Abstract
In previous functional magnetic resonance imaging (fMRI) studies concerning romantic love, several brain regions including the caudate and putamen have consistently been found to be more responsive to beloved‐related than control stimuli. In those studies, infatuated individuals were typically instructed to passively view the stimuli or to think of the viewed person. In the current study, we examined how the instruction to attend to, or ignore the beloved modulates the response of these brain areas. Infatuated individuals performed an oddball task in which pictures of their beloved and friend served as targets and distractors. The dorsal striatum showed greater activation for the beloved than friend, but only when they were targets. The dorsal striatum actually tended to show less activation for the beloved than the friend when they were distractors. The longer the love and relationship duration, the smaller the response of the dorsal striatum to beloved‐distractor stimuli was. We interpret our findings in terms of reinforcement learning. By virtue of using a cognitive task with a full factorial design, we show that the dorsal striatum is not activated by beloved‐related information per se, but only by beloved‐related information that is attended. Hum Brain Mapp 35:503–512, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: love, motivation, emotion, reward, functional magnetic resonance imaging (fMRI)
INTRODUCTION
The lifetime prevalence of romantic love is extremely high, as romantic love strikes nearly 100% of the people at one or more times during their life. As a comparison, the lifetime prevalence of experiencing any mental disorder is “only” 46.4% (National Institute of Mental Health, US). In addition, when people fall in love, it affects their lives to a great extent: People are often willing to change their clothing, hobbies, friends, job, country or even their religion to be able to be together with their beloved [Aron and Aron, 1997]. It may be clear that the phenomenon of romantic love requires thorough scientific investigation. In the last decade, the scientific community has begun to study how the brain processes beloved‐related information differently from other information.
In previous functional magnetic resonance imaging (fMRI) studies, participants viewed pictures of the face of the beloved, while pictures of the faces of friends or acquaintances, erotic pictures, autobiographical pictures, pictures of landscapes, and/or verbal or arithmetic tasks typically served as control stimuli [Acevedo et al., 2012; Aron et al., 2005; Bartels and Zeki, 2000; Fisher et al., 2010; Kim et al., 2009; Stoessel et al., 2011; Xu et al., 2011; Younger et al., 2010; Zeki and Romaya, 2010]. Participants were either instructed to passively view the stimuli, or to think of the viewed person. Differences between these studies include the populations studied, such as individuals who had fallen in love only recently [Aron et al., 2005] or longer ago [Acevedo et al., 2012; Bartels and Zeki, 2000], individuals who had a beloved of the same or the opposite sex [Zeki and Romaya, 2010], individuals who were happily or unhappily in love [Fisher et al., 2010; Stoessel et al., 2011], and individuals from Western or Eastern cultures [Xu et al., 2011], as well as the inclusion of pain conditions [Younger et al., 2010]. In another fMRI study [Ortigue et al., 2007], the name of the beloved, the name of a friend, and a word that described a passion of the participants were presented as subliminal primes in a lexical decision task. Despite these differences between studies, a number of brain areas have shown increased activation in response to beloved‐related information compared to control information in multiple studies, including the caudate, putamen, ventral tegmental area (VTA), insula, anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and inferior frontal gyrus (IFG). It is not obvious, though, how to interpret the observed activation for beloved‐related stimuli, as there is no one‐to‐one mapping between brain regions and their functions. For example, the caudate and putamen form the dorsal striatum that has been implicated in the control of movement, cognitive functions, and reward‐related processing [Balleine et al., 2007].
There is no doubt that the above‐mentioned pioneering studies have yielded valuable knowledge. Now the time has come to take the scientific investigation of the neurocognition of romantic love to the next level by examining the processing of beloved‐related information during cognitive tasks, employing full factorial designs. The advantages of this approach are threefold. First, the use of well‐established tasks helps to interpret the brain response in terms of cognitive operations. Second, the resulting behavioral data may aid in interpreting the neural findings. Finally, the use of full factorial designs allows for both main and interaction effects to be studied [Friston et al., 1996], which sheds light on the context‐dependence of effects.
Beloved‐related stimuli obviously are highly emotional and motivationally relevant for the infatuated individual. It is well known that attention is increased for emotional over neutral stimuli [Compton, 2003]. In an event‐related potential (ERP) study, in which infatuated individuals passively viewed pictures of their beloved, a friend, and a beautiful stranger, the late positive potential (LPP/P3) was increased in response to the beloved. In line with the notion that the LPP/P3 reflects motivated attention [Schupp et al., 2006], it was concluded that romantic love is associated with increased attention for the beloved [Langeslag et al., 2007]. In a subsequent ERP study, in which infatuated individuals performed a full factorial oddball task with pictures of the beloved and friend as target and distractor stimuli, it was shown that this increased attention for the beloved occurred even when the participants were explicitly instructed to ignore the beloved and to pay attention to the friend instead [Langeslag et al., 2008]. In the current fMRI study, we used the same full factorial oddball task to examine how the instruction to attend to, or ignore the beloved modulates the BOLD response in brain regions that are sensitive to beloved‐related information.
Some previous fMRI studies concerning romantic love included an attentional task that did not involve any beloved‐related stimuli, as an implicit baseline or as an explicit control condition [Acevedo et al., 2012; Aron et al., 2005; Younger et al., 2010]. The results of those studies are hence controlled for the general effects of paying attention to the task at hand. But because these attentional tasks did not involve beloved‐related information, they did not elucidate how attention modulates the processing of beloved‐related information.
Although the oddball task has not been used in previous fMRI studies regarding romantic love, it has been used in previous fMRI studies regarding emotion or reward. In most of those studies the emotional or motivational salience of the stimuli was varied within one task condition (i.e., targets or distractors) only [Aleman and Swart, 2008; Dichter et al., 2010; Pannu Hayes et al., 2009; Robertson et al., 2007; Tricomi et al., 2004; Wang et al., 2005, 2008a, 2008b, c, 2009; Yamasaki et al., 2002]. In one study, the emotionality of the stimuli was varied within the target condition in one group, and between the target and distractor conditions in another group of participants [Fichtenholtz et al., 2004]. To our knowledge no previous emotion or reward fMRI studies have used an oddball task with a full factorial design, and the current study could thus also inform future emotion and reward studies.
MATERIAL AND METHODS
Participants
Twenty students of the Erasmus University Rotterdam participated in this study. Five of these participants had to be excluded due to scanner malfunction (n = 2), loss of stimulus timing files (n = 2), and excessive head movement (n = 1). Thus, the analyses were based on 15 participants (six men; mean age = 20.8 years, range = 18–25). Only participants who had been in love (in Dutch: “verliefd,” meaning “in love” or “infatuated”) for a relatively short period of time (<9 months) and who's beloved was of the opposite sex were included. Other inclusion criteria were normal or corrected‐to‐normal vision, no medical diagnosis, no use of medication known to affect the central nervous system, and no fMRI contraindications. All participants were right‐handed as determined by a hand preference questionnaire [Van Strien, 1992]. A questionnaire score of −10 reflects extreme left‐handedness while a score of 10 reflects extreme right‐handedness, and all participants had a score of 7 or higher. The study was approved by the ethics committee of the Erasmus Medical Center, Rotterdam and was conducted in accordance with the Declaration of Helsinki. Participants provided written informed consent prior to testing and were remunerated with course credit or 20 Euros.
Procedure
To start with, participants rated the extent to which they experienced romantic love with the beloved on a nine‐point Likert scale (1 = not in love at all, 9 = very much in love) (i.e., love intensity). Then, participants indicated for how many months (1 week = 0.25 month) they had been in love (i.e., love duration), and whether they were involved in a romantic relationship with their beloved. If they were, they indicated for how many months (1 week = 0.25 months) they had been involved in a romantic relationship with their beloved (i.e., relationship duration). The participants also completed the Dutch version of the passionate love scale (PLS) [Langeslag et al., 2007, 2008], which assesses the extent to which someone experiences passionate or romantic love [Hatfield, 1998], (minimum mean score = 1, maximum = 9; Chronbach's alpha = 0.93).
The stimuli were photographs of the faces of the participants' beloved and friends, and of a person that was unknown to them. A friend was defined as someone of the same sex as the beloved that the participant knew well, but for whom they had no romantic feelings. The photographs of the beloved and friend were supplied by the participants and were digitally adjusted to meet the requirements of the experiment (gray‐scale, showing face only). The male participants viewed only female faces, whereas the female participants viewed male faces (i.e., the beloved, friend and the unknown person were of the opposite sex). A separate sample of ten participants (5 men; mean age = 23.5 years, range = 18–28) who did not know people in the photographs and were unaware of the purpose of the study rated the attractiveness of the faces and the quality of the images on nine‐point Likert scales (1 = very unattractive/poor quality, 9 = very attractive/high quality). The mean attractiveness ratings were 4.9 (SD = 0.3) for the beloved, 4.6 (SD = 0.3) for the friends, and 4.7 (SD = 0.6) for the unknown persons. Attractiveness did not differ between conditions, F(2,18) = 1.8, ε = 0.77, P = 0.20. The mean image quality ratings were 5.4 (SD = 0.8) for the beloved, 5.3 (SD = 0.8) for the friends, and 5.0 (SD = 2.0) for the unknown persons. Image quality did not differ between conditions, F(2,18) < 1, ns.
In the oddball task, a trial consisted of the presentation of a fixation cross with jittered duration between 1,550 and 2,050 ms, followed by the presentation of a face for 250 ms, with no inter‐trial interval. The face of the unknown person always served as the standard stimulus, occurring in 80% of the trials. The faces of the beloved and friends each appeared pseudo randomly in 10% of the trials, and were separated by three to five standard stimuli.
Participants performed a few practice trials outside the scanner. Inside the scanner the participants completed four experimental runs, which consisted of 200 trials each. In two of the runs, the beloved was the target stimulus and the friend was the distractor. In the other two runs, the friend was the target and the beloved was the distractor, making the design full factorial. These run types alternated and run order was counterbalanced across participants. Participants were instructed to respond to the target stimulus by pressing a button with their right thumb. Accuracy was stressed over speed.
fMRI Recording and Signal Processing
The MRI scans were acquired on a 3T scanner (General Electric, Milwaukee, USA). Functional images were obtained using echo‐planar imaging sequences (EPI) in four runs of 210 volumes each. At the beginning of each run, five dummy volumes were acquired but not stored, to allow for T1‐equilibration effects. The T2*‐weighted images were acquired in 26 axial slices (thickness = 3.5 mm, interslice gap = 0.5 mm) with a repetition time (TR) of 2,000 ms, echo time (TE) of 30 ms, field of view (FOV) of 220 mm, voxels of 1.72 × 1.72 × 3.50 mm3, and sequential slice acquisition order from superior to inferior. For anatomical reference, a 3D high resolution inversion recovery fast spoiled gradient recalled echo T1‐weighted sequence was collected, covering the whole brain in 192 slices (thickness = 1.6 mm, overlap = 0.8 mm) with a FOV of 250 mm, and voxels of 0.49 × 0.49 × 0.80 mm3.
The data were preprocessed using Analysis of Functional NeuroImages (AFNI, http://afni.nimh.nih.gov/) [Cox, 1996]. Six‐parameter rigid‐body motion correction within and across runs was performed using Fourier interpolation [Cox and Jesmanowicz, 1999] such that all volumes were spatially registered to the volume acquired closest in time to the particular participant's anatomical volume. In three participants, the third and fourth runs were excluded from further analysis because of excessive head movement (i.e., more than 3 mm in the x, y, or z direction). To account for the timing offset between slices, slice timing correction was performed using Fourier interpolation such that all slices were realigned to the first slice of the associated volume. To normalize the functional data to Talairach space [Talairach and Tournoux, 1988], initially each subject's high‐resolution anatomical volume was spatially registered to the so‐called TT_N27 template (in Talairach space) using a 12‐parameter affine transformation; the same transformation was then applied to the functional data. All volumes were spatially smoothed using a Gaussian filter with a full‐width at half maximum of 6 mm. Finally, the signal intensity of each voxel was scaled to a mean of 100.
Statistical Analyses
Hit (i.e., proportion button presses for target stimuli) and false alarms rates (i.e., proportion button presses for distractor stimuli) were computed using the correction recommended by Snodgrass and Corwin 1988. These data, as well as the median response times (RTs) for hits, were analyzed with repeated measures analyses of variance (rmANOVA) with the factor love (beloved, friend) using a significance level of 5%. Only significant effects are mentioned.
Single‐subject fMRI analyses were performed using AFNI. There were four event types of interest, namely beloved‐target, beloved‐distractor, friend‐target, friend‐distractor, and a nuisance event type that included all incorrect trials. Constant, linear, and quadratic terms were included for each run separately (as regressors of no interest) to model the baseline and drifts of the MR signal. Correct standard trials were not modeled explicitly and constituted the implicit baseline in the model. Therefore, all parameter estimates reported in this study are with respect to correct standard trials as a baseline. Because we did not want to assume the shape of the BOLD response, responses were estimated from stimulus onset until 14 s after stimulus onset via a deconvolution model using cubic spline basis functions. Because the estimated BOLD response averaged across subjects peaked around 4–6 s after stimulus onset, see Figure 1, the BOLD response averaged over the third and fourth time points (i.e., 4–6 s) after stimulus onset was fed into the group‐level analyses.
Figure 1.
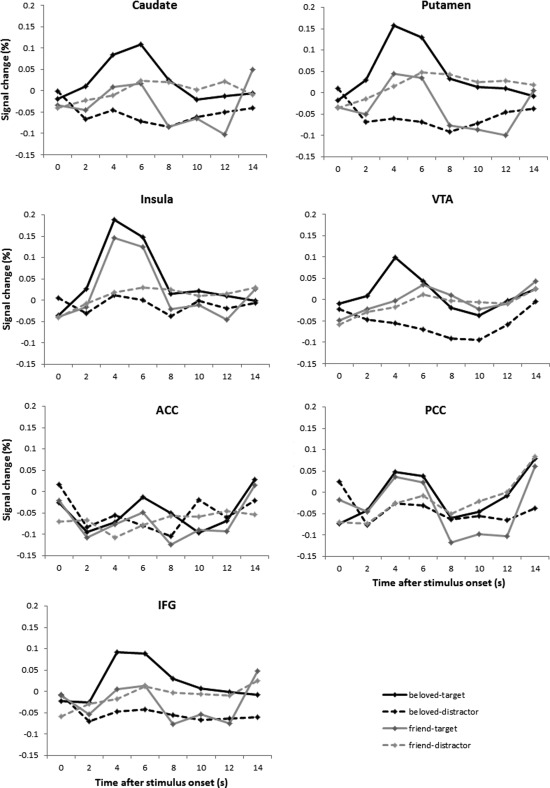
Estimated BOLD responses to the four event types of interest compared to the baseline (i.e., correct standard trials) in the seven bilateral ROIs.
We focused our group‐level analysis on a set of regions‐of‐interest (ROIs) that have been found to be activated more to beloved‐related than control stimuli in previous studies [Acevedo et al., 2012; Aron et al., 2005; Bartels and Zeki, 2000; Fisher et al., 2010; Kim et al., 2009; Stoessel et al., 2011; Xu et al., 2011; Zeki and Romaya, 2010], as discussed in the introduction. Six bilateral anatomical ROIs were selected using the Talairach‐Tournoux Atlas in AFNI: caudate, putamen, insula, ACC, PCC, and IFG, see Figure 2. An ROI that included the VTA was defined using the ventral diencephalon region of the DD_Desai_MPM atlas in AFNI, see Figure 2. For each ROI and condition, the voxel‐wise betas were averaged to get one beta per condition per ROI. For each ROI, the beta values were entered into a 2x2 rmANOVA with the factors love (beloved, friend) and task (target, distractor), which was performed with SPSS 18. Because preliminary analyses including the factor hemisphere (left, right) did not reveal any significant interactions involving both the factors love and hemisphere, all Ps > 0.10, activity was collapsed across hemispheres. In the Supporting Information, we present analyses for the left and right ROIs separately. A significance level of 5% was selected and significant love × task interactions were clarified by paired‐samples t tests.
Figure 2.
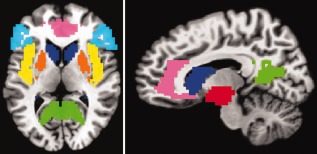
The seven bilateral ROIs at z = 7 (left panel) and x = −10 (right panel). Dark blue = caudate, orange = putamen, red = VTA, yellow = insula, pink = ACC, green = PCC, light blue = IFG. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
For completeness, we also report the results of a whole‐brain voxel‐wise (excluding white matter voxels) 2x2 rmANOVA with the factors love (beloved, friend) and task (target, distractor). A combined uncorrected threshold of P < 0.001 at the voxel level and a minimum cluster size of 25 voxels were used, which resulted in a corrected significance level of 5% as determined by the 3dClustSim program in AFNI.
RESULTS
Love Characteristics
The mean duration of the participants' romantic love was 5.1 months (SD = 1.6, range = 2.5–8.0). All but one female participant were involved in a romantic relationship with their beloved and the mean duration of these relationships was 3.9 months (SD = 1.7, range = 1.0–6.5). The participants' mean self‐reported love intensity was 7.9 (SD = 1.0, range = 6–9) and their mean PLS score was 7.2 (SD = 0.9, range = 5.1–8.8).
Behavioral Data
Mean accuracy on the oddball task was very high, namely 98% (range = 92–100), which indicates that participants were alert and adhered to the instructions. Hit rates did not differ significantly between the beloved‐target (M = 0.98, SD = 0.02) and the friend‐target (M = 0.96, SD = 0.07) conditions, F(1,14) = 1.4, P = 0.25. False alarm rates, however, were higher in the beloved‐distractor (M = 0.05, SD = 0.04) than in the friend‐distractor (M = 0.03, SD = 0.02) condition, F(1,14) = 7.5, P = 0.016. Also, RTs tended to be shorter for beloved‐target (Mdn = 528 ms, SD = 64) than for friend‐target (Mdn = 561 ms, SD = 80) stimuli, F(1,14) = 3.6, P = 0.079.
ROI Analyses
See Figure 1 for the BOLD responses at 0–14 s after stimulus onset to the four stimulus types in each ROI, and see Table 1 for the results of the 2x2 rmANOVA on the average BOLD response at 4–6 s after stimulus onset. None of the ROIs displayed a significant main effect of love. In the putamen, the love × task interaction was significant. The average responses at 4–6 s after stimulus onset in the putamen are displayed in Figure 3. The BOLD response was significantly larger for the beloved‐target than for the friend‐target stimuli, t(14) = 3.0, P = 0.010. The response was smaller for beloved‐distractor than friend‐distractor stimuli, but this difference did not reach significance, t(14) = −1.9, P = 0.076. Furthermore, the response was significantly larger for beloved‐target than for beloved‐distractor stimuli, t(14) = 4.7, P < 0.001, while the responses for friend‐target and friend‐distractor stimuli did not differ, t(14) = 0.2, P = 0.87.
Table 1.
Overview of the results of the 2 × 2 rmANOVAs on the average BOLD signal at 4–6 s after stimulus onset in the seven bilateral ROIs
| ROI | Main effect of love | Main effect of task | Love × task interaction |
|---|---|---|---|
| Caudate | F(1,14) < 1, ns | F(1,14) = 43.3, P < 0.001a | F(1,14) = 3.7, P = 0.073 |
| Putamen | F(1,14) < 1, ns | F(1,14) = 40.8, P < 0.001a | F(1,14) = 6.6, P = 0.022a |
| VTA | F(1,14) < 1, ns | F(1,14) = 9.4, P < 0.008a | F(1,14) = 2.3, P = 0.16 |
| Insula | F(1,14) < 1, ns | F(1,14) = 76.6, P < 0.001a | F(1,14) < 1, ns |
| ACC | F(1,14) = 3.3, P = 0.089 | F(1,14) = 3.1, P = 0.10 | F(1,14) < 1, ns |
| PCC | F(1,14) < 1, ns | F(1,14) = 3.8, P = 0.071 | F(1,14) < 1, ns |
| IFG | F(1,14) < 1, ns | F(1,14) = 24.4, P < 0.001a | F(1,14) = 2.1, P = 0.17 |
significant.
Figure 3.
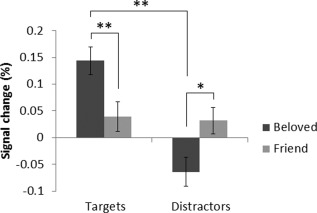
Average percent signal change in putamen at 4–6 s after stimulus onset for each of the four stimuli types compared to the baseline (i.e., standard correct trials). The BOLD response was significantly larger for beloved‐target than for friend‐target and beloved‐distractor stimuli, which is indicated by the double asterisks. The BOLD response tended to be smaller for beloved‐distractor than for friend‐distractor stimuli, which is indicated by the single asterisk.
The caudate, VTA, and IFG showed the same activation pattern as the putamen, see Figure 1. The love x task interaction was not significant in the VTA or IFG though, and only approached significance in the caudate. When subdividing the caudate into its head, body, and tail using the Talairach‐Tournoux Atlas in AFNI, trends toward significant love × task interactions were observed in the head, F(1,14) = 3.5, P = 0.081, and body, F(1,14) = 3.7, P = 0.076, but not in the tail, F(1,14) < 1, ns.
The main effect of task was highly significant in the caudate, putamen, VTA, insula, and IFG, with the responses being larger for target than distractor stimuli, see Figure 1.
Correlational Analyses
Next, we examined whether the observed differences between conditions in putamen response were associated with the love intensity, the mean PLS score, and the duration of the romantic feelings. The variables love duration and relationship duration were highly correlated, r(12) = 0.86, P < 0.001. To increase statistical power, we used the average of the two in these correlational analyses. Pearson correlation coefficients between the love characteristics (i.e., love intensity, mean PLS score, and the average between love and relationship duration) and the beta values for each of the four contrasts were computed. Neither love intensity, nor mean PLS score correlated significantly with the beta values of any of the contrasts, −0.29 < all rs(13) < 0.41, all Ps > 0.13. The average between love and relationship duration correlated significantly with the difference in putamen response between beloved‐distractor and friend‐distractor, r(13) = −0.72, P = 0.003, between beloved‐target and beloved‐distractor, r(13) = 0.59, P = 0.020, and between friend‐target and friend‐distractor, r(13) = −0.66, P = 0.008. To clarify these findings, Pearson correlation coefficients were computed between the average between love and relationship duration and the putamen response in each of the conditions. The average between love and relationship duration was negatively correlated with the putamen response to beloved‐distractor stimuli, r(13) = −0.59, P = 0.022, and positively correlated with the response to friend‐distractor stimuli, r(13) = 0.69, P = 0.005, but not significantly correlated with beloved‐target stimuli, r(13) = 0.29, P = 0.29, or friend‐target stimuli, r(13) = −0.34, P = 0.21. To summarize, the longer the love and relationship duration, the smaller the putamen response to beloved‐distractor stimuli and the larger the putamen response to friend‐distractor stimuli.
Whole‐Brain Analyses
In the whole‐brain analysis, no significant clusters appeared for the main effect of love, or for the love × task interaction. See Table 2 for the eight significant clusters for the main effect of task. The largest of these clusters extended anterior and posterior of the left central sulcus. All of these clusters showed a larger response for target than distractor stimuli.
Table 2.
Location and Talairach coordinates for the peak voxel of significant clusters for the main effect of task (P < 0.05 corrected) at 4–6 s after stimulus onset in the whole‐brain analysis
| Location | x | y | z | F(1,14) |
|---|---|---|---|---|
| Left postcentral gyrus | 49 | 31 | 50 | 190.9 |
| Right culmen | −10 | 52 | −15 | 107.3 |
| Left middle occipital gyrus | 49 | 64 | −3 | 84.5 |
| Right middle frontal gyrus | −43 | −40 | 11 | 80.1 |
| Left culmen | 22 | 52 | −18 | 28.1 |
| Left middle frontal gyrus | 28 | −37 | 26 | 46.1 |
| Right precuneus | −16 | 67 | 38 | 26.0 |
| Left cuneus | 13 | 76 | 35 | 24.7 |
All of these clusters showed a greater BOLD response for target than distractor stimuli.
DISCUSSION
The goal of this study was to examine how the instruction to attend to, or ignore the beloved modulates the BOLD response in brain regions that are sensitive to beloved‐related information. To this end, infatuated participants performed an oddball task in which pictures of the faces of their beloved and friend served as target and distractor stimuli alternately.
We focused our analysis on brain areas that have consistently been shown to be more responsive to beloved‐related information than control information in previous fMRI studies: the caudate, putamen, VTA, insula, ACC, PCC, and IFG [Acevedo et al., 2012; Aron et al., 2005; Bartels and Zeki, 2000; Fisher et al., 2010; Kim et al., 2009; Ortigue et al., 2007; Stoessel et al., 2011; Xu et al., 2011; Zeki and Romaya, 2010]. In the current study, none of these brain regions was differentially activated in response to the beloved compared to friend stimuli when collapsing across target and distractor conditions. Only when the beloved and friend stimuli were targets, the dorsal striatum showed greater activation for beloved compared to friend stimuli. In many previous studies, increased dorsal striatum activation for beloved compared to control stimuli has been observed [Acevedo et al., 2012; Aron et al., 2005; Bartels and Zeki, 2000; Fisher et al., 2010; Ortigue et al., 2007; Zeki and Romaya, 2010]. In most of those studies, no cognitive task was associated with the beloved and control stimuli, which were presented in a blocked design with relatively long stimulus durations. We show that increased dorsal striatum activation for beloved‐related information can also be observed during an event‐related cognitive task with very short stimulus durations.
Although dorsal striatum activation was increased for beloved‐related information when it was attended, the responses of the dorsal striatum for beloved‐distractor and friend‐distractor stimuli did not differ significantly. If anything, the dorsal striatum was actually less responsive to beloved compared to friend stimuli when they were distractors. By virtue of the full factorial design, the current study extends existing knowledge from previous studies by showing that the dorsal striatum is not activated by beloved‐related information per se, but only by beloved‐related information that is attended.
Because in some previous studies the activation by beloved‐related information was unilateral rather than bilateral [Acevedo et al., 2012; Aron et al., 2005], we presented additional analyses separately for the left and right ROIs as Supporting Information. The only difference with the bilateral analyses was that the love × task interaction additionally reached significance in the left caudate, particularly in the left caudate body. In many previous studies, beloved‐related information has activated bilateral putamen [Acevedo et al., 2012; Bartels and Zeki, 2000; Fisher et al., 2010] and bilateral caudate [Bartels and Zeki, 2000; Zeki and Romaya, 2010], and in those studies in which unilateral caudate activation was observed it was mostly right‐lateralized [Acevedo et al., 2012; Aron et al., 2005; Ortigue et al., 2007] as opposed to left‐lateralized in the current study. It is as of yet unclear what causes the lateralization of the caudate response to (attended) beloved‐related information, and this intriguing issue deserves further investigation.
The dorsal striatum is highly heterogeneous in terms of connectivity and functionality, which gives it the ultimate position to influence goal‐directed behavior by integrating information regarding cognition, motivation, and motor control [Balleine et al., 2007; Delgado, 2007]. In a previous study, caudate activation was specifically associated with the amount of obsessive thinking about the beloved [Acevedo et al., 2012]. It has also been suggested that the caudate plays a role in the attentional aspects of romantic love [Aron et al., 2005], and the current results support this suggestion. Generally, the increased dorsal striatum response to attended beloved‐related stimuli in the current and previous studies fits the notion that romantic love is a motivational state that is associated with approach behavior [Acevedo et al., 2012; Aron et al., 2005; Langeslag, 2006].
While the ventral striatum has been associated with reward anticipation, the dorsal striatum is more involved in using reward outcomes to guide future cognitions and actions [Knutson et al., 2008], including social behavior, with the goal to maximize the reward obtained [Delgado, 2007]. The dorsal striatum has specifically been implicated in reinforcement learning [Balleine et al., 2007; Bornstein and Daw, 2011], which entails that previously rewarded behavior is likely to be repeated while previously punished behavior is not. It has been shown that gambling actions that were in accordance with reinforcement learning activated the dorsal striatum more than gambling actions that were in conflict with reinforcement learning [Jessup and O'Doherty, 2011]. The currently observed increased dorsal striatum activation for the beloved stimuli that were targets resonates with the notion that attending/responding to one's beloved is associated with positive reinforcement more than attending/responding to one's friend, or ignoring one's beloved is. Interestingly, the BOLD response in the dorsal striatum to beloved stimuli that were distractors was smaller in participants who had fallen in love with their beloved longer ago and who had been in a romantic relationship with their beloved for a longer time. The association between the BOLD response in the dorsal striatum to friend stimuli that were distractors showed the opposite pattern, with larger responses in participants that had fallen in love and had become involved in a relationship longer ago. The association between the response of the dorsal striatum and the duration of the romantic love supports our interpretation of the dorsal striatum response as reflecting prior reinforcement of social actions.
This reinforcement learning would lead infatuated individuals to preferentially pay attention to their beloved, perhaps at the cost of paying attention to their friend. Correspondingly, the participants in the current study tended to respond faster to their beloved than to their friend. Also, the increased false alarm rate for the beloved indicates that infatuated individuals find it harder to ignore their beloved than to ignore their friend. These behavioral findings corroborate the ERP‐based conclusion that romantic love is associated with increased attention for the beloved [Langeslag et al., 2007, 2008].
The current study has a couple of limitations. First, because the dorsal striatum plays a role in motor functions [Delgado, 2007], our findings could be confounded by the motor responses. We feel confident though that the responses of the dorsal striatum do not reflect the button presses, because no difference was observed between the dorsal striatum responses for friend‐target and friend‐distractor stimuli, even though the former were associated with button presses and the latter were not. Second, given the known gender differences in romantic love [Geary et al., 2004; Harris, 2002; Langeslag et al., 2012; Meston and Buss, 2007], it would have been interesting to examine gender differences in the current study. Unfortunately, the inclusion of only six men in our data analyses rendered the examination of gender differences unfeasible. Third, the mask that was used to extract the signal from the VTA contained the entire ventral diencephalon instead of just the VTA. Although the VTA is a small region, the limited spatial resolution of fMRI combined with spatial resampling and spatial smoothing during preprocessing justifies the use of a larger mask. Nevertheless, the use of this larger mask may have limited the power to observe a significant main effect of love or a love x task interaction. Future studies could focus on the VTA by scanning only a part of the brain with higher resolution.
The oddball task used in the current study was adapted from a previous ERP study [Langeslag et al., 2008]. In that study, it was observed that the LPP/P3 was larger for target compared to distractor stimuli, which reflected task‐related attention. This main effect of task in the ERP was very robust, and so was main effect of task in the current study. Many brain areas showed an increased response to targets compared to distractors, including a large cluster extending anterior and posterior of the central sulcus of the left hemisphere, which obviously reflects the required button presses for the target stimuli. In the prior ERP study, the LPP/P3 also showed a main effect of love, being larger for beloved compared to friend stimuli, reflecting love‐related attention. The target‐related LPP/P3 for visual stimuli is thought to emerge from a widespread network including the parietal, temporal, and cingulate cortices [Linden, 2005], and it has been shown that the emotional modulation of the LPP/P3 originates in the prefrontal, occipital, parietal, and temporal cortices [Moratti et al., 2011; Sabatinelli et al., 2007]. In the current study, no main effects of love occurred in the ROI or whole‐brain analyses. The former may be due to the fact that involvement in the LPP/P3 was not an ROI selection criterion, and the latter may be due to reduced power in the whole‐brain analysis. In the previous ERP study, the LPP/P3 did not display a love × task interaction. Signals from certain neural populations, such as those oriented tangentially to the scalp, with closed field configurations, and located in subcortical areas, are not reflected in the ERP signal [Luck, 2005]. The currently observed love × task interaction in the subcortical dorsal striatum could thus not have been observed in the ERP. It may be clear that ERP and fMRI studies yield different types of information, and that both modalities are valuable when trying to elucidate the neurocognition of romantic love.
It is known that reward and motivation modulate attention [Franken et al., 2003; Namkoong et al., 2004; Peck et al., 2009], but less is known about how attention modulates reward processing. The current study raises the question to what extent the current findings are generalizable to other types of reward processing. On the one hand, it may be that attention modulates the dorsal striatum response to any kind of reward stimuli. This hypothesis may be tested using a similar full factorial oddball task with reward stimuli other than pictures of the beloved, such as drug‐related or sexual stimuli, or monetary gains or losses. On the other hand, the current correlational analyses suggest that it is (social) reinforcement learning instead of attention per se that may be the key factor in modulating the dorsal striatum response to reward stimuli. More research is needed to clarify the roles of attention and reinforcement learning on the processing of beloved‐related information, and on reward processing in general. It will remain important to disentangle the effects of reward and attention on neural processing, as they are often confounded [Baldassi and Simoncini, 2011; Maunsell, 2004].
To conclude, we present here the first fMRI study in which the neurocognition of romantic love is studied using a cognitive task with a full factorial design. We show that the dorsal striatum responds preferentially to beloved‐related information only when it is attended. We explain this finding by interpreting the dorsal striatum response as reflecting previous reinforcement of social actions. This study greatly advances our understanding of the role of the dorsal striatum in romantic love. More research is needed to further elucidate the roles of the multiple brain regions that are activated by beloved‐related information.
Supporting information
Supporting Information
Acknowledgments
The authors thank Gianna Baak for her help with the data collection and Srikanth Padmala for his valuable suggestions regarding the data analysis and the manuscript.
REFERENCES
- Acevedo BP, Aron A, Fisher HE, Brown LL (2012): Neural correlates of long‐term intense romantic love. Social Cogn Affect Neurosci 7:145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman A, Swart M (2008): Sex differences in neural activation to facial expressions denoting contempt and disgust. Plos ONE 3:E3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek D, Strong G, Li H, Brown LL (2005): Reward, motivation and emotion systems associated with early‐stage intense romantic love. J Neurophysiol 94:327–337. [DOI] [PubMed] [Google Scholar]
- Aron EN, Aron A (1997): Extremities of love: The sudden sacrifice of career, family, dignity. J Social Clin Psychol 16:200–212. [Google Scholar]
- Baldassi S, Simoncini C (2011): Reward sharpens orientation coding independently of attention. Front Neurosci 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O (2007): The role of the dorsal striatum in reward and decision‐making. J Neurosci 27:8161–8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S (2000): The neural basis of romantic love. Neuroreport 11:3829–3834. [DOI] [PubMed] [Google Scholar]
- Bornstein AM, Daw ND (2011): Multiplicity of control in the basal ganglia: Computational roles of striatal subregions. Curr Opin Neurobiol 21:374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton RJ (2003): The interface between emotion and attention: A review of evidence from psychology and neuroscience. Behav Cogn Neurosci Rev 2:115–129. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996): AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A (1999): Real‐time 3D image registration for functional MRI. Magn Reson Med 42:1014–1018. [DOI] [PubMed] [Google Scholar]
- Delgado MR (2007): Reward‐related responses in the human striatum. Anna N Y Acad Sci 1104:70–88. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Bellion C, Casp M, Belger A (2010): Impaired modulation of attention and emotion in schizofrenia. Schizophr Bull 36:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtenholtz HM, Dean HL, Dillon DG, Yamasaki H, Mccarthy G, Labar KS (2004): Emotion‐attention network interactions during a visual oddball task. Cogn Brain Res 20:67–80. [DOI] [PubMed] [Google Scholar]
- Fisher HE, Brown LL, Aron A, Strong G, Mashek D (2010): Reward, addiction, and emotion regulation systems associated with rejection in love. J Neurophysiol 104:51–60. [DOI] [PubMed] [Google Scholar]
- Franken IHA, Stam CJ, Hendriks VM, Van Den Brink W (2003): Neurophysiological evidence for abnormal cognitive processing of drug cues in heroin dependence. Psychopharmacology 170:205–212. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ, Fletcher P, Moore C, Frackowiak RS, Dolan RJ (1996): The trouble with cognitive subtraction. Neuroimage 4:97–104. [DOI] [PubMed] [Google Scholar]
- Geary DC, Vigil J, Byrd‐Craven J (2004): Evolution of human mate choice. J Sex Res 41:27–42. [DOI] [PubMed] [Google Scholar]
- Harris CR (2002): Sexual and romantic jealousy in heterosexual and homosexual adults. Psychol Sci 13:7–12. [DOI] [PubMed] [Google Scholar]
- Hatfield E (1998): The passionate love scale In: Davis CM, Yarber WL, Bauserman R, Schreer G, Davis SL, editors.Handbook of Sexuality‐Related Measures.Thousand Oaks:Sage Publications; pp449–451. [Google Scholar]
- Jessup RK, O'Doherty JP (2011): Human dorsal striatal activity during choice discriminates reinforcement learning behavior from the gambler's fallacy. J Neurosci 31:6296–6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Kim S, Jeong J, Lee KU, Ahn KJ, Chung YA, Hong KY, Chae JH (2009): Temporal changes in functional magnetic resonance imaging activation of heterosexual couples for visual stimuli of loved partners. Psychiatry Investig 6:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Delgado MR, Phillips PEM (2008): Representation of subjective value in the striatum In: Glimcher PW, Camerer CF, Fehr E, Poldrack RA, editors.Neuroeconomics: Decision Making and the Brain.London:Elsevier Academic Press; pp389–406. [Google Scholar]
- Langeslag SJE (2006): Liefde is een motivatie, niet een emotie: Een neurobiologische benadering (Love is a motivational state, not an emotion: A neurobiological approach). De Psycholoog 41:260–265. [Google Scholar]
- Langeslag SJE, Jansma BM, Franken IHA, Van Strien JW (2007): Event‐related potential responses to love‐related facial stimuli. Biol Psychol 76:109–115. [DOI] [PubMed] [Google Scholar]
- Langeslag SJE, Franken IHA, Van Strien JW (2008): Dissociating love‐related attention from task‐related attention: An event‐related potential oddball study. Neurosci Lett 431:236–240. [DOI] [PubMed] [Google Scholar]
- Langeslag SJE, Van Der Veen FM, Fekkes D (2012): Blood levels of serotonin are differentially affected by romantic love in men and women. J Psychophysiol 26:92–98. [Google Scholar]
- Linden DEJ (2005): The P300: Where in the brain is it produced and what does it tell us? Neuroscientist 11:563–576. [DOI] [PubMed] [Google Scholar]
- Luck SJ (2005):An Introduction to the Event‐Related Potential Technique.Cambridge:The MIT Press. [Google Scholar]
- Maunsell JHR (2004): Neuronal representations of cognitive state: Reward or attention? Trends Cogn Sci 8:261–265. [DOI] [PubMed] [Google Scholar]
- Meston CM, Buss DM (2007): Why humans have sex. Arch Sex Behav 36:477–507. [DOI] [PubMed] [Google Scholar]
- Moratti S, Saugar C, Strange BA (2011): Prefrontal‐occipitoparietal coupling underlies late latency human neuronal responses to emotion. J Neurosci 23:17278–17286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkoong K, Lee E, Lee CH, Lee BO, An SK (2004): Increased P3 amplitudes induced by alcohol‐related pictures in patients with alcohol dependence. Alcohol Clin Exp Res 28:1317–1323. [DOI] [PubMed] [Google Scholar]
- Ortigue S, Bianchi‐Demicheli F, Hamilton Afdc, Grafton ST (2007): The neural basis of love as a subliminal prime: An event‐related functional magnetic resonance imaging study. J Cogn Neurosci 19:1218–1230. [DOI] [PubMed] [Google Scholar]
- Pannu Hayes J, Labar KS, Petty CM, Mccarthy G, Morey RA (2009): Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatr Res 172:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck CJ, Jangraw DC, Suzuki M, Efem R, Gottlieb J (2009): Reward modulates attention independently of action value in posterior parietal cortex. J Neurosci 29:11182–11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B, Wang L, Diaz MT, Aiello M, Gersing K, Beyer J, Mukundan S, Mccarthy G, Doraiswamy PM (2007): Effect of bupropion extended release on negative emotion processing in major depressive disorder: A pilot functional magnetic resonance imaging study. J Clin Psychiatry 68:261–267. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, Bradley MM (2007): Emotional perception: Correlation of functional MRI and event‐related potentials. Cereb Cortex 17:1085–1091. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Flaish T, Stockburger J, Junghöfer M (2006): Emotion and attention: Event‐related brain potential studies In: Anders S, Ende G, Junghöfer M, Kissler J, Wildgruber D, editors.Progress in Brain Research: Understanding Emotions.Amsterdam, The Netherlands:Elsevier Science; pp31–51. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J (1988): Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen 117:34–50. [DOI] [PubMed] [Google Scholar]
- Stoessel C, Stiller J, Bleich S, Boensch D, Doerfler A, Garcia M, Richter‐Schmidinger T, Kornhuber J, Forster C (2011): Differences and similarities on neuronal activities of people being happily and unhappily in love: A functional magnetic resonance imaging study. Neuropsychobiology 64:52–60. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988):Co‐planar Stereotaxis Atlas of the Human Brain.New York:Thieme Medical. [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA (2004): Modulation of caudate activity by action contingency. Neuron 41:281–292. [DOI] [PubMed] [Google Scholar]
- Van Strien JW (1992): Classificatie van links‐ en rechtshandige proefpersonen (classification of left‐handed and right‐handed test subjects). Nederlands Tijdschrift Voor De Psychol 47:88–92. [Google Scholar]
- Wang L, Mccarthy G, Song AW, Labar KS (2005): Amygdala activation to sad pictures during high‐field (4 Tesla) functional magnetic resonance imaging. Emotion 5:12–22. [DOI] [PubMed] [Google Scholar]
- Wang L, Huettel S, De Bellis MD (2008a): Neural substrates for processing task‐irrelevant sad images in adolescents. Dev Sci 11:23–32. [DOI] [PubMed] [Google Scholar]
- Wang L, Krishnan KR, Steffens DC, Potter GG, Dolcos F, Mccarthy G (2008b): Depressive state‐ and disease‐related alterations in neural responses to affective and executive challenges in geriatric depression. Am J Psychiatry 165:863–871. [DOI] [PubMed] [Google Scholar]
- Wang L, Labar KS, Smoski M, Rosenthal MZ, Dolcos F, Lynch TR, Krishnan RR, Mccarthy G (2008c): Prefrontal mechanisms for executive control over emotional distraction are altered in major depression. Psychiatry Res 163:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mullette‐Gillman OA, Gadde KM, Kuhn CM, Mccarthy G, Huettel SA (2009): The effect of acute tryptophan depletion on emotional distraction and subsequent memory. Social Cogn Affect Neurosci 4:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Aron A, Brown L, Cao G, Feng T, Weng X (2011): Reward and motivation systems: A brain mapping study of early‐stage intense romantic love in Chinese participants. Hum Brain Mapp 32:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Labar KS, Mccarthy G (2002): Dissociable prefrontal brain systems for attention and emotion. Proc Natl Acad Sci USA 99:11447–11451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger J, Aron A, Parke S, Chatterjee N, Mackey S (2010): Viewing pictures of a romantic partner reduces experimental pain: Involvement of neural reward systems. Plos ONE 5:E13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Romaya JP (2010): The brain reaction to viewing faces of opposite‐and same‐sex romantic partners. Plos ONE 5:E15802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


