC homing into the IVD.
MSC homing could represent a potential treatment strategy to prevent the onset of the degenerative cascade in IVDs.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the McGill Scoliosis and Spine Group for providing the non-degenerated human IVD tissue, N.M. Goudsouzian and D. Nehrbass for their assistance with histological preparations.
Footnotes
The BD FACSAria III was donated by the Innovationsstiftung Graubünden.
The manuscript submitted does not contain information about medical device(s)/drug(s).
AO Foundation and AOSpine International funds were received in support of this work.
Relevant financial activities outside the submitted work: grants.
References
- 1.Lin W, Xu L, Zwingenberger S, et al. Mesenchymal stem cells homing to improve bone healing. J Orthop Translat 2017; 9:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eseonu OI, De Bari C. Homing of mesenchymal stem cells: mechanistic or stochastic? Implications for targeted delivery in arthritis. Rheumatology (Oxford) 2015; 54:210–218. [DOI] [PubMed] [Google Scholar]
- 3.Nitzsche F, Muller C, Lukomska B, et al. Concise review: MSC adhesion cascade-insights into homing and transendothelial migration. Stem Cells 2017; 35:1446–1460. [DOI] [PubMed] [Google Scholar]
- 4.Sakai D, Nishimura K, Tanaka M, et al. Migration of bone marrow-derived cells for endogenous repair in a new tail-looping disc degeneration model in the mouse: a pilot study. Spine J 2015; 15:1356–1365. [DOI] [PubMed] [Google Scholar]
- 5.Illien-Junger S, Pattappa G, Peroglio M, et al. Homing of mesenchymal stem cells in induced degenerative intervertebral discs in a whole organ culture system. Spine (Phila Pa 1976) 2012; 37:1865–1873. [DOI] [PubMed] [Google Scholar]
- 6.Pereira CL, Goncalves RM, Peroglio M, et al. The effect of hyaluronan-based delivery of stromal cell-derived factor-1 on the recruitment of MSCs in degenerating intervertebral discs. Biomaterials 2014; 35:8144–8153. [DOI] [PubMed] [Google Scholar]
- 7.Pereira CL, Teixeira GQ, Ribeiro-Machado C, et al. Mesenchymal stem/stromal cells seeded on cartilaginous endplates promote intervertebral disc regeneration through extracellular matrix remodeling. Sci Rep 2016; 6:33836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wangler S, Menzel U, Li Z, et al. CD146/MCAM distinguishes stem cell subpopulations with distinct migration and regenerative potential in degenerative intervertebral discs. Osteoarthritis and cartilage 2019; 27:1094–1105. [DOI] [PubMed] [Google Scholar]
- 9.Clouet J, Fusellier M, Camus A, et al. Intervertebral disc regeneration: from cell therapy to the development of novel bioinspired endogenous repair strategies. Adv Drug Deliv Rev 2018. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Peroglio M, Alini M, et al. Potential and limitations of intervertebral disc endogenous repair. Curr Stem Cell Res Ther 2015; 10:329–338. [DOI] [PubMed] [Google Scholar]
- 11.Huang YC, Leung VY, Lu WW, et al. The effects of microenvironment in mesenchymal stem cell-based regeneration of intervertebral disc. Spine J 2013; 13:352–362. [DOI] [PubMed] [Google Scholar]
- 12.Wuertz K, Godburn K, Iatridis JC. MSC response to pH levels found in degenerating intervertebral discs. Biochem Biophys Res Commun 2009; 379:824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korecki CL, Costi JJ, Iatridis JC. Needle puncture injury affects intervertebral disc mechanics and biology in an organ culture model. Spine (Phila Pa 1976) 2008; 33:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofer HR, Tuan RS. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther 2016; 7:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strassburg S, Richardson SM, Freemont AJ, et al. Co-culture induces mesenchymal stem cell differentiation and modulation of the degenerate human nucleus pulposus cell phenotype. RegenMed 2010; 5:701–711. [DOI] [PubMed] [Google Scholar]
- 16.Bertolo A, Thiede T, Aebli N, et al. Human mesenchymal stem cell co-culture modulates the immunological properties of human intervertebral disc tissue fragments in vitro. Eur Spine J 2011; 20:592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shim EK, Lee JS, Kim DE, et al. Autogenous mesenchymal stem cells from the vertebral body enhance intervertebral disc regeneration by paracrine interaction: an in vitro pilot study. Cell Transplant 2016; 25:1819–1832. [DOI] [PubMed] [Google Scholar]
- 18.Matta A, Karim MZ, Isenman DE, et al. Molecular therapy for degenerative disc disease: clues from secretome analysis of the notochordal cell-rich nucleus pulposus. Sci Rep 2017; 7:45623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin H, Shen J, Wang B, et al. TGF-beta signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett 2011; 585:1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakai D, Nakamura Y, Nakai T, et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nature Commun 2012; 3:1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tekari A, Chan SCW, Sakai D, et al. Angiopoietin-1 receptor Tie2 distinguishes multipotent differentiation capability in bovine coccygeal nucleus pulposus cells. Stem Cell Res Ther 2016; 7:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bara JJ, Herrmann M, Menzel U, et al. Three-dimensional culture and characterization of mononuclear cells from human bone marrow. Cytotherapy 2015; 17:458–472. [DOI] [PubMed] [Google Scholar]
- 23.Illien-Junger S, Gantenbein-Ritter B, Grad S, et al. The combined effects of limited nutrition and high-frequency loading on intervertebral discs with endplates. Spine (Phila Pa 1976) 2010; 35:1744–1752. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 25.Gawri R, Mwale F, Ouellet J, et al. Development of an organ culture system for long-term survival of the intact human intervertebral disc. Spine (Phila Pa 1976) 2011; 36:1835–1842. [DOI] [PubMed] [Google Scholar]
- 26.Rosenzweig DH, Gawri R, Moir J, et al. Dynamic loading, matrix maintenance and cell injection therapy of human intervertebral discs cultured in a bioreactor. Eur Cell Mater 2016; 31:26–39. [DOI] [PubMed] [Google Scholar]
- 27.Sakai D, Schol J, Bach FC, et al. Successful fishing for nucleus pulposus progenitor cells of the intervertebral disc across species. JOR SPINE 2018; 1:e1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XC, Tang Y, Wu JH, et al. Characteristics and potentials of stem cells derived from human degenerated nucleus pulposus: potential for regeneration of the intervertebral disc. BMC Musculoskelet Disord 2017; 18:242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Becker A, Van Hummelen P, Bakkus M, et al. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica 2007; 92:440–449. [DOI] [PubMed] [Google Scholar]
- 30.Zhang F, Zhao X, Shen H, et al. Molecular mechanisms of cell death in intervertebral disc degeneration (review). Int J Mol Med 2016; 37:1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams JM, Cory S. Bcl-2-regulated apoptosis: mechanism and therapeutic potential. Curr Opin Immunol 2007; 19:488–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J 2007; 16:1858–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghiselli G, Wang JC, Bhatia NN, et al. Adjacent segment degeneration in the lumbar spine. J Bone Joint Surg Am 2004; 86:1497–1503. [DOI] [PubMed] [Google Scholar]
- 34.Saavedra-Pozo FM. Adjacent segment disease perspective and review of the literature. Oschner 2014; 14:78–83. [PMC free article] [PubMed] [Google Scholar]
- 35.Schroeder JE, Dettori JR, Brodt ED, et al. Disc degeneration after disc herniation: are we accelerating the process? Evid Based Spine Care J 2012; 3:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fingar KR, Stocks C, Weiss AJ, et al. 2018. Most Frequent Operating Room Procedures Performed in U.S. Hospitals, 2003–2012: Statistical Brief #186. [Google Scholar]
- 37.Vadala G, Russo F, Pattappa G, et al. The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine (Phila Pa 1976) 2013; 38:E319–E324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


