FIGURE 1.
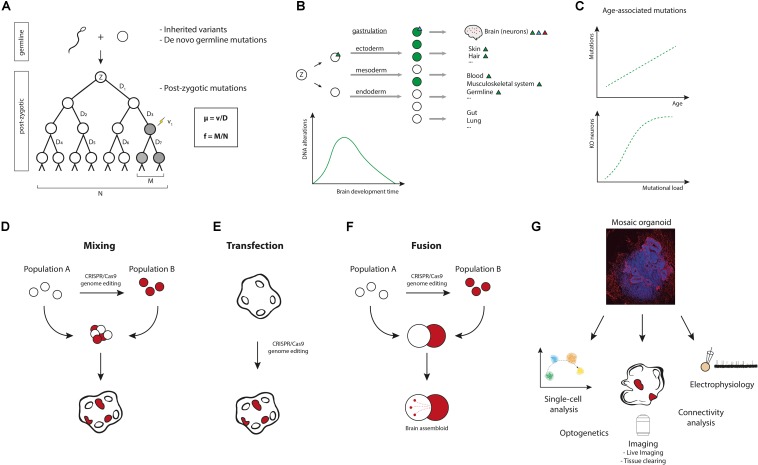
Modeling brain somatic mosaicism in cerebral organoids. (A) From zygote (Z) to birth and after, somatic mutations occur in the genomes of cells. These mutations result in somatic mosaicism, i.e., the presence of genetically distinct populations of cells within an individual. Normal tissues, including the brain, are mosaics of clones of various sizes wherein each cell’s genome is unique. Through genome sequencing, somatic mutations can actually be used to trace complete cell lineage trees (Behjati, 2016; McKenna and Gagnon, 2019). The mutation rate μ (probability of a mutation occurring per cell division) equals the number of mutation events (v) divided by the number of cell divisions (D). In the example μ = 1/7 mutations per genome per cell division. The mutation frequency f (proportion of mutant cells in a population) is the number of mutant cells in a population (M) divided by the total number of cells in a population (N). In the example f = 2/8. (B) Somatic mutations occur throughout development and aging. Mutations in early development can affect a large number of cells and will be shared among various tissues (green triangle). Mutations occurring later in development will be limited to a smaller number of cells (blue triangle), e.g., brain-specific mutations. Mutations occurring in post-mitotic cells result in very fine changes, as these are confined to single cells (red triangle). The estimated average mutation rate during neurogenesis (∼5.1 SNVs per day per progenitor, corresponding to ∼8.6 SNVs per division per progenitor) has been found to be higher than the mutation rate during early embryogenesis (∼1.3 SNVs per division per cell) (Bae et al., 2018). From an evolutionary point of view, this ramping up of mutation rate during neurogenesis is not very surprising. After all, protecting the genome at early embryonic stages is more important than at later stages of differentiation, where these mutations will affect fewer cells. Postnatally, after the rapid cellular expansion that happens during development, the mutation rate slows down considerably. (C) Somatic mutations continue to accumulate over a lifetime. It has been suggested that accumulation of mutations could cause aging (Failla, 1958; Szilard, 1959), but this remains to be proven (Niedernhofer et al., 2018; Zhang and Vijg, 2018). Recent data support a model wherein mutations accumulate age-dependently in single neurons (Lodato et al., 2018), and it was proposed that age-related accumulation of mutations in a diploid genome could provide a model for the exponential occurrence of age-related disease (following Gompertz kinetics). Most genes can function with one remaining allele, so for many years single mutations would have little effect on gene function (although a lot of genes are dosage-sensitive). During aging, mutations would then increasingly knockout the remaining allele or genes, creating “zombie cells” that are complete knockouts for essential genes. In neurodegenerative diseases like AD, oxidative stress and DNA damage are increased, making it likely that somatic mutation burden is increased in affected neurons. (D–F) Various approaches can be devised to model somatic mosaicism in cerebral organoids, including: generating cerebral organoids from mixed cultures of genetically different hiPSCs (D), transfection of cerebral organoids with gene-editing constructs (E), or combining genetically different hiPSC-derived cells into fused cerebral organoids (F). (G) Mosaic cerebral organoids can be analyzed by multiple methods, such as single-cell sequencing, proteomics, epigenetic analysis, live imaging, tissue clearing and 3D reconstruction, optogenetic probing, and electrophysiology (e.g., patch-clamping or multi-electrode recordings) (Amin and Paşca, 2018). Xenotransplantation of the organoids to mouse brains can be considered to study in vivo effects (Mansour et al., 2018). Cerebral organoids can also be used for pharmacological testing.