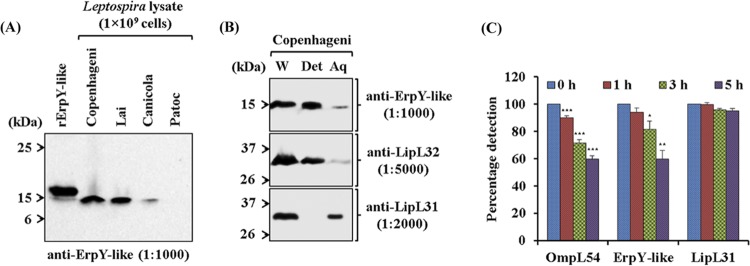
FIG 2.
Characterization of the recombinant Leptospira ErpY-like protein. (A) Immunoblot analysis of recombinant and native ErpY-like antigen using anti-ErpY-like protein antibody. Laboratory animal (mouse)-generated polyclonal antisera (1:1,000) after immunization with rErpY-like protein can detect both the native ErpY-like antigen in the pathogenic Leptospira (L. interrogans serovar Copenhageni, Lai, or Canicola) and the purified rErpY-like antigen (200 ng). The lysates of nonpathogenic L. biflexa serovar Patoc were used as a negative control in the immunoblot. (B) Phase partitioning of whole-cell protein of L. interrogans serovar Copenhageni using Triton X-114 and its immunoblot with the anti-rErpY-like antibody. Live leptospires were subjected to 1% Triton X-114 to partition proteins into detergent (Det) and aqueous-phase (Aq) fractions. Phase-partitioned proteins were resolved on 12% SDS-polyacrylamide gel, electroblotted on nitrocellulose membrane, and probed with anti-ErpY-like (1:1,000), anti-LipL32 (1:5,000), or anti-LipL31 (1:2,000) antibody. (Top) The ErpY-like protein was detected (15 kDa) predominantly in the detergent phase. LipL32, a known outer membrane protein of Leptospira, was detected predominantly in the detergent phase (middle), whereas a leptospiral cytoplasmic membrane protein, LipL31, was detected in the aqueous phase (bottom). Whole-cell lysate of L. interrogans serovar Copenhageni (W) was used in the immunoblot as a marker for ErpY-like protein, LipL32, and LipL31. (C) Protease accessibility assay of L. interrogans serovar Copenhageni using proteinase K (PK). Live leptospire suspensions (5 × 108 cells per suspension) were incubated with 5 μg of PK at the indicated time points. The suspensions were harvested, washed, resuspended in PBS, and coated on a microtiter plate (5 × 107 cells per well). In ELISA, the reactivity of ErpY-like protein with its antiserum showed a perpetual decrease in signal from 0 to 5 h of PK treatment. A similar trend of reduction in signal was observed for OmpL54, a known surface-exposed outer membrane protein of Leptospira. The serological detection of antigen LipL31, a known cytoplasmic membrane protein of Leptospira, demonstrates the uncompromised integrity of leptospire inner membrane during treatment with PK. Error bars represent SD from three replicates and are indicative of two independent experiments. Statistical analysis was performed using Student's t test by comparing the signals obtained for 0 h and other time points of treatment with proteinase K (*, P < 0.05; **, P < 0.01; ***, P < 0.001).