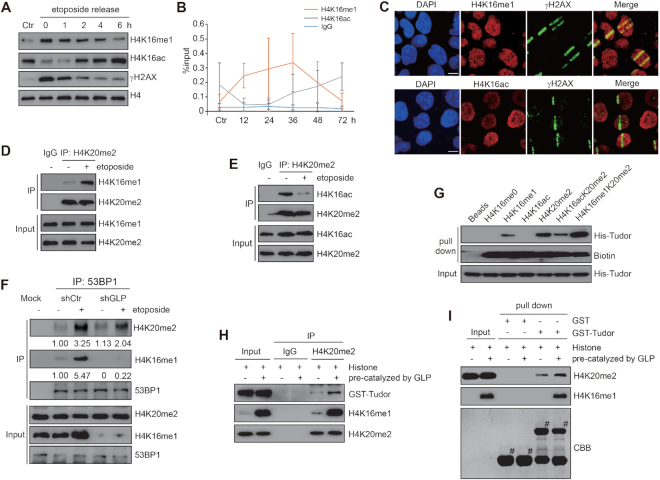
Figure 5.
H4K16me1 differs from H4K16ac in mediating the DDR and in regulating the H4K20me2–53BP1 interaction. (A) HeLa cells were treated with 40 μM etoposide for 30 min. The cells were then washed four times with PBS and re-cultured in fresh medium. Histones were extracted at each release time (indicated) and analyzed by western blotting. (B) DR-U2OS cells were transfected with I-SceI and then collected at each indicated time point. H4K16me1 and H4K16ac enrichment at I-SceI sites was detected by three independent ChIP assays. (C) HeLa cells were micro-irradiated, fixed and stained with the indicated antibodies. Images show an enrichment in H4K16me1 and a decrease in H4K16ac at the irradiated strips. Scale bars: 10 μm. (D and E) Mononucleosomes were extracted from HCT116 cells that had been exposed to 40 μM etoposide treatment for 30 min. H4K16me1 or H4K16ac levels were normalized to each sample. The mononucleosomes were immunoprecipitated with an anti-H4K20me2 antibody. The H4K16me1 (D) or H4K16ac (E) levels on H4K20me2-enriched mononucleosomes were analyzed by western blotting. (F) 53BP1 was immunoprecipitated from etoposide-treated or untreated shCtr or shGLP cells, and the interactive components were analyzed by western blotting. (G) A peptide pull-down assay was performed to detect the interactions between the 53BP1 Tudor domain and several H4 peptides. (H) Histones were extracted from HCT116 cells and incubated with GLP in methylation reaction buffer with or without SAM for 1 h. Subsequently, the differently modified histones were incubated with GST-Tudor for 8 h before anti-H4K20me2 immunoprecipitation and western blotting. (I) Histones were extracted from HCT116 cells and incubated with GLP in methylation reaction buffer with or without SAM for 1 h. Subsequently, the differently modified histones were incubated with beads-bound GST or GST-Tudor for 2 h at 4°C. The beads were washed three times before western blotting.