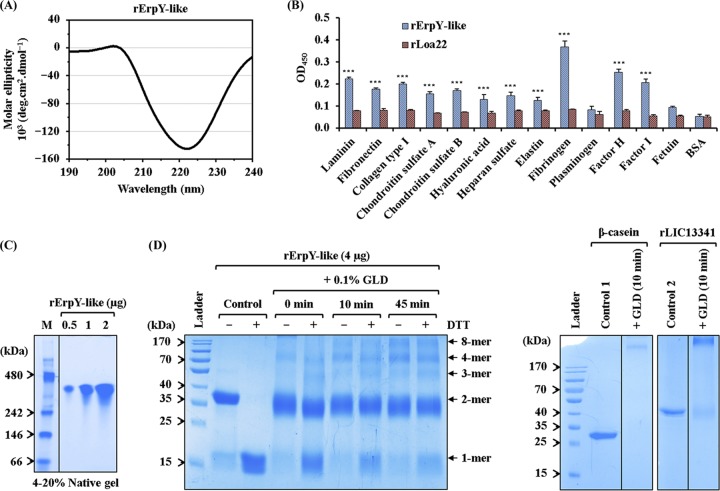
FIG 4.
Recombinant ErpY-like protein binds to host extracellular matrix and plasma components. (A) Far-UV (190 to 240 nm) CD spectra of rErpY-like protein. The analyzed CD spectral data show the predominance of α-helix (87.6%), which is in agreement with the theoretically predicted secondary structure of the rErpY-like protein. The spectra are represented as averages from three scans with a scanning speed of 100 nm min−1. (B) Interaction between rErpY-like protein and commercial host ECM components. The binding of rErpY-like protein to ECM components (laminin, fibronectin, collagen type I, chondroitin sulfate A and B, hyaluronic acid, heparan sulfate, and elastin) or plasma components (fibrinogen, plasminogen, factor H, and factor I) was analyzed through ELISA. The plasma components fetuin (highly glycosylated protein) and bovine serum albumin fraction V (nonglycosylated protein) were included as negative controls for ligands. rLoa22, which is known to interact moderately with host ECM components, was included in the assay as a negative-control protein. Protein-specific antisera (anti-ErpY-like protein or anti-Loa22 antibody) were used to measure the interaction of recombinant proteins with host ligands. The rErpY-like protein was recorded to interact preferentially with plasma fibrinogen, followed by factor H and other host ligands. The bar represents mean absorbance values (450 nm) for a ligand. Error bars are indicative of SD from two independent experiments, each performed in triplicate. For statistical analyses, the binding of rErpY-like protein with host ligands was compared to that with fetuin or BSA by the two-tailed t test (***, P < 0.001). (C) Pure rErpY-like protein forms a supramolecule. The purified rErpY-like protein (0.5, 1, and 2 μg) was resolved on a 4 to 20% native-PAGE and stained with Coomassie blue. The standard native molecular weight marker (M) was run in lane 1. The rErpY-like protein resolved at around 300 kDa. The gel in this figure has been spliced for labeling purposes. (D) Denaturing polyacrylamide gel electrophoresis of rErpY-like protein after glutaraldehyde (GLD) cross-linking. Chemical cross-linking of rErpY-like protein with glutaraldehyde (0 to 45 min) shows multiple higher-molecular-weight bands when resolved in the presence (+) or absence (−) of DTT. In the panel on the right, the cross-linked products of two control proteins, β-casein and rLIC13341, demonstrate only one band of larger size.