Figure 3.
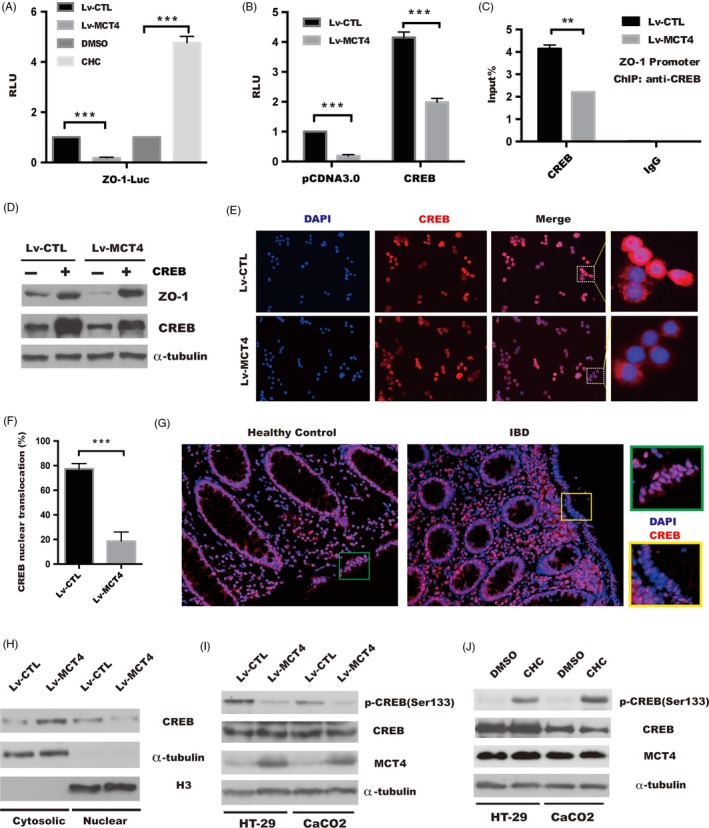
MCT4 inhibits phosphorylation of CREB(Ser133) and attenuates CREB‐mediated ZO‐1 transactivity. A, Luciferase activity in cells which was transiently transfected with the pGL4.17‐ZO‐1 promoter plasmid together with renilia plasmid in stable HT‐29 cells or in HT‐29 cell treated as indicated. The Renilla luciferase activities were used as internal controls. One‐way analysis of variance (ANOVA) and Dunnett's multiple comparison test, ***P < .001, n = 3; error bars indicate s.d. B, pGL4.17‐ZO‐1 promoter plasmid together with pCDNA 3.0 or CREB plasmid was transfected into indicated stable HT‐29 cells, and the Renilla luciferase activities were used as internal controls. Two‐way analysis of variance (ANOVA) and Dunnett's multiple comparison test, ***P < .001, n = 3; error bars indicate s.d. C, ChIP analysis of binding of CREB protein to ZO‐1 gene promoter in CaCO2 cells treated as indicated. Student's t test, **P < .05, n = 3; error bars indicated s.d. D, Western blotting was performed to analyse ZO‐1 expression in indicated CaCO2 cells transfected with CREB or control plasmid, α‐tubulin served as internal control. E, Immunofluorescence was employed to detect CREB nuclear translocation in indicated HT‐29 cells, scale bar: 200 μm. F, Percentage of cells that exhibited CREB nuclear translocation. Data represent the means ± s.d. of three independent experiments and were analysed by t test, ***P < .05. G, CREB nuclear location in intestinal tissue from patients with IBD and healthy control was performed by IF, scale bar: 100 μm. H, Levels of nuclear (Nuclear) and cytosolic (Cytosolic) CREB were determined by immunoblotting analysis. α‐tubulin and H3 were used as internal controls for the cytosolic and nuclear fractions, respectively. I‐J, Whole cell lysates were separated by SDS‐PAGE and assayed with the antibodies against the indicated proteins, inducing pCREB(Ser133), CREB and MCT4, in indicated group of HT‐29 and CaCO2 cells, and α‐tubulin was determined to ensure equal loading
