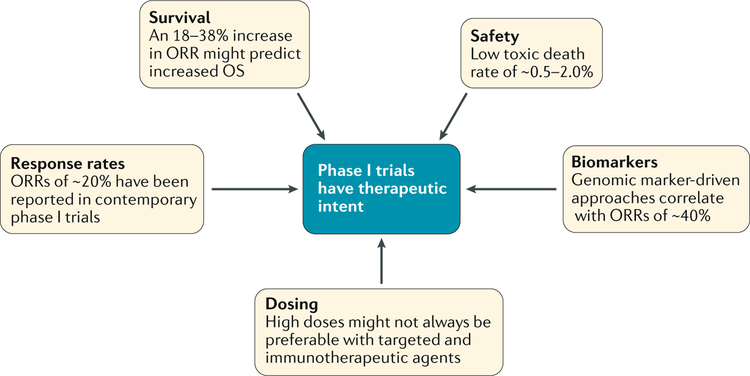
Fig. 1 |. Phase I trials as valid therapeutic options.
The design adaptations made in phase I trials in the past few years (in aspects including dosing63, biomarkers8,9,17,30,56,70, safety14, survival71 and responses18) have helped them to become valid therapeutic options. We propose that researchers can anticipate therapeutic responses in contemporary phase I trials and therefore these trials can be considered to have therapeutic intent.