Fig. 1. Targeted GALC CLEA NP synthesis and characterization.
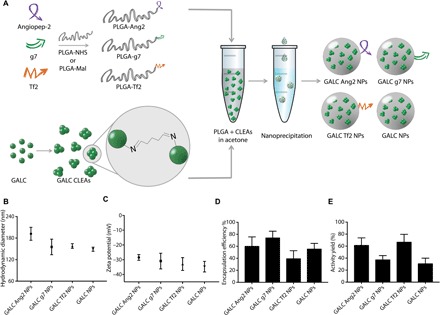
(A) Graphical summary of the experiment. Peptide-modified PLGA was produced by covalent linking of each peptide to a previously activated form of PLGA. GALC CLEAs were obtained by precipitation of GALC in acetone in the presence of glutaraldehyde, resulting in Schiff base formation between enzyme molecules. Last, targeted GALC CLEA NPs were obtained by nanoprecipitation. (B) Hydrodynamic Diameter of GALC CLEA NPs. Error bars represent the SEM of four independently synthesized NP batches. (C) Zeta potential of GALC CLEA NPs. Error bars represent the SEM of four independently synthesized NP batches. (D) Encapsulation efficiency of GALC CLEA NPs. Encapsulation efficiency is expressed as percentage of encapsulated GALC with respect to the amount of enzyme used in each synthesis. Error bars represent the SEM of four independently synthesized NP batches. (E) Activity yield of GALC CLEA NPs. Activity yield is expressed as percentage of specific activity (nanomole substrate hydrolyzed in 1 hour by 1 μg of enzyme) with respect to the free unaltered enzyme. Error bars represent the SEM of four independently synthesized NP batches.
