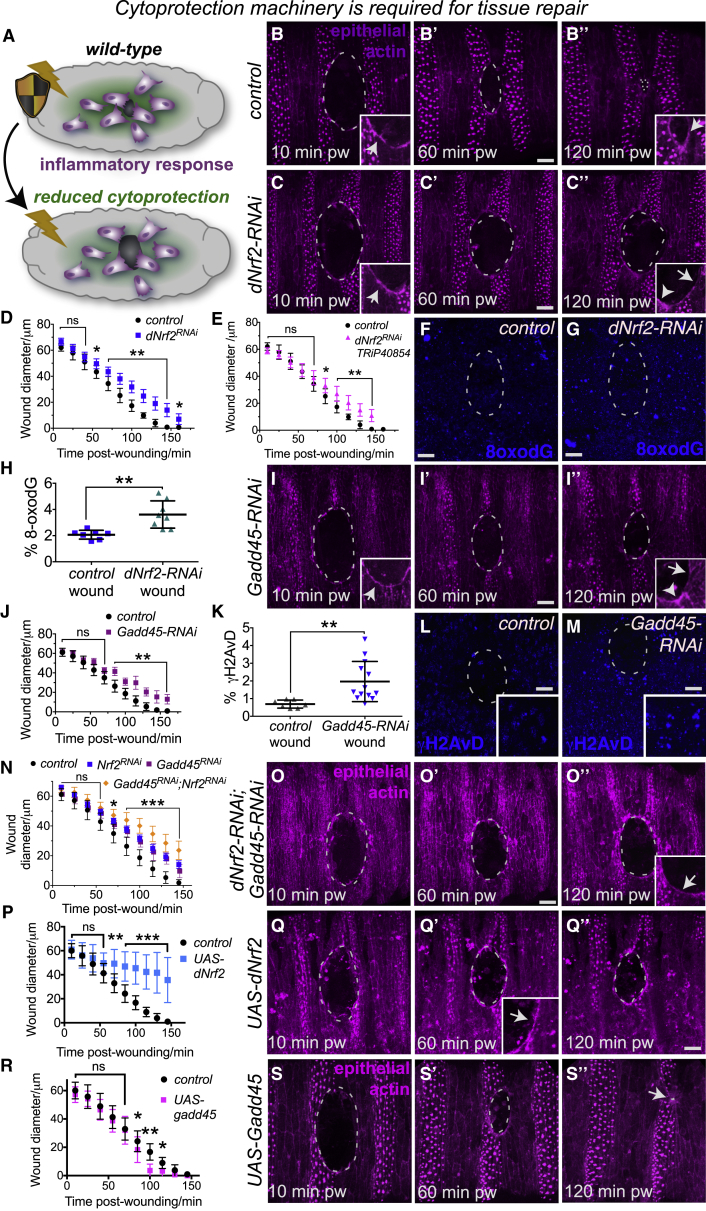
Figure 5.
Wound-Induced Cytoprotective Pathways Are Required for Efficient Wound Repair In Vivo
RNAi-mediated inhibition of either dNrf2 (A, schematic and B–H; independent dNrf2-RNAi lines were used in C and D, as in [22], and E, dNrf2-RNAi TRiP40854) or Gadd45 expression (I–M) caused a delay in epithelial wound repair (C–C″ and quantified in D and E for dNrf2-RNAi; I–I″ and quantified in J for Gadd45-RNAi, n > 20 for each condition) compared to controls (B–B″; epithelium labeled using Moesin-mCherry in B, C, and I) despite the initial assembly of actin cables at the wound margin (arrowheads, insets, B, C, and I). By 120 min post-wounding, the actin cable had been lost (insets, C″ and I″) compared to controls (inset, B″). Impaired wound healing was associated with elevated levels of oxidative DNA damage (quantified in H; blue, 8-oxo-dG in F and G) following dNrf2-RNAi and elevated γH2AvD punctae (quantified in K; blue, γH2AvD in L and M) following Gadd45-RNAi. Simultaneous knockdown of dNrf2 and Gadd45 caused more severe delays in wound repair (N and O; inset in O″ indicates loss of actin cable by 120 min post-wounding). Overexpression of dNrf2 significantly delayed wound repair (P and Q) despite assembly of robust actin cable (inset, Q′), but Gadd45 overexpression slightly accelerated wound closure (R and S). pw, post-wounding. % 8-oxo-dG and % γH2AvD refer to percent (%) of area measured that is positive for marker of interest after thresholding. Scale bars represent 10 μm in all panels. Data represented as mean ± SEM; ns, not significant, ∗p < 0.05 and ∗∗p < 0.01 via the Mann-Whitney Test (H and K) or multiple t tests followed by Holm-Sidak multiple comparisons correction (D, E, J, N, P, and R).