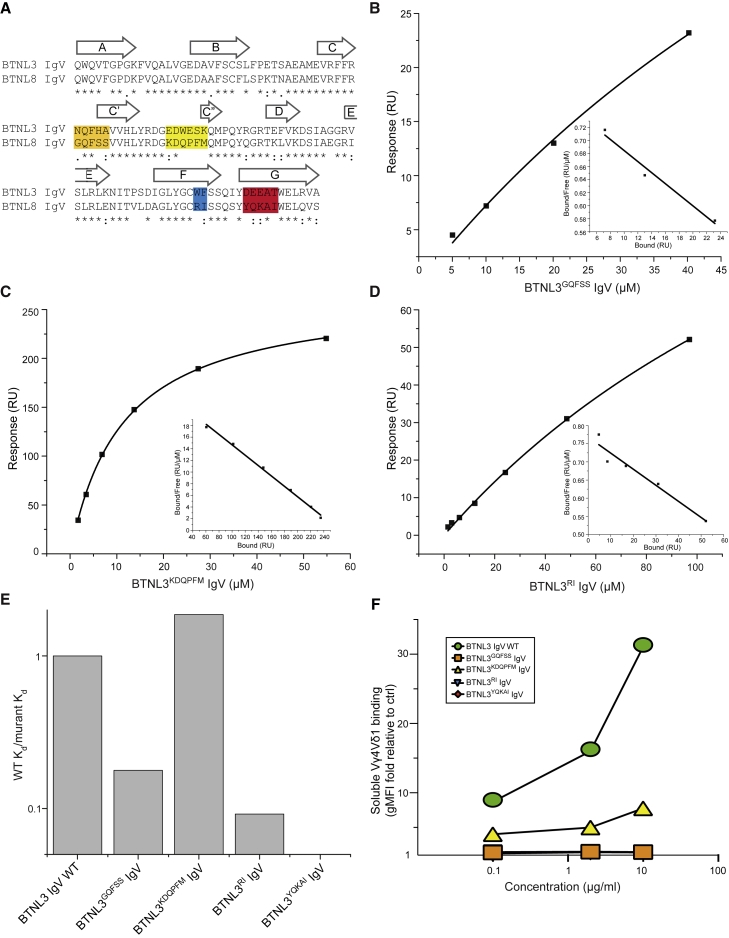
Figure 4.
Regions of BTNL3 IgV Involved in Vγ4 Binding
(A) Alignment of BTNL3 and BTNL8 IgV domains showing mutants generated.
(B–D) Equilibrium affinity analysis of the binding of (B) BTNL3GQFSS IgV (Kd = 117.6 μM), (C) BTNL3KDQPFM mutant (Kd = 11.2 μM), or (D) BTNL3RI mutant (Kd = 217.3 μM) to Vγ4 TCR.
(E) Binding affinity of indicated mutants of BTNL3 (mutant Kd) relative to WT LES TCR affinity (WT Kd) measured in the same experiment. Data are representative of two experiments.
(F) Flow cytometry analysis of 293T cells co-transduced with BTNL3 variants (as shown in the legend) and BTNL8 and stained with increasing concentrations (x axis) of soluble Vγ4Vδ1 and anti-His mAb. Results are presented as geometric mean fluorescence intensity (gMFI) of staining with the sTCR plus antibody to the His tag, normalized to the staining of 293T.EV cells under the same conditions.
See also Figure S4.