Figure 3.
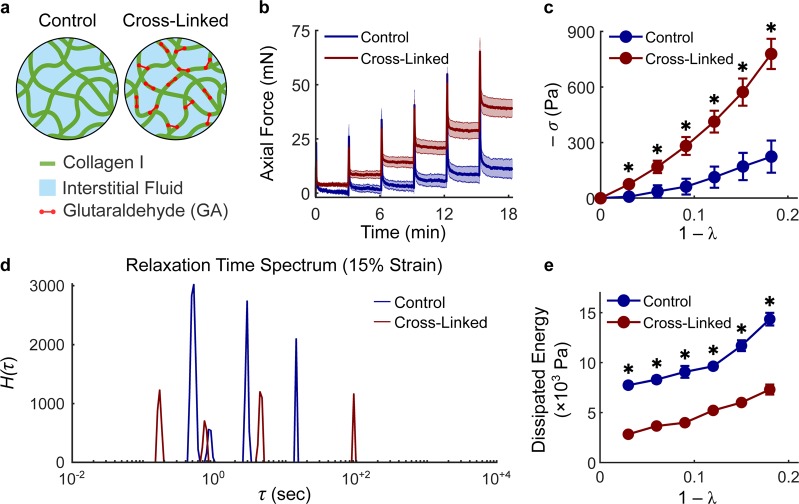
Collagen cross-linking increases hydrogel stiffness and reduces energy dissipation. At the microstructural level (a), a collagen gel is a network of collagen fibers (green) with pores filled by interstitial fluid (light blue), constituted in this study by either PBS or cell culture media. The collagen network can be cross-linked chemically using glutaraldehyde (GA), a bifunctional molecule (red) that strengthens both intrafibrillar and interfibrillar bonds. GA cross-linking influences both transient (b) and equilibrium (c) responses of 4 mg/mL collagen gels to confined compression. Control (blue lines) and cross-linked (red lines) gels were compressed to 18% of their original height by means of 3% steps. Cross-linked gels are characterized by a lower force decay (b) and a mechanical behavior that is both stiffer and nonlinearly elastic with respect to control gels (c). Representative relaxation time spectra H(τ) evaluated at 15% strain reveal that collagen gels display three to four distinct peaks which increase in height for increasing deformations (Supplementary Fig. 3) and are consistently lower for cross-linked, with respect to control samples (d). The energy dissipated at each compression step is quantified as the area under the curve of the relaxation time spectrum H(τ). Cross-linked gels display lower energy dissipation with respect to controls at all strains (e). Data are presented as mean ± SEM (control: n = 5, cross-linked: n = 9) and * indicates statistically significant differences with respect to controls at p < 0.05.