Abstract
BACKGROUND AND AIMS
The non-medical use of over-the-counter or prescribed analgesics (NMUA) is a significant public health problem. Little is known about the genetic and environmental etiology of NMUA and how these risks relate to other classes of substance use and misuse. Our aims were to estimate the heritability NMUA and sources of genetic and environmental covariance with cannabis and nicotine use, cannabis and alcohol use disorders and nicotine dependence in Australian twins.
DESIGN
Biometrical genetic analyses or twin methods using structural equation univariate and multivariate modeling.
SETTING
Australia.
PARTICIPANTS
2,007 young adult twins (66% female; μage=25.9, SD=3.6, range=18–38) from the Brisbane Longitudinal Twin Study retrospectively assessed between 2009 and 2016.
MEASUREMENTS
Self-reported NMUA (non-opioid or opioid-based), lifetime nicotine, cannabis and opioid use, DSM-V cannabis and alcohol use disorders and the Fagerström Test for Nicotine Dependence.
FINDINGS
Lifetime NMUA was reported by 19.4% of the sample. Univariate heritability explained 46% (95% CI = 0.29–0.57) of the risks in NMUA. Multivariate analyses revealed that NMUA is moderately associated genetically with cannabis (rg=0.41) and nicotine (rg=0.45) use, and nicotine dependence (rg=0.34). In contrast, the genetic correlations with cannabis (rg=0.15) and alcohol (rg=0.07) use disorders are weak.
CONCLUSIONS
In young male and female adults in Australia, the non-medical use of over-the-counter or prescribed analgesics (NMUA) appears to have moderate heritability. NMUA is moderately associated with cannabis and nicotine use and nicotine dependence. Its genetic etiology is largely distinct from that of cannabis and alcohol use disorders.
Keywords: non-medical use, over-the-counter, prescribed analgesics, twin, gene, comorbidity, substance use
Introduction
The non-medical use of over-the-counter or prescribed analgesics is one of the fastest growing drug trends in the U.S. (1–3). However, very little is known about the sources of individual differences in this emerging class of substance use or how these differences relate to the genetic and environmental risks that are known to predict other major classes of substance use and misuse.
The non-medical use of either prescribed or over-the-counter analgesics (NMUA) is a clear public health threat. In the U.S., deaths related to NMUA now exceed those for all other illicit substances including cocaine and heroin, and continue to increase (4). Between 1993 and 2005 the prevalence of the non-medical use of prescribed opioids among U.S. college students increased by 343% (5). Among the 1.2 million emergency department visits in the U.S in 2009 involving nonmedical use of pharmaceuticals or dietary supplements, approximately 50% involved the nonmedical use of prescribed opioid-based analgesics (6). In Australia in 2016, prescribed and over-the-counter analgesics were the most commonly misused pharmaceuticals in the past 12 months, which made this class the second most illicitly used substance after cannabis (7). The non-medical use of opiate or non-steroidal analgesics is associated with a variety of negative physical effects including, tachycardia, seizures, agitation, dependence, and death (8, 9). In terms of comorbid substance use, the non-medical use of prescribed opioids has been linked to the risk of transitioning to other classes of illicit SU and SUDs (10–13).
Despite these trends and consequences, the genetic epidemiology of this class of substance use remains completely unknown. Specifically, the degree to which the genetic risk factors underpinning comorbid licit and illicit substance use and misuse are also responsible for individual differences in NMUA remains to be determined.
We hypothesize that familial aggregation in the NMUA will be largely explained by genetic risks and that these risks will be correlated with the genetic risks in other forms of licit and illicit substance use including opioid use as well as common classes of substance use disorders involving cannabis, alcohol and nicotine. These predictions are based on widely accepted findings showing heritability estimates ranging from 40–70% across substances (14–17), along with evidence that genetic risks in licit and illicit substance abuse or dependence, at least in males, are largely common across substances (18, 19) and indeed are shared more broadly with the spectrum of externalizing psychopathology (20, 21). Although evidence supports two distinct genetic risk factors underpinning individual differences in substance use disorders (22) with one predisposing to illicit (cannabis and cocaine) and the other to licit (alcohol, caffeine and nicotine) drug dependence, both factors are highly correlated (22) and recent studies demonstrate moderate to high genetic correlations between licit and illicit abuse and dependence disorders in both males and females (23). And because of the degree of shared genetic risks between licit and illicit substance use despite their diverse pharmacology (18, 19), we hypothesize that the genetic correlation between NMUA and common classes of substance use and misuse will be high.
The degree to which environmental risk factors related to NMUA are shared with other drug classes is unclear. Quantifying heritability and establishing if the genetic and environmental pathways leading to NMUA are linked to other major classes of substance use and misuse will provide valuable insight into the aetiology of NMUA, which may, in turn, inform future intervention and prevention programs.
Aims
This report has two aims. The first is to estimate the contribution of genes and environment to the NMUA. This includes determining if there are significant sex differences in the prevalence of use, including sex differences in the relative proportions of genetic and environmental risks. The second aim is to determine if the genetic and environmental risks in lifetime cannabis and nicotine use are correlated with NMUA. This aim will also determine if the genetic and environmental risks in cannabis use disorder, nicotine dependence, and alcohol use disorder are likewise correlated with NMUA.
Methods
Participants
The sample consists of male and female adult twins from the ongoing and population-based Brisbane Longitudinal Twin Study (BLTS) (24–26). Participants are of European ancestry, predominately Anglo-Saxon, who were ascertained beginning 1992 to study melanocytic naevi, and have since been followed up on multiple occasions. The BLTS is a longitudinal, phenotypically rich collection of psychiatric phenotypes, environmental and psychological risk factors, and neurobiological correlates of psychiatric disorders (24–26). The sample comprises 2,900 twins (including 700 siblings and 2,100 parents) with assessments at 12, 14, 16, and 21 years. Typical response rates across the BLTS projects since 1992 range from 73% to 85% (24–26).
BLTS data for this report come from the 19UP Project (66% female; μage = 25.9, SD=3.6, range=18–38) collected between 2009–2015 and which relied on a combination of telephone and online self-report surveys to assess SU and SUDs (25–27). The 19UP was a US National Institute on Drug Abuse (NIDA) and Australian National Health and Medical Research Council (NHMRC) funded project to study the pathways to cannabis use and misuse (25, 26), comorbid substance use and misuse, internalizing and externalizing disorders along with a wide array of general health, behavioural, and lifestyle measures (27).
Measures
The non-clinical data used to test our hypotheses included lifetime nicotine, cannabis and opioid use (e.g. heroin morphine, methadone, codeine, etc.), as well as the non-medical use of over-the-counter or prescribed analgesics (NMUA). NMUA included codeine-based and nonsteroidal anti-inflammatory drugs (e.g. cough medicine, acetaminophen, codeine phosphate hemihydrate, doxylamine succinate, ibuprofen, acetaminophen, acetaminophen & codeine phosphate hemihydrate, codeine, hydrocodone etc). Non-medical use was defined as substances not taken in quantities or in a manner prescribed by a medical practitioner. All four lifetime use measures were assessed as dichotomous outcomes beginning with the phrase ‘In your life, have you ever used [substance]’. Alcohol use was not included because the lifetime prevalence was 98% at the time of assessment.
Diagnostic data included criteria for the Fagerström Test for Nicotine Dependence (FTND) (28), DSM-V Alcohol Use Disorder and DSM-V Cannabis Use Disorder (CUD) (marijuana, hashish, ‘THC’ or ganja) (29). All three diagnoses were based on the period(s) when subjects reported using each substance the most. Subjects answered the AUD psychiatric criteria only if they endorsed having consumed 5 (males) / 4 (females) or more drinks at least once a week for one month or more. Subjects answered the CUD psychiatric criteria if they endorsed having smoked cannabis 6 or more times lifetime or 11 or more times in a month. Finally, subjects answered FTND psychiatric criteria if they reported having smoked 100 or more cigarettes lifetime.
In order to avoid sparse data and improve computational efficiency when using raw ordinal data methods, we recoded the AUD, CUD and FTND criteria sum scores. The total AUD and CUD criteria were recoded onto 3-point ordinal scales (0–1, 2–3,≥4), which combined the DSM-V categories of ‘moderate’ and ‘high’. The total FTND criteria were also recoded onto a 3-point ordinal scale (0–1, 2–3,≥4). Here, we combined (i) ‘low’ and ‘low to moderate dependence’ and (ii) ‘moderate dependence’ and ‘high dependence’.
For the FTND and CUD diagnoses, nicotine and cannabis non-users were excluded and their diagnosis coded as missing. Our rationale was based on the possibility that non-users are potentially heterogeneous and comprise individuals with varying degrees of environmental risk (including exposure opportunities) and levels of genetic liability that cannot be accurately assessed here. Recoding non-users to ‘zero’, instead of missing, falsely assumes that knowledge of an individual’s diagnosis status can be known in the absence of self-reported data on either the use or exposure to a substance. Assigning non-users to zero inflates the denominator in prevalence estimates, thereby altering not only the item threshold but all subsequent variance-covariance estimates. Although only 1.7% of the sample (N=34) reported no lifetime alcohol use, the same procedure was followed for AUD.
Among the N = 2,773 twins eligible to participate in the 19UP Project, N = 2,007 (72%) provided complete responses to the non-medical use of analgesics item for lifetime use. This included 214 complete and 56 incomplete same-sex MZ female twin pairs, 132 complete and 86 incomplete same-sex MZ male twin pairs, 157 complete and 37 incomplete same-sex DZ female twin pairs, 97 complete and 66 incomplete same-sex DZ male twin pairs, and 216 complete and 130 incomplete opposite-sex MZ female twin pairs.
Statistical analyses
Prevalence and measures of association
The prevalence of the non-medical use of analgesics, along with pairwise polychoric correlations between all the above binary and ordinal measures of substance use and misuse were calculated using the Full Information Maximum Likelihood (FIML) raw data method using the OpenMx software package (Version 2.9.9.1) (30) in R (Version 3.4.1) (31). We did not use Weighted Least Squares (WLS) since considerably larger samples are required to arrive at reliable weight matrix estimates (32). Given the numbers of incomplete twin pairs (see Supplement Table S2), WLS would result in significant listwise deletion thereby altering the accuracy of the threshold estimates. The raw ordinal data FIML option (30) has the advantage of not only being more robust to violations of non-normality. Critically, FIML enables the analysis of missing or incomplete data as well as the direct estimation of covariate effects e.g. age and sex, on the item thresholds. More accurate thresholds improve the estimation of the polychoric correlations. Polychoric correlations were first proposed by Pearson (33, 34). They are based on the central limit theorem of theoretical statistics, which assumes that underlying an observed binary or ordinal variable, there exists a continuous, normally distributed latent liability and that the joint distribution of each scale with the liability scales underlying other items is bivariate normal (35, 36). Polychoric (or tetrachoric for binary or dichotomous variables) represent correlations between the underlying liability distributions rather than observed dichotomous or ordinal distributions.
Burnham and Anderson have argued that choice between AIC and alternatives such as the Bayesian Information Criterion (BIC) should be determined by the philosophical context of what is assumed about reality (37). We have argued elsewhere that the advantage of AIC is its deep theoretical connections to cross-validation (38). Specifically, in large samples, the AIC is expected to select that model in the candidate set which minimizes the error of prediction in new samples of the same size from the population (where the error is based on a log-likelihood function) (38). Specifically, the AIC is expected to minimize the Kullback–Leibler (KL) divergence from full reality at the given sample size. A sensible objective of model selection is to choose the model that has the smallest KL divergence from full reality. The full reality, of course, is not known, and may not even be knowable. Indeed, a complete description of full reality would be infinitely long. If we accept the possibility that no statistical model can completely describe reality, then the premise of there being a ‘true model’ that generated the data becomes rather dubious. In summary, because full reality may be unknowable, we do not presume that the true model is knowable from our data and consequently, chose our fit index based on this philosophy. Rather than proposing to identify the true model, the AIC selects the best-approximating model based on an optimal balance of parsimony and model fit.
Univariate twin modelling
To test the hypothesis that familial aggregation in the non-medical (lifetime) use of analgesics is entirely explained by additive genetic risk factors, we fitted univariate biometrical genetic models (32) that exploit the expected genetic and environmental correlations between monozygotic (MZ) and dizygotic (DZ). Specifically, we fitted twin models using the Full Information Maximum Likelihood (FIML) raw ordinal data methods in the OpenMx software package (Version 2.9.9.1) (30) in R (Version 3.4.1) (31). This approach assumes that the categories in a binary or ordinal variable are imprecise indicators of a latent normal liability distribution. These categorical thresholds are conceived of as cut-points along a standard normal distribution that relate category frequencies to cumulative probabilities indicating increasing levels of risk. In OpenMx2.0 (39), thresholds can be adjusted for covariates such as age and sex. Based on the Classical Twin Design (32, 40), our method of univariate modelling also assumes that individuals differences in substance use or variance in an observed behaviour can be decomposed into additive (A) genetic, shared environmental (C), and non-shared or unique (E) environmental variance components (32, 40). Since MZ twin pairs are genetically identical and DZ twin pairs share, on average, half of their genes, the expected twin pair correlations for additive genetic effects are 1.0 and 0.5 respectively. An important assumption is that the common environments (C) are equal in MZ and DZ twin pairs and because non-shared environments (E) are uncorrelated, E necessarily includes measurement error. All models include the covariates of age and sex.
The univariate A, C and E parameters were estimated using a ‘variance components’ or Direct Symmetric approach, which directly estimates a set of symmetrical variance components matrices (41). This approach may return nonsensical values in some situations (e.g. heritability estimates larger than 1, or non-positive definite covariance matrices). However, the absence of boundaries on the estimates (as in the pathway coefficients approach) yields asymptotically unbiased parameter estimates and corrects for Type I and Type II errors (41).
Multivariate twin modelling
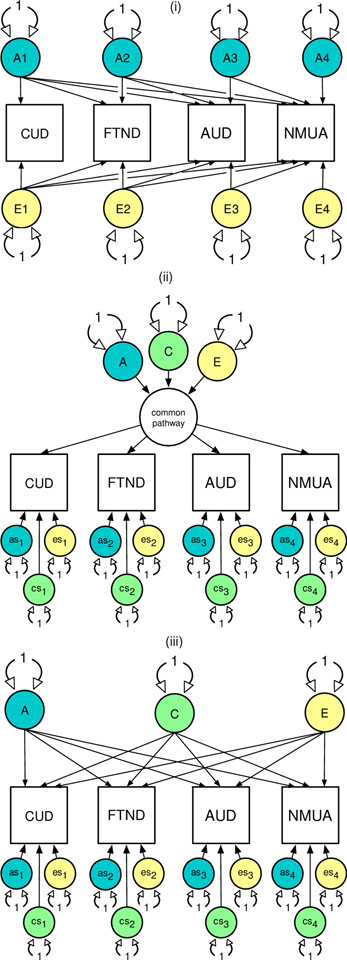
To test the hypotheses that genetic risk factors in the NMUA are shared with common forms of licit and illicit substance use and misuse we fitted common and independent pathway models (see Figure 1) (Neale and Cardon, 1992) again using the OpenMx software package (Version 2.9.9.1) (30) in R (Version 3.4.1) (31). In Figure 1, the reference model is the Cholesky decomposition (i) is a method of triangular decomposition where the first observed phenotypic measure is assumed to be caused by a latent factor (A1) that can explain the variance in the remaining variables. The second variable is assumed to be caused by a second latent factor (A2) that explains variation in the second as well as the remaining observed variables. This pattern continues until the final observed variable is explained by a latent variable which is constrained from explaining the variance in any of the previously observed variables. A ‘Cholesky Decomposition’ is specified for each latent source of additive genetic (A), shared environmental (C), and individual-specific environmental variance (E).
Figure 1.
The Cholesky decomposition (i) common (ii) and independent (iii) pathway models to explain sources comorbid substance use (or misuse) in terms of genetic (A), shared environmental (C) and non-shared (E) environmental risks. For brevity, the shared environmental risk factors are omitted from the Cholesky. The common and independent pathway models include variable specific genetic (as1–4) and environmental (cs1–4, es1–4) risks unique to each substance. All latent variables (circles) are standardized. All pathways with single-headed arrows are estimated.
The common pathway model (ii) predicts that a single, common latent liability to substance use or misuse, which can be decomposed into A, C, and E components of variance. The common pathway is ‘indicated’ by the strength of the factor loadings to each of the observed phenotypic measures. Residual variance or risks unique to each measure of substance use or misuse can be further decomposed into variable specific ‘as’, ‘cs’, and ‘es’ components. In contrast, the independent pathway model (iii) predicts that latent genetic and environmental risk factors each independently account for any observed comorbidity between the substance use and misuse phenotypes. For each aim, the best fitting model was determined based on an optimal balance of complexity and explanatory power using the Akaike’s Information Criterion (AIC) (27). For each best fitting model, the A and C parameters are successively fixed to zero and their significance determined using a likelihood ratio test.
Results
Prevalence of lifetime non-medical use of over-the-counter or prescribed analgesics (NMUA)
Table 1 shows prevalence and age initiation for lifetime NMUA, cannabis, nicotine, and opioid use along with the age of onset for AUD, ND and CUD. Psychiatric criteria for AUD, ND and CUD were based on the period of heaviest use. The prevalence of lifetime NMUA was marginally higher among females (20.2% vs 18.4%). The prevalence of lifetime NMUA was lower compared to lifetime use of cannabis or nicotine, but higher than the lifetime prevalence of opioids. For males and females alike, the average age of NMUA initiation occurred after nicotine but before cannabis and opioid use. Finally, the prevalence of NMUA was marginally lower among males.
Table 1.
Prevalence of lifetime cannabis, nicotine, illicit opioid, alcohol use disorder, nicotine dependence, cannabis use disorder, and lifetime non-medical use of over-the-counter or prescription analgesics (NMUA).
| Sample size | Prevalence | Age of Initiation (SD) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | Male | Female | |
| 1. Cannabis use | 2,100 | 910 | 1,190 | 56.0% | 63.7% | 50.0% | 17.5 (2.73) | 17.6 (2.81) |
| 2. Nicotine use | 2,012 | 873 | 1,139 | 45.2% | 37.7% | 51.0% | 16.0 (3.07) | 15.3 (2.28) |
| 3. Opioid use | 2,005 | 870 | 1,135 | 6.1% | 6.4% | 5.8% | 20.4 (0.03) | 19.8 (0.04) |
| 4. Alcohol use disorder | 1,989 | 846 | 1,126 | 45.5% | 56.0% | 37.5% | 15.8 (1.80) | 16.1 (1.77) |
| 5. FTND | 1,162 | 557 | 605 | 36.9% | 40.0% | 34.2% | NA | NA |
| 6. Cannabis use disorder | 1,024 | 512 | 512 | 24.2% | 29.5% | 18.9% | NA | NA |
| 7. NMUA | 2,007 | 871 | 1,136 | 19.4% | 18.4% | 20.2% | 16.2 (0.05) | 15.9 (0.06) |
FTND = Fagerström Test for Nicotine Dependence. SD=standard deviation. Substance use disorders based on the period when subjects reported using the most. All non-users coded as missing.
Measures of association
Among males and females, NMUA was correlated with lifetime opioid use (r=0.60–0.67) (see Table 2a). In contrast, the phenotypic correlations between NMUA and lifetime cannabis or nicotine use were smaller in males (r=0.26–0.29) and much smaller in females (r=0.10–0.15). As expected, the phenotypic correlations between cannabis and nicotine use were high. The correlations between opioid and cannabis (r=0.42–0.60) or between opioid and nicotine (r=0.39–0.43) use were higher than the correlations between NMUA and cannabis (r=0.10–0.26) or between NMUA and nicotine (r=0.15–0.29) use.
Table 2.
Pairwise polychoric phenotypic correlations (and standard errors) between lifetime non-medical use of over-the-counter or prescription analgesics (NMUA) and measures of (a) substance use and (b) substance use disorders. Males are below the diagonal.
| (a) Correlations with lifetime substance use | (b) Correlations with substance use disorders | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 1. | 2. | 3. | 4. | ||
| 1. Cannabis use | 1 | 0.74(0.04) | 0.42(0.07) | 0.10(0.05) | 1. CUD | 1 | 0.44(0.04) | 0.50(0.05) | 0.11(0.00) |
| 2. Nicotine use | 0.78(0.03) | 1 | 0.43(0.07) | 0.15(0.05) | 2. FTND | 0.43(0.05) | 1 | 0.72(0.04) | 0.22(0.00) |
| 3. Opioid use | 0.60(0.08) | 0.39(0.08) | 1 | 0.60(0.06) | 3. AUD | 0.46(0.05) | 0.70(0.04) | 1 | 0.24(0.00) |
| 4. NMUA | 0.26(0.06) | 0.29(0.06) | 0.67(0.06) | 1 | 4. NMUA | 0.15(0.00) | 0.24(0.00) | 0.32(0.00) | 1 |
Notes: CUD = DSM-V cannabis use disorder, FTND = Fagerström Test for Nicotine Dependence, AUD = DSM-V alcohol use disorder (AUD). Substance use disorders based on the period when subjects reported using the most. All non-users coded as missing.
In terms of the associations between NMUA and substance misuse, NMUA did not correlate phenotypically very highly with AUD, ND or CUD (see Table 2b) with point estimates ranging from 0.15 to 0.32 among males and from 0.11 to 0.24 among females. In contrast, correlations between the three measures of substance misuse were moderate to high (r=0.43–0.72).
Sex differences
Before modelling the genetic aetiology of NMUA we first tested the significance of age and sex effects on the prevalence of each variable (see Supplement Table S1). Specifically, we tested age and sex effects on the mean latent liability. For NMUA, a model without any age and sex differences did not deteriorate significantly (Δχ2=1.97, Δdf=2, p=0.37). Likewise, there were no sex differences in the prevalence of lifetime opioid use. In contrast, males were significantly more likely to report lifetime cannabis and nicotine use and be diagnosed with DSM-V alcohol use disorder, cannabis use disorder and nicotine dependence. Older subjects were also more likely to endorse lifetime cannabis, nicotine and opioid use, as well as receive a diagnosis of nicotine dependence.
Twin pair correlations
Monozygotic (MZ) and dizygotic (DZ) twin pair polychoric correlations including 95% confidence intervals based on combined male and female data with sex and age included as covariates are shown in the Supplement Table S2. For NMUA, the DZ twin pair correlation is approximately one-half of the MZ counterpart, which is consistent with the hypothesis that familial aggregation is entirely explained by additive genetic risk factors. For nicotine and opioid use as well as alcohol use disorder the DZ twin pair correlations did not exceed one-half of the MZ correlations suggesting familial aggregation attributable to additive genetic risks. In contrast, the DZ correlations for FTND and cannabis use disorder suggest familial aggregation attributable to a combination of shared environmental and additive genetic risks. Note however that the 95% confidence intervals for most of the twin pair correlations are wide.
Univariate results
When fitting univariate models to estimate the proportions of genetic and environmental risks in each variable, we first determined if the genetic (A) and environmental (C and E) risk factors could be constrained equal across sex (see Supplement S3). For NMUA, constraining these variance components did not result in a significant deterioration in model fit (Δχ2=6.13, Δdf=3, p=0.11), which suggests that the relative contribution of these risk factors is unchanged with respect to sex. Likewise, for all remaining variables, there were no significant sex differences in the variance components. Henceforth, all male and female data were combined and modelled with age and sex effects on the variable means.
Table 3 includes the standardized variance components based on each of the best fitting univariate models (see Supplement Table S4). With the exception of lifetime opioid use, all shared environmental risk factors could be removed from each univariate model without any significant deterioration in model fit. For lifetime NMUA additive genetic risk factors explained 46% of the total variation. Relative to cannabis use, nicotine use, nicotine dependence and cannabis use disorder, the genetic risk factors for NMUA explained a much smaller proportion of the total variance. Instead, the remaining proportion of variance was entirely explained by non-shared or random environmental risk factors including measurement error.
Table 3.
Standardized components of variance (and 95% confidence intervals) attributable to additive genetic (A), shared environmental (C), and non-share or random environmental (E) risks based on the best fitting univariate models for substance use and misuse including.
| A | C | E | |
|---|---|---|---|
| 1. Cannabis use | 0.77 (0.68–0.85) | - | 0.23 (0.15–0.32) |
| 2. Nicotine use | 0.70 (0.60–0.79) | - | 0.30 (0.21–0.40) |
| 3. Opioid use | 0.49 (−0.52–0.99) | −0.20 (−0.79– 0.57) | 0.71 (0.40–0.99) |
| 4. AUD | 0.49 (0.38–0.60) | - | 0.51 (0.40–0.62) |
| 5. FTND | 0.72 (0.60–0.81) | - | 0.28 (0.19–0.40) |
| 6. CUD | 0.65 (0.47–0.80) | - | 0.34 (0.20–0.53) |
| 7. NMUA | 0.46 (0.29–0.57) | - | 0.54 (0.43–0.71) |
Notes: A = additive genetic, C = common environmental risks, E = non-shared environment risk factors, AUD=DSM-V Alcohol Use Disorder, FTND = Fagerström Test for Nicotine Dependence, CUD = DSM-V Cannabis Use Disorder, NMUA = lifetime non-medical use of over-the-counter or prescription analgesics (NMUA). Substance use disorders based on the period when subjects reported using the most. All non-users coded as missing.
For lifetime opioid use, neither the AE nor CE models deteriorated significantly when compared to the full ACE model and all three AICs were in close proximity. Therefore, the ACE was retained in Table 3 despite the non-significant estimate for A and the nonsensical negative variance estimate for C. In samples where there is greater sampling distribution variability, the observed MZ twin pair correlations can be underestimated and the DZ correlations overestimated by chance alone. When this occurs, variance component estimates will often be negative but not significant, implying that the parameter is not statistically distinguishable from zero (41). Negative shared environmental variance components may be due to stochastic variation in the estimate or to a genuinely different source of variation such as genetic dominance (41). Post-hoc power calculations using the R-based acePowOrd function (42) revealed insufficient power (19%) to detect an additive genetic variance of 25% based on the AE model in Table S4. Given the lack of statistical power to resolve the sources of familial aggregation, lifetime opioid use was excluded from all subsequent analyses.
Multivariate results
Lifetime cannabis, nicotine and opioid use and NMUA
To test the hypothesis that comorbid cannabis and nicotine use and NMUA can be explained by common genetic risks, we first fitted an ACE Cholesky as a reference for comparing the common independent pathway models (see Supplement Table S5). When compared to the full Cholesky, neither of the hypothesis-driven models provided a better fit when judged by the AIC.
We then determined if the additive genetic or the shared environmental risks could be removed from the Cholesky. As shown in Table S5, all shared environmental risks could be removed from the model. Table 4 shows the standardized proportions of variance attributed to the additive genetic and non-shared environmental variance for each variable based on the multivariate AE Cholesky. We then estimated the latent genetic and environmental factor correlations, which revealed that the additive genetic risks in NMUA were modestly correlated with those for cannabis and nicotine use. In contrast, aspects of the unique environment that comprise individual differences in NMUA were unrelated to those for lifetime cannabis and nicotine use.
Table 4.
Standardized proportions of variance along with additive genetic and non-shared environmental risk factor correlations (95% confidence intervals) based on the best fitting multivariate AE Cholesky decomposition of cannabis use, nicotine use and lifetime non-medical use of over-the-counter or prescription analgesics (NMUA).
| Variance components | Additive genetic (below diagonal) & non-shared environmental correlations | ||||
|---|---|---|---|---|---|
| A | E | 1. | 2. | 3. | |
| 1. Cannabis use | 0.80 (0.71–0.87) | 0.20 (0.13–0.29) | 1 | 0.71 (0.50 – 0.88) | −0.28 (−0.54 – 0.00) |
| 2. Nicotine use | 0.72 (0.62–0.80) | 0.28 (0.20–0.38) | 0.78 (0.71 – 0.85) | 1 | −0.18 (−0.42 – 0.00) |
| 3. NMUA | 0.47 (0.41–0.61) | 0.53 (0.39–0.59) | 0.41 (0.24 – 0.56) | 0.45 (0.27 – 0.60) | 1 |
Notes: A = additive genetic, E = non-shared environment risk factors
Lifetime alcohol use disorder (AUD), nicotine dependence (ND), cannabis use disorder (CUD) and NMUA.
To test the hypothesis that cannabis use disorder, nicotine dependence, alcohol use disorder and NMUA can be explained by common risks, we again fitted a Cholesky as a reference, followed by the common and independent pathway models (see Supplement Table S6). Neither the common nor independent pathway models provided a good fit to the data. Subsequent hypothesis testing revealed that shared environmental risk factors could be entirely removed from the Cholesky without any significant deterioration in fit. Standardized multivariate components of variance are shown in Table 5 along with the additive genetic and non-shared environmental correlations. The correlations between the additive genetic risks for NMUA and the three substance use disorders ranged from small to moderate (0.07 to 0.34). The highest genetic correlation was with FTND. The additive genetic correlation between NMUA and AUD was non-significant. Finally, the unique environments risks in NMUA were unrelated to those in substance use disorders.
Table 5.
Standardized proportions of variance along with additive genetic and non-shared environmental risk factor correlations (95% confidence intervals) based on the best fitting multivariate AE Cholesky decomposition of CUD, FTND, AUD and lifetime non-medical use of over-the-counter or prescription analgesics (NMUA).
| Variance components | Additive genetic (below diagonal) & non-shared environmental correlations | |||||
|---|---|---|---|---|---|---|
| A | E | 1. | 2. | 3. | 4. | |
| 1. CUD | 0.68 (0.50–0.81) | 0.32 (0.19–0.50) | 1 | 0.71 (0.50 – 0.88) | −0.28 (−0.54 – 0.00) | −0.28 (−0.54 – 0.00) |
| 2. FTND | 0.76 (0.65–0.84) | 0.24 (0.16–0.35) | 0.67 (0.54 – 0.82) | 1 | −0.18 (−0.42 – 0.00) | −0.18 (−0.42 – 0.00) |
| 3. AUD | 0.51 (0.40–0.61) | 0.49 (0.39–0.60) | 0.40 (0.18 – 0.62) | 0.25 (0.08 – 0.41) | 1 | −0.18 (−0.42 – 0.00) |
| 4. NMUA | 0.46 (0.30–0.61) | 0.54 (0.39–0.70) | 0.15 (0.06 – 0.41) | 0.34 (0.14 – 0.54) | 0.07 (−0.14 – 0.29) | 1 |
Notes: A = additive genetic, E = non-shared environment risk factors, AUD=DSM-V Alcohol Use Disorder, FTND = Fagerström Test for Nicotine Dependence, CUD = DSM-V Cannabis Use Disorder. Substance use disorders based on the period when subjects reported using the most. All non-users coded as missing
Discussion
Almost one-fifth of this Australian sample of young adults reported lifetime non-medical use of over-the-counter or prescribed analgesics (NMUA). There were no sex and age differences in the prevalence of this class of substance use. Regarding the aetiology, lifetime NMUA could be explained by a combination of genes and random aspects of the environment. Commensurate with other family studies on substance use and misuse (18, 21, 43), the shared familial environment played no significant role in the risk of NMUA. Contrary to our hypothesis, genes that increase the risk of NMUA were only moderately related to the genes for lifetime cannabis and nicotine use. In terms of substance misuse, this class of substance use was genetically unrelated to alcohol use disorder, and while the genetic correlations with cannabis and nicotine use disorders were significant, they were small to very modest. Overall, the genetic risks in this newer class of substance use were mostly distinct from the more prevalent classes of licit and illicit substances and misuse.
Our finding of no significant sex differences in lifetime NMUA is commensurate with the 2013 National Drug Strategy Household Survey (NDSHS) in Australia based on a nationally representative sample of 23,855 respondents, which found the prevalence of past 12-month use was similar among males (3.3%) and females (3.2%) (44).
In the 2016 NDSHS (45), pain-killers and opioids were combined into one section while the use of non-opioid over-the-counter (OTC) substances such as paracetamol and aspirin were removed. This was because they were not known to be misused for cosmetic purposes, to induce or enhance a drug experience, or to enhance performance (45). Despite the removal of all non-opioid OTCs from the 2016 survey, the past 12-month prevalence of NMUA increased slightly to 3.6% (45), suggesting that non-opioid OTCs were not being misused nor were they being endorsed by respondents in the 2013 survey.
The finding of no significant shared environmental risks in the lifetime NMUA contrasts with reports that have investigated cannabis (46–49) and nicotine (50, 51) initiation, as well as individual differences in the frequency of nicotine, alcohol, cannabis and other classes of substance use (52–55), nearly all of which have revealed evidence of significant shared environmental risks. The decline in shared environmental risk factors over time is characteristic of the progression to more frequent substance use and the variation in psychiatric criteria indicative of misuse (56). Beyond the associations with other forms of substance use examined here, it is plausible that the liability to NMUA represents a more severe, emerging class of substance use. For instance, NMUA has been linked to psychiatric symptoms by us (57–60) and others (61). In non-genetically information studies, we have documented numerous adverse associations between NMUA and stimulants with behaviours such as high-risk sexual behaviour (62, 63), driving under the influence (60) and sexual assault (64, 65). However, attempts to determine empirically the degree of impairment associated with this class of substance use vis-à-vis other substances are currently hampered by a lack of available abuse and dependence criteria and the appropriate application of item response theory analysis (66) beyond the scope of the present analyses.
Limitations
Our findings must be interpreted in the context of four potential limitations. First, our sample comprises a population-based sample of young adult Australians, predominately of Anglo-Saxon ancestry. Although our findings may not generalize to other populations, given the higher rates of prescribed opioid use (67) and opioid-related mortality (68) in Anglo-Saxon ancestral populations, this is an ideal sample for preliminary investigation and one of the few with genetically informative NMUA data. With respect to genetic relatedness, we have detected no significant genetic differences between our sample, large population-based samples from the U.S., Western and Eastern Europe (69, 70).
Second, opioids refer to the entire family of natural, synthetic, and semi-synthetic forms. Our self-report assessment of lifetime opioid use included heroin (semi-synthetic), morphine (opium alkaloid), methadone (fully synthetic), and codeine (opium alkaloid). At the time of assessment, many over-the-counter analgesics in Australia contained codeine (71). Codeine was also included among the list of NMUA examples. This may have inflated the phenotypic association with lifetime self-reported illicit opioid use. However, if subjects were responding to lifetime codeine use in both items, then the prevalence and components of genetic and environmental variance ought to have been identical. Future research would benefit from more fine-grained assessment of illicit opioids, non-medical use of opioid prescription medications and non-medical use of over-the-counter medications. We note also that Australia has seen an increase in both codeine dependence and death-related to overdose from codeine-containing over-the-counter products (9). Consequently, as of February 2018 codeine-based drugs were rescheduled to be available only by prescription (71). Changes in the rescheduling of codeine-based medications are likely to impact the prevalence and individual differences in use, and potentially the relative contribution of genes and environment to its use and misuse.
Third, the NMUA assessment included non-steroidal or non-opioid analgesics. Their inclusion and any ensuing heterogeneity may have attenuated the association between lifetime non-medical use of opioid-based analgesics and other classes of SU and SUD. We note that the prevalence of NMUA in the NDSHS surveys between 2013 and 2016 did not change despite the removal of non-opioid OTC from the list of survey items (45). This is consistent with non-opioid analgesics not being known to be misused for cosmetic purposes or to induce or to enhance a drug experience or to enhance performance (7).
Fourth, non-medical use was defined as not taken in quantities or manner prescribed by a medical practitioner. This definition may have benefited with an expanded description that included ‘exceeding the recommendations on the label’ for the non-medical use of OTC medications.
Fifth, the NMUA assessment was lifetime. Psychiatric criteria for abuse and dependence were not assessed. The extent to which the genetic and environmental risks in this measure predict the risk of transitioning to chronic NMUA is unknown. Our work has previously shown that the genetic and environmental risks in licit and illicit substance use are partly, but not entirely related to corresponding diagnoses of substance misuse (46, 48, 72). Although very high genetic correlations between major classes of illicit and licit substance use disorders have been observed (55), it is unclear if the genetic risks in chronic non-medically prescribed or over-the-counter analgesics use will be highly correlated with those for CUD, AUD, and ND.
Conclusion
Lifetime non-medical or over-the-counter use of analgesics is moderately heritable and there is no evidence that aspects of the familial or shared environmental risks are etiologically significant. Twin modelling suggests that the genetic risks in this emergent class of substance use are mostly etiologically distinct. There was no genetic overlap with alcohol use disorder and very little overlap with cannabis use disorder. There was, however, a moderate degree of genetic overlap between NMUA and lifetime cannabis use, nicotine use and nicotine dependence.
Supplementary Material
Acknowledgments
We are very grateful to the twins for their generosity of time and willingness to participate in our studies. We thank Marlene Grace and Ann Eldridge for twin recruitment and data collection from 1992–2009, Lenore Sullivan, Lorelle Nunn, Mary Ferguson, Kerri McAloney, and Lucy Winkler for 19Up data collection, Daniel Park, David Smyth and Anthony Conciatore for IT support, and finally, Anjali Henders and Richard Parker for project management. The Australian NHMRC [APP10499110] and the United States NIH / NIDA [R00DA023549, R21DA038852]. EGB is supported by NIH [R21DA038852]. SEM is supported by an NHMRC fellowship [APP1103623]. IBH is supported by an NHMRC Fellowship [APP10499110].
References
- 1.Johnston LD O’Malley PM Bachman JG Psychotherapeutic, licit, and illicit use of drugs among adolescents. An epidemiological perspective, J Adolesc Health Care 1987: 8: 36–51. [DOI] [PubMed] [Google Scholar]
- 2.Johnston LD Prescription drug use by adolescents: what we are learning and what we still need to know, J Adolesc Health 2009: 45: 539–540. [DOI] [PubMed] [Google Scholar]
- 3.McCabe SE West BT Wechsler H Trends and college-level characteristics associated with the non-medical use of prescription drugs among US college students from 1993 to 2001, Addiction 2007: 102: 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report, 2011: 60: 1489. [Google Scholar]
- 5.National Center on Addiction and Substance Abuse (NCASA) at Columbia University. Wasting the best and the brightest: Substance abuse at America’s colleges and universities.; 2007. [Google Scholar]
- 6.Substance Abuse and Mental Health Services Administration C. f. B. H. S. a. Q. f. t. O. o. A. S. The DAWN Report: Highlights of the 2009 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits, Rockville, MD: Author.; 2010. [PubMed] [Google Scholar]
- 7.Australian Institute of Health and Welfare 2017. National Drug Strategy Household Survey 2016: detailed findings Drug Statistics series no. 31. Cat. no. PHE 214 Canberra: AIHW. [Google Scholar]
- 8.Ford JA Misuse of over-the-counter cough or cold medications among adolescents: prevalence and correlates in a national sample, J Adolesc Health 2009: 44: 505–507. [DOI] [PubMed] [Google Scholar]
- 9.Hopkins RE Dobbin M Pilgrim JL Unintentional mortality associated with paracetamol and codeine preparations, with and without doxylamine, in Australia, Forensic Sci Int 2018: 282: 122–126. [DOI] [PubMed] [Google Scholar]
- 10.Lankenau SE Teti M Silva K Jackson Bloom J Harocopos A Treese M Initiation into prescription opioid misuse amongst young injection drug users, Int J Drug Policy 2012: 23: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulozzi LJ Prescription drug overdoses: a review, Journal of safety research 2012: 43: 283–289. [DOI] [PubMed] [Google Scholar]
- 12.Roxburgh A Burns L Drummer OH Pilgrim J Farrell M Degenhardt L Trends in fentanyl prescriptions and fentanyl-related mortality in Australia, Drug Alcohol Rev 2013: 32: 269–275. [DOI] [PubMed] [Google Scholar]
- 13.Silva K Schrager SM Kecojevic A Lankenau SE Factors associated with history of non-fatal overdose among young nonmedical users of prescription drugs, Drug Alcohol Depend 2013: 128: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman D Oroszi G Ducci F The genetics of addictions: uncovering the genes, Nature reviews Genetics 2005: 6: 521–532. [DOI] [PubMed] [Google Scholar]
- 15.Bienvenu OJ Davydow DS Kendler KS Psychiatric ‘diseases’ versus behavioral disorders and degree of genetic influence, Psychol Med 2011: 41: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li MD The genetics of smoking related behavior: a brief review, Am J Med Sci 2003: 326: 168–173. [DOI] [PubMed] [Google Scholar]
- 17.Kendler KS Chen X Dick D Maes H Gillespie N Neale MC et al. Recent advances in the genetic epidemiology and molecular genetics of substance use disorders, Nat Neurosci 2012: 15: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kendler KS Jacobson KC Prescott CA Neale MC Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins, Am J Psychiatry 2003: 160: 687–495. [DOI] [PubMed] [Google Scholar]
- 19.Tsuang MT Lyons MJ Meyer JM Doyle T Eisen SA Goldberg J et al. Co-occurrence of abuse of different drugs in men: the role of drug-specific and shared vulnerabilities, Arch Gen Psychiatry 1998: 55: 967–972. [DOI] [PubMed] [Google Scholar]
- 20.Hicks BM Krueger RF Iacono WG McGue M Patrick CJ Family transmission and heritability of externalizing disorders: a twin-family study, Arch Gen Psychiatry 2004: 61: 922–928. [DOI] [PubMed] [Google Scholar]
- 21.Kendler KS Prescott CA Myers J Neale MC The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women, Arch Gen Psychiatry 2003: 60: 929–937. [DOI] [PubMed] [Google Scholar]
- 22.Kendler KS Myers J Prescott CA The specificity of genetic and environmental risk factors for symptoms of cannabis, cocaine, alcohol, caffeine and nicotine dependence, Ann Gen Psychiatry 2007: 64: 1313–1320. [DOI] [PubMed] [Google Scholar]
- 23.Grant JD Lynskey MT Madden PA Nelson EC Few LR Bucholz KK et al. The role of conduct disorder in the relationship between alcohol, nicotine and cannabis use disorders, Psychol Med 2015: 45: 3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright MJ Martin N The Brisbane Adolescent Twin Study: outline of study methods and research projects., The Australian Journal of Psychology 2004: 56: 65–78. [Google Scholar]
- 25.Gillespie NA Henders AK Davenport TA Hermens DF Wright MJ Martin NG et al. The Brisbane Longitudinal Twin Study: Pathways to Cannabis Use, Abuse, and Dependence project-current status, preliminary results, and future directions, Twin Res Hum Genet 2013: 16: 21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Couvy-Duchesne B O’Callaghan V Parker R Mills N Kirk KM Scott J et al. Nineteen and Up study (19Up): understanding pathways to mental health disorders in young Australian twins, BMJ Open 2018: 8: e018959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang LH Couvy-Duchesne B Medland SE Gillespie NA Hickie IB Parker R et al. The Genetic Relationship Between Psychological Distress, Somatic Distress, Affective Disorders, and Substance Use in Young Australian Adults: A Multivariate Twin Study, Twin Res Hum Genet 2018: 1–14. [DOI] [PubMed] [Google Scholar]
- 28.Heatherton TF Kozlowski LT Frecker RC Fagerstrom KO The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire, Br J Addict 1991: 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM–5) Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 30.Boker S Neale M Maes H Wilde M Spiegel M Brick T et al. OpenMx: An Open Source Extended Structural Equation Modeling Framework, Psychometrika 2011: 76: 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.R Development Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. 2018. [Google Scholar]
- 32.Neale MC Cardon LR Methodology for Genetic Studies of Twins and Families Dordrecht: Kluwer Academic Publishers; 1992. [Google Scholar]
- 33.Pearson K Mathematical contributions to the theory of evolution. vii.on the correlation of characters not quantitatively measurable.PhilosophicalTransactions of the Royal Society of London A: Mathematical, Physical andEngineering Sciences, 195(262–273):1–405., 1900. [Google Scholar]
- 34.Pearson K Pearson E On polychoric coefficients of correlation.Biometrika, 14:127–156., 1922. [Google Scholar]
- 35.Tallis GM The maximum likelihood estimation of correlation from contingency tables, Biometrics 1962: 18: 342–353. [Google Scholar]
- 36.Jöreskog K Sörbom D New features in PRELIS 2 Chicago: Scientific Software International; 1993. [Google Scholar]
- 37.Burnham KP Anderson DR Multimodel Inference: Understanding AIC and BIC in Model Selection, Sociological Methods Research 2004: 33: 261–304. [Google Scholar]
- 38.Kirkpatrick RM McGue M Iacono WG Replication of a gene-environment interaction Via Multimodel inference: additive-genetic variance in adolescents’ general cognitive ability increases with family-of-origin socioeconomic status, Behav Genet 2015: 45: 200–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neale MC Hunter MD Pritikin JN Zahery M Brick TR Kirkpatrick RM et al. OpenMx 2.0: Extended Structural Equation and Statistical Modeling, Psychometrika 2016: 81: 535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jinks JL Fulker DW Comparison of the biometrical genetical, MAVA, and classical approaches to the analysis of human behavior, Psychol Bull 1970: 73: 311–349. [DOI] [PubMed] [Google Scholar]
- 41.Verhulst B Prom-Wormley E Keller M Medland S Neale MC Type I Error Rates and Parameter Bias in Multivariate Behavioral Genetic Models, Behav Genet 2019: 49: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhulst B A Power Calculator for the Classical Twin Design, Behav Genet 2017: 47: 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kendler KS Aggen SH Prescott CA Crabbe J Neale MC Evidence for multiple genetic factors underlying the DSM-IV criteria for alcohol dependence, Mol Psychiatry 2012: 17: 1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Australian Institute of Health and Welfare 2014. National Drug Strategy Household Survey detailed report 2013 Drug statistics series no. 28. Cat. no. PHE 183 Canberra: AIHW. [Google Scholar]
- 45.Australian Institute of Health and Welfare 2017. National Drug Strategy Household Survey 2016: detailed findings Drug Statistics series no. 31. Cat. no. PHE 214 Canberra: AIHW. [Google Scholar]
- 46.Agrawal A Neale MC Jacobson KC Prescott CA Kendler KS Illicit drug use and abuse/dependence: modeling of two-stage variables using the CCC approach, Addict Behav 2005: 30: 1043–1048. [DOI] [PubMed] [Google Scholar]
- 47.Verweij KJ Vinkhuyzen AA Benyamin B Lynskey MT Quaye L Agrawal A et al. The genetic aetiology of cannabis use initiation: a meta-analysis of genome-wide association studies and a SNP-based heritability estimation, Addict Biol 2013: 18: 846–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gillespie NA Neale MC Kendler KS Pathways to cannabis abuse: a multi-stage model from cannabis availability, cannabis initiation and progression to abuse, Addiction 2009: 104: 430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minică CC Verweij KJH Mbarek H Bernard M Derringer J van Eijk KR et al. Genome-wide survival meta-analysis of age at first cannabis use, Addiction in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maes HH Prom-Wormley E Eaves LJ Rhee SH Hewitt JK Young S et al. A Genetic Epidemiological Mega Analysis of Smoking Initiation in Adolescents, Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 2017: 19: 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan PF Kendler KS The genetic epidemiology of smoking, Nicotine and Tobacco Research 1999: 1 Suppl 2: S51–57; Discussion S69–70. [DOI] [PubMed] [Google Scholar]
- 52.Kendler KS Karkowski LM Neale MC Prescott CA Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins, Arch Gen Psychiatry 2000: 57: 261–269. [DOI] [PubMed] [Google Scholar]
- 53.Silberg J Rutter M D’Onofrio B Eaves L Genetic and environmental risk factors in adolescent substance use, J Child Psychol Psychiatry 2003: 44: 664–676. [DOI] [PubMed] [Google Scholar]
- 54.Han C McGue MK Iacono WG Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses, Addiction 1999: 94: 981–993. [DOI] [PubMed] [Google Scholar]
- 55.Kendler KS Schmitt E Aggen SH Prescott CA Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood, Arch Gen Psychiatry 2008: 65: 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kendler KS Prescott CA Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders New York: The Guilford Press; 2006. [Google Scholar]
- 57.Benotsch EG Koester S Martin AM Cejka A Luckman D Jeffers AJ Intentional Misuse of Over-the-Counter Medications, Mental Health, and Polysubstance Use in Young Adults, J Community Health 2013. [DOI] [PubMed] [Google Scholar]
- 58.Benotsch EG Snipes DJ Martin AM Bull SS Sexting, substance use, and sexual risk behavior in young adults, J Adolesc Health 2013: 52: 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benotsch EG Zimmerman R Cathers L McNulty S Pierce J Heck T et al. Non-medical use of prescription drugs, polysubstance use, and mental health in transgender adults, Drug Alcohol Depend 2013: 132: 391–394. [DOI] [PubMed] [Google Scholar]
- 60.Benotsch EG Martin AM Koester S Mason MJ Jeffers AJ Snipes D Driving under the influence of prescription drugs used non-medically: associations in a young adult sample., Substance Abuse 2015: 36: 99–105. [DOI] [PubMed] [Google Scholar]
- 61.Zullig KJ Divin AL The association between non-medical prescription drug use, depressive symptoms, and suicidality among college students, Addict Behav 2012: 37: 890–899. [DOI] [PubMed] [Google Scholar]
- 62.Benotsch EG Koester S Luckman D Martin AM Cejka A Non-medical use of prescription drugs and sexual risk behavior in young adults, Addict Behav 2011: 36: 152–155. [DOI] [PubMed] [Google Scholar]
- 63.Benotsch EG Martin AM Koester S Cejka A Luckman D Nonmedical use of prescription drugs and HIV risk behavior in gay and bisexual men, Sex Transm Dis 2011: 38: 105–110. [DOI] [PubMed] [Google Scholar]
- 64.Snipes DJ Green BA Javier SJ Perrin PB Benotsch EG The use of alcohol mixed with energy drinks and experiences of sexual victimization among male and female college students, Addict Behav 2014: 39: 259–264. [DOI] [PubMed] [Google Scholar]
- 65.Snipes DJ Green BA Benotsch EG Perrin PB The Non-Medical Use of Prescription Drugs and Lifetime Experiences of Sexual Victimization Among College Men, Journal of interpersonal violence 2014. [DOI] [PubMed] [Google Scholar]
- 66.Gillespie NA Neale MC Prescott CA Aggen SH Kendler KS Factor and item-response analysis DSM-IV criteria for abuse of and dependence on cannabis, cocaine, hallucinogens, sedatives, stimulants and opioids, Addiction 2007: 102: 920–930. [DOI] [PubMed] [Google Scholar]
- 67.Mojtabai R National trends in long-term use of prescription opioids, Pharmacoepidemiology and drug safety 2018: 27: 526–534. [DOI] [PubMed] [Google Scholar]
- 68.Alexander MJ Kiang MV Barbieri M Trends in Black and White Opioid Mortality in the United States, 1979–2015, Epidemiology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pasman JA Verweij KJH Gerring Z Stringer S Sanchez-Roige S Treur JL et al. Genome-wide association analysis of lifetime cannabis use (N=184,765) identifies new risk loci, genetic overlap with mental health, and a causal influence of schizophrenia on cannabis use, Nat Neurosci accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walters RK Adams MJ Adkins AE Aliev F Bacanu SA Batzler A et al. Trans-ancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders., Nat Neurosci submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nielsen S MacDonald T Johnson JL Identifying and treating codeine dependence: a systematic review, Med J Aust 2018: 208: 451–461. [DOI] [PubMed] [Google Scholar]
- 72.Maes HH Sullivan PF Bulik CM Neale MC Prescott CA Eaves LJ et al. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use and nicotine dependence, Psychol Med 2004: 34: 1251–1261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.