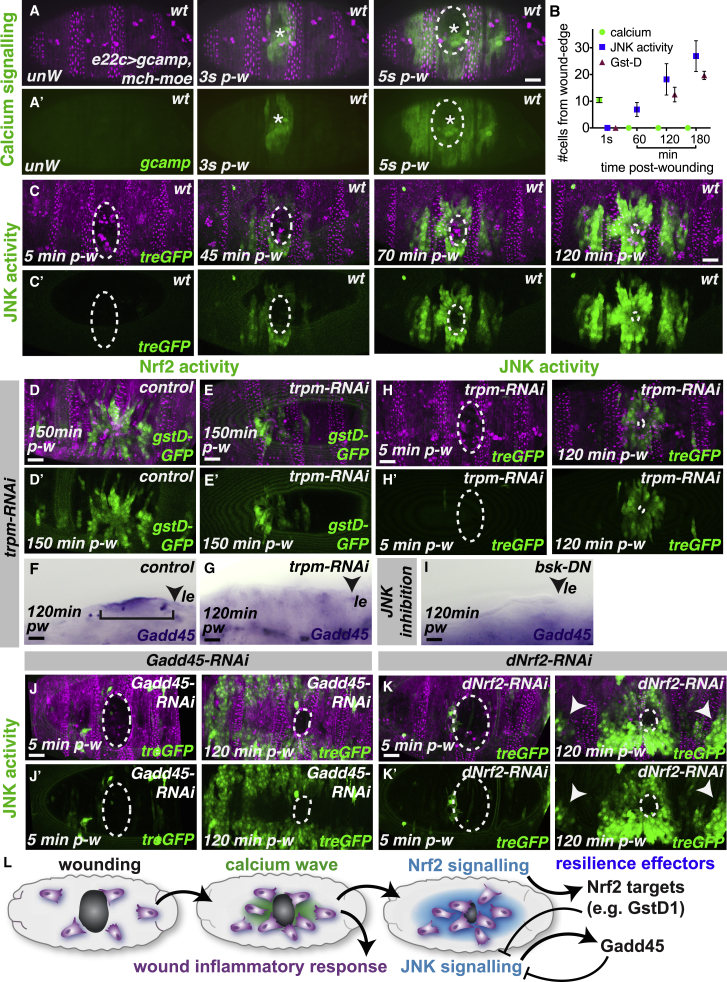
Figure 6.
A Complex Network of Wound-Induced Signaling Drives the Expression of Multiple Cytoprotective Factors within the Wounded Epithelium
(A–C) Epithelial (magenta Moesin-mCherry, A) wounding triggers a rapid wave of calcium (green, Gcamp3 reporter, A and A′) through the epithelium spreading up to 10 cell diameters from the wound edge (B). Wounding also activates JNK signaling (green, tre-GFP reporter, C) in the surrounding epithelium (magenta, Moesin-mCherry) but with slower dynamics (B and C).
(D–H) Inhibition of the wound-induced calcium wave using trpm-RNAi causes reduced expression of the Nrf2 target GstD1 (green, E compared to control in D), loss of Gadd45 expression (purple, in situ hybridization; F and G) in the wounded epithelium, and reduced activation of JNK signaling (green, H compared to controls in C).
(I–K) Wound-induced Gadd45 expression is also lost following inhibition of JNK signaling using bsk-dominant negative (arrowhead, I). Wound-induced JNK signaling (green, treGFP in J and K) is elevated in areas further from the wound site following RNAi-mediated inhibition of either Gadd45 (J) or dNrf2 (K) compared to controls (C).
(L) Schematic illustrates cascading and cross-regulatory network of wound-induced signaling that leads to the upregulation of multiple cytoprotective pathways within the wounded epithelium. Pw, post-wounding; le, leading edge.
Scale bars represent 15 μm in (A), (C), (D)–(F), (J), and (K) and 10 μm in (G)–(I).