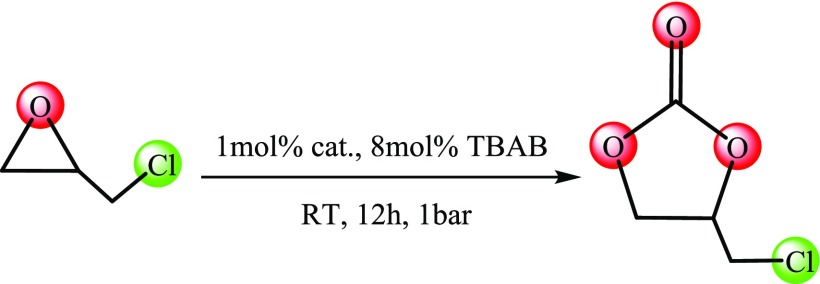
Table 1. Catalytic Investigation of the Cycloaddition of CO2 with Epichlorohydrina.
| entry | catalysts | conversion (%)b | TON | TOF (h–1) |
|---|---|---|---|---|
| 1 | Zr-Bipy-UiO-67 | 81 ± 1 | 81 | 6.71 |
| 2 | Hf-Bipy-UiO-67 | 82 ± 2 | 83 | 6.83 |
| 3 | 2,2′-Bipy-5,5′-dicarboxylic acid | 46 ± 2 | 46 | 3.85 |
| 4 | ZrCl4 | 39 ± 3 | 39 | 3.26 |
| 5 | HfCl4 | 38 ± 2 | 38 | 3.14 |
| 6 | Zr-Bipy-UiO-67(MnCl2) | 46 ± 3 | 46 | 3.81 |
| 7 | Zr-Bipy-UiO-67(CuCl2) | 44 ± 3 | 44 | 3.69 |
| 8 | Hf-Bipy-UiO-67(MnCl2) | 48 ± 2 | 48 | 3.98 |
| 9 | Hf-Bipy-UiO-67(CuCl2) | 49 ± 2 | 49 | 4.05 |
| 10 | Hf-Bipy-UiO-67(CoCl2) | 48 ± 1 | 48 | 4.02 |
| 11 | Hf-Bipy-UiO-67(ZnCl2) | 43 ± 4 | 43 | 3.57 |
| 12 | Hf-Bipy-UiO-67(Mn(NO3)2) | 66 ± 2 | 66 | 5.50 |
| 13 | Hf-Bipy-UiO-67(Cu(NO3)2) | 74 ± 2 | 74 | 6.17 |
| 14 | Hf-Bipy-UiO-67(Co(NO3)2) | 84 ± 1 | 84 | 7.01 |
| 15 | Hf-Bipy-UiO-67(Zn(NO3)2) | 86 ± 2 | 86 | 7.16 |
| 16 | Zr-Bipy-UiO-67(Mn(OAc)2) | 85 ± 1 | 85 | 7.08 |
| 17 | Zr-Bipy-UiO-67(Cu(OAc)2) | 52 ± 3 | 52 | 4.31 |
| 18 | Hf-Bipy-UiO-67(Mn(OAc)2) | >99 | 99 | 8.25 |
| 19 | Hf-Bipy-UiO-67(Cu(OAc)2) | 85 ± 2 | 85 | 7.08 |
| 20 | Hf-Bipy-UiO-67(Co(OAc)2) | 74 ± 1 | 74 | 6.19 |
| 21 | Hf-Bipy-UiO-67(Zn(OAc)2) | 55 ± 3 | 55 | 4.62 |
| 22 | Mn(OAc)2 | 43 ± 4 | 43 | 3.58 |
Reaction conditions: epichlorohydrin (4.3 mmol), catalysts (1 mol % based on open metal sites; for entries 13 and 17, 1.2 mol %), n-Bu4NBr (8 mol %), 1 bar CO2, room temperature (ca. 29 °C), 12 h. All reactions were run at least three times, and the reported data are averages.
Determined by liquid nuclear magnetic resonance (NMR) in CDCl3.