Abstract
In depression, patients suffer from emotional and cognitive deficits, among others in semantic processing. If these semantic deficits are cognitive or interact with emotional dysfunctions, is still an open question. The aim of the current study was to investigate the influence of emotional valence on the neural correlates of semantic priming in major depression. In a lexical decision task, positive, negative, and neutral word pairs were presented during fMRI measurement. Nineteen inpatients and 19 demographically matched controls were recruited. Behaviorally, positive and neutral valence induced a priming effect whereas negative valence induced no effect (controls) or even inhibition (slower RT for related stimuli) in patients. At the neural level, the semantic relation effect revealed similar neural activation in right middle frontal regions for patients and controls. Group differences emerged in the right fusiform gyrus and the ACC. Activity associated with positive valence differed at the DLPFC and amygdala and for negative valence at putamen and cerebellum. The activation of amygdala and DLPFC correlated negatively with the severity of depression. To conclude, semantic processing deficits in depression are modulated by emotional valence of the stimulus on the behavioral as well as on neural level in right‐lateralized prefrontal areas and the amygdala. The results highlighted an influence of depression severity on emotion information processing as the severity of symptoms correlated negatively with neural responses to positively and negatively valenced information. Hence, the dysfunctional emotion processing may further enhance the cognitive deficits in depression. Hum Brain Mapp 35:471–482, 2014. © 2012 Wiley Periodicals, Inc.
Keywords: semantic priming, emotional valence, DLPFC, amygdale, right hemisphere, major depression
INTRODUCTION
Cognitive and affective symptoms are well documented in depression. For example, studies found an influence of emotional content of words on memory recognition in depression [Dietrich et al., 2000], deficits in cognitive control related to reduced emotion‐regulation strategies [Joormann and Gotlib, 2010], and functional imaging studies showed that emotional load in cognitive tasks led to abnormal modulation in the ventral and dorsal part of superior and medial frontal gyrus and ventral parts of the inferior and middle frontal gyri [Northoff et al., 2004]. However, there are still open questions regarding the mutual influence of emotion on cognition and particularly on the neural basis of semantic processing in depression.
One approach to investigate the emotional influence on semantic processing is semantic priming. Advantages of this approach are that no additional executive functions or nonsemantic processes, strategies, or expectancies are involved if automatic processing is addressed [stimulus onset asynchrony [SOA] below 400 ms; Neely, 1991]. In a classical task, a prime (e.g., car) is presented, followed by a target that can be a real word (e.g., garage) or a pseudoword (e.g., fubber). The participant is asked to decide if the target is a real word by button press (lexical decision). In general, related prime‐target pairs (car–garage) lead to a faster reaction time (RT) than unrelated word pairs (car–bottle) indicating facilitated word recognition. The differential change in RT is called the semantic priming effect. A possible cause of the semantic priming effect is an automatic spread of activation between related concepts within the semantic network [Neely, 1991]. Here, concepts are represented as nodes that are interconnected via associative pathways. If a prime is presented, its node is activated and the spread of activation will “preactivate” corresponding (related) nodes.
Bower's 1981 “affect priming theory” and “affect infusion model [Bower and Forgas, 2001] extended the model of spreading activation to the domain of emotion. It is assumed that each emotion is represented by a specific node. The “arousal of an […] emotion spreads activation throughout a network of associations surrounding that […] emotion” [Bower and Forgas, 2001]. Hence, mood‐congruent information is processed faster than mood‐incongruent information. In depression, there might be a (pre‐)activation of “negative nodes” and associations between emotionally congruent, negative nodes, may be stronger [Bower, 1981; Ingram, 1984] as patients are not able to interrupt or suppress the automatic activation [Bradley et al., 1995] leading to an enhanced reactivity to negative information [=negative potentiation hypothesis; Cohen et al., 2005].
Behavioral studies investigating the interaction of semantic priming and the “emotion” network in depression revealed controversial results, i.e., depressed patients showed slower RT than healthy controls in response to positive and negative words [Matthews and Southall, 1991] or faster processing only for negative information [Klumpp and Deldin, 2010]. Hence, the presence of emotional (negative and in some studies positive) stimuli interferes with performance, impairs attention and influences the RT of verbal processing in depression [Power et al., 1996]. Based on these results, the open questions are (a) if semantic processing per se is affected in depression or rather specifically semantic emotion processing and (b) what neurocognitive connection of the semantic and the “emotion”‐network exists.
Regarding the neural correlates of semantic priming in healthy subjects, activation was found in left temporoparietal (concept retrieval and integration), lateral and medial prefrontal (semantic processing and executive functions), and parietal areas [episodic and visuospatial memory; Binder et al., 2009]. In addition, on a neural level semantic priming induces either response suppression or response enhancement. Suppression was found within lateral superior temporal and inferior frontal regions [e.g., Rissman et al., 2003; Wible et al., 2006] and refers to reduced activity for unrelated > related stimuli reflecting the consequence of priming, i.e., the ease to retrieve primed targets. In contrast, response enhancement was found within left middle temporal and bilateral prefrontal regions and is normally defined as an increase in the hemodynamic response to priming relative to unpriming stimuli [Kotz et al., 2002; Raposo et al., 2006]. It is assumed that these signal changes are a correlate of cognitive processes that involve primed words and index the spread of activation itself [Henson, 2003; Marinkovic et al., 2003].
The influence of emotion on semantic priming on the neural level in healthy subjects revealed activation in the anterior medial frontal gyrus, superior and inferior frontal gyrus and the posterior cingulate cortex [e.g., Kuchinke et al., 2005; Sass et al., 2012]. In depression, Canli et al. 2004 presented emotional and neutral words and asked patients and controls to make a lexical decision, i.e., subjects had to decide if letter strings were real words or pseudowords. They found reduced activity for positive stimuli (happy) in emotion‐associated regions (amygdala) and enhanced activation for negative stimuli (sad) in the inferior parietal cortex reflecting attentional processing of emotional stimuli [Davidson et al., 2002]. These data suggest that depression might lead to decreased neural activation in response to positive word stimuli in areas related to language and affect while processing of negative words is associated with enhanced parietal activation mirroring attention‐related processes. However, this study makes no claims about a possible interaction of semantic priming and affective priming as suggested by Bower and Forgas 2001.
Hence, the aim of the current study was to map the influence of emotional valence on semantic priming in depression on a neural level. The novelty of our design compared with existing studies includes the usage of a semantic priming task with implicit emotional influence, and the correlation of the neural correlates with the current mood state and severity of depression. Behaviorally, we hypothesized differences for negative valence between controls and patients with facilitated negative information processing in depression [Bower, 1981]. On a neural level, we compared the semantic relation effect (independent of emotional valence), the valence effect and the influence of current mood (assessed by the Positive and Negative Affective Schedule [Watson et al., 1988] and self‐reported severity of depression [assessed by Beck Depression Inventory (BDI‐II); Beck et al., 1996]. We suggested neural responses within frontotemporal areas for the semantic relation [Sass et al., 2012] whereas the valence effect should lead to group differences in regions related to emotion regulation and attention (dorsolateral prefrontal cortex [DLPFC]) as well as emotion processing [anterior cingulate cortex (ACC), amygdala; Canli et al., 2004; Sass et al., 2012]. These group differences might depend on the direction of activation (response suppression vs. response enhancement) rather than on different areas of the brain. According to the negative potentiation hypothesis patients should show a higher neural priming effect in correlation with severity of depression or current mood. For this correlation, we decided to restrict the analysis to three regions of interest that show consistent functional and structural changes in depression: amygdala, DLPFC and ACC.
MATERIALS AND METHODS
Participants
Nineteen inpatients meeting DSM‐IV criteria for major depressive disorder (clinical diagnoses confirmed by the SCID interviews) were recruited from the Department of Psychiatry, Psychotherapy and Psychosomatics, RWTH Aachen University, Germany. All patients were receiving SSRIs antidepressant. Nineteen healthy subjects matched for age, gender, and education served as control group. All subjects were native speakers of German, had normal or corrected‐to‐normal vision and were right handed. Exclusion criteria for all subjects were past or present medical or neurological diseases or trauma which could affect the nervous system, comorbid mental disorders, and a history of substance abuse (at least 4 weeks before scanning). Demographic, neuropsychological, and psychopathological characteristics are listed in Table 1. The study was approved by the local ethics committee and all participants gave informed consent to participate in the study.
Table 1.
Characteristics of subjects
| Patients | Controls | Differences P value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Mean age in years | 31.2 | 7.1 | 28.2 | 2.7 | 0.10a |
| Gender | 10 f/9 m | 10 f/9 m | 1.0b | ||
| Education in years | 13 | 3 | 14 | 2 | 0.26 |
| Verbal IQ | 102.05 | 8.9 | 108.88 | 12.9 | 0.09a |
| TMT‐A | 24 | 11 | 19 | 8 | 0.13a |
| TMT‐B | 41 | 17 | 32 | 10 | 0.10a |
| Digit span forward | 8.4 | 1.9 | 9.3 | 1.1 | 0.09a |
| Digit span backward | 7.7 | 1.9 | 7.4 | 2.3 | 0.62a |
| BDI‐II | 23.26 | 9.24 | |||
| HAMD | 14.53 | 4.21 | |||
| PANAS positive | 2.99 | 0.60 | 3.44 | 0.40 | 0.01a |
| PANAS negative | 1.81 | 0.58 | 1.34 | 0.24 | 0.01a |
Patients were clinically assessed using two different scales Beck Depression Inventory [BDI‐II]; Hamilton scale [HAMD]. By applying the trial making test [TMT‐A and B], attention and task switching were tested. The premorbid IQ was determined by using a German verbal crystallized intelligence estimation Mehrfachwahlwortschatztest [Verbal IQ]. Working memory storage capacity was tested by the digit‐span test. Additionally, the Positive and Negative Affect Schedule PANAS assessed current mood.
SD = standard deviation.
Independent sample t‐test.
Pearson χ2 test.
Stimuli and Design
During the fMRI experiment, the trials started with an attention cue “+” (500 ms) followed by the prime (200 ms). A visually presented string of letters (target) followed the prime (1,000 ms). Subjects were asked to make a lexical decision, i.e., decide if the target was a real word or not (pseudoword) by pressing one of two buttons with the left hand (real word—index finger or pseudoword—middle finger). A hash sign appeared as intertrial‐interval (small jitter: M = 2 s; long jitter: M = 4 s; see Fig. 1). The visual stimuli were shown in Arial font at 24 pts. Seven experimental conditions were used: positive (heaven‐angel), negative (torture–force), and neutral related (map–geography); positive (sun–terror), negative (grave–luck), and neutral unrelated (concert–water) and nonwords (bus–reinsa). The stimulus set used was developed and validated as described in greater detail earlier [for validation of the stimulus set see Sass et al., 2012 and Supporting Information].
Figure 1.
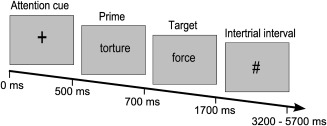
Schematic display of the semantic priming task.
fMRI Procedure
A rapid event‐related design was used to present the stimuli. The idea behind the design was that the presentation of trials from the same condition in a sequence leads to a better sampling of the hemodynamic response function (HRF) curve and hence, to a better signal. Therefore, small blocks of two to three related or unrelated word pairs were constructed in a pseudorandomized fashion. Within these blocks, the intertrial interval (ITI) was shorter than the duration of the HRF generated from previous trials. Between the blocks, the ITI was longer in order to allow BOLD responses to return to baseline [see Sass et al., 2012, 2009, for further description of fMRI procedure]. The fMRI experiment started with a digitalized version of the PANAS. The stimuli display was controlled using Presentation (Version 11.0 software package Neurobehavioral Systems; available at: http://www.neurobs.com/) and MR‐compatible video goggles (VisuaStim XGA, Resonance Technology, Inc.; available at: http://www.mri-video.com/).
fMRI Data Acquisition
Scanning was performed on a 3T scanner (Siemens Medical Systems, Erlangen, Germany) using standard gradients and a circular polarized phase array head coil. Stimuli were presented in a rapid erfMRI design fashion, with 30 (all related and unrelated conditions) or 90 stimuli per condition (nonword) and a trial length of approximately 5 s. The scans covered the whole brain, including five initial dummy scans parallel to the AC/PC line with the following parameters: number of slices (NS), 34; slice thickness (ST), 3.5 mm; interslice gap (IG), 0.30 mm; matrix size (MS), 64 × 64; field of view (FOV), 240 × 240 mm; echo time (TE), 30 ms; repetition time (TR), 2 s.
Behavioral Data Analysis
For each group (controls, patients) reaction time was measured from the target onset until the participant made a correct response. Data were entered into a repeated‐measures ANOVA with VALENCE (positive, negative, neutral) and RELATION (related, unrelated) as within‐subject factors and GROUP (depression, control) as between‐subject factor. Paired t‐tests were conducted to decompose significant interactions. To assess the relationship between current mood and priming effects, a Pearson product‐moment correlation was calculated for (a) positive PANAS values and size of priming effects and (b) negative PANAS values and size of priming effects within each group.
fMRI Data Analysis
Image processing and statistical analyses were performed using statistical parametric mapping software (SPM5; available at: http://www.fil.ion.ucl.ac.uk) implemented in MATLAB 7.0 (Mathworks Inc., Sherborn, MA). For preprocessing, the first five volumes were discarded for all protocols due to initial recording burst. FMRI images of each participant were realigned to the first functional image in order to correct for head movement. The resliced volumes were normalized to the standard stereotaxic anatomical MNI‐space by using the transformation matrix calculated from the mean image of each participant and the EPI‐template. For the normalization the default SPM5 settings with 16 nonlinear iterations and the standard EPI‐template of SPM5 were used. Each normalized image was then smoothed using an 8‐mm Gaussian kernel to accommodate differences in anatomy between participants. Low frequencies were removed using a high‐pass filter with a cut‐off period of 128 s. The first‐order autocorrelations of the data were estimated and corrected for. After preprocessing, statistical analyses for each individual participant were conducted. The delta‐functions of the seven experimental conditions with the onsets of stimuli were convolved with the canonical HRF and used as regressors in subject‐specific general linear models (GLM). Parameter estimates of the HRF regressor were calculated from the least mean squares fit of the model to the time series. At group level, parameter estimates of the six experimental conditions were entered into a repeated‐measures ANOVA (flexible factorial design). This mixed two factorial design consisted of the within‐subject factor condition (valence: positive, negative, neutral; relation: related, unrelated) and the between‐subjects factor GROUP (patients and controls).
The first contrast of interest at the second level was the semantic relation effect (independent of valence) across the two groups, i.e., the comparison of the semantic priming effects for each condition. The priming effects refer to response enhancement (related over unrelated) and response suppression (unrelated over related). Even if both signal changes represent different processes, both reflect the neural processes that underlie semantic priming. Hence, we decided to investigate the signal changes by F‐contrasts which reveal any priming effect regardless of directionality and emotionality, e.g., to contrast related versus unrelated implies an F‐test of “related > unrelated” and “unrelated > related”. The emotionality implies that priming effects were not averaged across emotions but rather assessed independently. The first contrast of interest was the semantic relation effect:
Conjunction: depression (positive [related vs. unrelated] ∩ negative [related vs. unrelated] ∩ neutral [related vs. unrelated]) ∩ controls (positive [related vs. unrelated] ∩ negative [related vs. unrelated] ∩ neutral [related vs. unrelated]). In order to investigate group differences, the following F‐contrast was conducted:
Depression (positive [related vs. unrelated] + negative [related vs. unrelated] + neutral [related vs. unrelated]) vs. controls (positive [related vs. unrelated] + negative [related vs. unrelated] + neutral [related vs. unrelated]).
The results were inclusively masked with (a) a conjunction of priming effects for the patients to examine the contribution of depression and (b) a conjunction of priming effects of controls to examine the contribution of healthy controls. The rationale behind the masking was that this masking procedure yields only those regions for the interaction “group by semantic priming effect” which also show a priming effect for the depressed patients or healthy controls, respectively. It should be emphasized that ensuring one of the groups showed a priming effect does not imply the absence of a priming effect in the respective other group, e.g., a contribution by depression does not imply that the interaction is driven by the patients alone (for further evidence see contrast estimate plots). However, as response suppression and enhancement represent are established neural priming effects that might represent distinct neural processes, we investigated our contrasts in detail using t‐contrast in a post hoc analysis. For instance, to analyze if patients showed response enhancement for the semantic relation the following contrast was conducted:
Depression (positive [related > unrelated] + negative [related > unrelated] + neutral [related > unrelated]) > controls (positive [related > unrelated] + negative [related > unrelated] + neutral[related > unrelated]); inclusively masked with the same contrast as mentioned above.
This was also done for response suppression in patients and response enhancement/suppression in controls (for a detailed description of the contrasts, please see Supporting Information).
Because we assume that the differences between the processing of different semantic relations might be small, we chose to employ Monte‐Carlo simulation of the brain volume to establish an appropriate voxel contiguity threshold [Slotnick, 2003]. This correction has the advantage of higher sensitivity to smaller effect sizes, while still correcting for multiple comparisons across the whole brain volume. Assuming an individual voxel Type I error of P < 0.05 a cluster extent of 29 contiguous resampled voxels was indicated as necessary to correct for multiple comparisons at P < 0.05. In addition, the mask was thresholded at P < 0.10 for the first contrast (semantic relation effect) and at P < 0.05 for the second contrast (group differences). The logic behind was that the first mask includes the conjunction of six independent contrasts, so that the probability of each voxel surviving the conjunction is approximately 0.0174. For the second group difference contrast this was true at P < 0.05 (three independent contrasts).
The second contrast of interest was the valence effect. For the investigation of group differences, we compared the (a) positive priming effect versus neutral and negative priming effect and (b) the negative priming effect versus neutral and positive priming effect. For example, within the set of voxels that show differences between the positive priming effects and the other two semantic priming effects (neutral and negative) we were looking for voxels that are differentially activated across the two groups. The following contrasts were conducted (for a detailed description see Supporting Information):
Depression (positive [related vs. unrelated] VS (negative [related vs. unrelated] + neutral [related vs. unrelated])) VS. Controls (positive [related vs. unrelated] vs. (negative [related vs. unrelated] + neutral[related vs. unrelated]))
For the between‐group comparisons of the valence effects, the same individual voxel Type I error of P < 0.0005 that was already used in the preceding study [corrected for multiple comparison based on the Monte‐Carlo simulation; Sass et al., 2012] was assumed (cluster with contiguous voxel extent of 8). Again, to investigate possible difference in directionality (enhancement vs. suppression) directed t‐contrasts were calculated in a post hoc analysis, e.g., to investigate if patients showed response enhancement for positive stimuli while controls show response suppression the following contrast was calculated (see also Supporting Information):
Depression (positive [related > unrelated] > negative [related > unrelated] + neutral [related > unrelated]) > controls (positive [related > unrelated] > negative [related > unrelated] + neutral [related > unrelated])
The third contrast of interest was a Pearson product‐moment correlation between current mood (PANAS), self‐report on severity of depression (BDI‐II), and functional signal changes within three regions of interest (amygdala, ACC, DLPFC). In other words, we wanted to investigate the modulation induced by the current mood and severity of depression. Here, we focused on three regions of interest where structural and functional differences between depressive patients and controls are well‐established: amygdala, anterior cingulate cortex, and dorsolateral prefrontal cortex. For all regions, the peak coordinates were defined by the main contrasts of effect (valence and relation effect; see Table 3 for the chosen coordinates that are marked with the symbol “a”). Since we are interested in the relationship between mood and semantic priming per se—i.e. regardless of response enhancement or suppression—unsigned contrast estimates were extracted from each region. The contrast weights for each priming effect (i.e., positive, negative, and neutral) were extracted from the first‐level analysis of every subject. The contrast weights and the values of the positive and negative PANAS scale from every subject and the BDI and HAMD values for the patients were then entered into a Pearson product‐moment correlation to investigate the dependence of these variables.
Table 3.
Neural correlates of relation effects and emotional valence effects
| Anatomical Region | BA | Coordinates | ||||
|---|---|---|---|---|---|---|
| x | y | z | z‐Value | No. voxels | ||
| Relation effects | ||||||
| Common activation for semantic relations | ||||||
| Right middle frontal gyrus | 8 | 30 | 24 | 52 | 2.16 | 85 |
| Group differences: Contribution of patients | ||||||
| Right fusiform gyrus | 19 | 42 | −70 | −16 | 4.02 | 14 |
| Group differences: Contribution of controls | ||||||
| Right anterior cingulate cortexa | 24 | 2 | 38 | 6 | 3.12 | 31 |
| Emotional valence effect | ||||||
| Group differences for positive vs. neutral and negative* | ||||||
| Left cerebellum | −2 | −40 | −10 | 4.07 | 16 | |
| Right dorsolateral prefrontal cortexa | 10 | 40 | 40 | 24 | 3.64 | 22 |
| Right dorsolateral prefrontal cortexa | 46 | 48 | 44 | 14 | 3.59 | 11 |
| Right amygdalaa | 30 | −6 | −20 | 3.65 | 10 | |
| Group differences for negative vs. neutral and positive | ||||||
| Right putamen | 30 | 4 | 14 | 3.72 | 26 | |
| Left cerebellum | −2 | −42 | −12 | 3.88 | 19 | |
Coordinates are listed in MNI space. BA is the Brodmann area nearest to the coordinate and should be considered approximate.
Areas that were considered as regions of interest.
The reported voxel coordinates of activation peaks are in MNI space (ICBM standard). For the anatomical localization the functional data were referenced to probabilistic cytoarchitectonic maps and to the SPM Anatomy toolbox [Eickhoff et al., 2005]. MNI coordinates were transformed to Talairach space [icbm2tal; Lancaster et al., 2007] to assess the nearest corresponding Brodman areas referenced to the Talairach daemon [Lancaster et al., 2000].
RESULTS
Behavioral Data
Accuracy
Incorrect responses were excluded from further analyses (controls: 3.8%, patients: 3.4%). For error rates, the two‐factorial ANOVA revealed no significant group difference (P > 0.19).
Reaction time
The ANOVA revealed a main effect for VALENCE (F (2,72) = 8.63, P < 0.001), RELATION (F (1,36) = 41.04, P < 0.001), and GROUP (F (1,36) = 5.25, P < 0.05) as well as a significant interaction of GROUP and VALENCE (F(2,72) = 5.38, P < 0.05). All other interactions were not significant (P > 0.17). Post hoc t‐tests showed that both groups showed a significant priming effect for the positive and neutral condition (t18 > 3.2, P > 0.005). The negative condition revealed no effect in controls but a significant effect in patients (t18 = −3.32, P < 0.005). Comparison of priming effects (unrelated–related) between groups, revealed no significant differences for the size of priming effects (all P > 0.09). The statistical results and mean values are represented in Table 2.
Table 2.
Behavioral data and analysis
| Group | Emotion | Relation | Mean RT (in ms) | S.D. (in ms) | Priming (in ms) | S.D. (in ms) | Mean error (in %) | S.D. (in %) | |
|---|---|---|---|---|---|---|---|---|---|
| Patients | Positive | Related | 682 | 134 | 40a | 55 | 2.3 | 3.9 | |
| Unrelated | 722 | 114 | 3.9 | 4.2 | |||||
| Negative | Related | 730 | 162 | −24a | 62 | 2.8 | 3.6 | ||
| Unrelated | 706 | 150 | 3.9 | 5.5 | |||||
| neutral | Related | 668 | 133 | 54a | 43 | 2.3 | 2.3 | ||
| Unrelated | 722 | 119 | 3.3 | 3.3 | |||||
| Controls | positive | Related | 610 | 57 | 66a | 35 | 2.3 | 3 | |
| Unrelated | 677 | 71 | 4 | 4.2 | |||||
| negative | Related | 630 | 73 | −8 | 39 | 2.5 | 3.8 | ||
| Unrelated | 622 | 64 | 1.4 | 2.8 | |||||
| neutral | Related | 587 | 62 | 52a | 33 | 1.6 | 2.8 | ||
| Unrelated | 639 | 73 | 2.3 | 2.5 | |||||
| Main effects ANOVA | F (1,36) = 5.25, P < 0.05 | F (2,72) = 8.63, P < 0.001 | F (1,36) = 41.04, P < 0.001 | No significant main effects and interactions (P > 0.19) |
Priming refers to semantic priming effects (unrelated − related).
RT = reaction time; SD = standard deviation/ within‐group comparisons.
Significant priming effects with p < 0.005 (for detailed information please see Results section).
The correlation between current mood and size of priming effects indicated that for controls the positive priming effect increased with positive mood (r = 0.61, P < 0.01). For patients, only the positive priming effect correlated negatively with negative mood (r = −0.73, P < 0.01).
Imaging Data
Semantic relation effect
The F‐contrast for common activation revealed significant signal changes within the right middle frontal gyrus (MFG/BA8; see Fig. 2A). The post hoc analysis of direction revealed that the semantic relation effect was based on response enhancement, i.e., the related conditions showed higher activation than the unrelated conditions. To test the group differences, the contribution of each group was tested separately. The relation effect influenced by patients revealed no significant signal changes. However, lowering the cluster extent revealed significant changes within the right fusiform gyrus (14 voxels; see Fig. 2B) with patients showing response suppression and nearly no semantic relation effect for controls. The effect influenced by controls revealed significant signal changes in the left ACC (response suppression; see Fig. 2C and Table 3).
Figure 2.
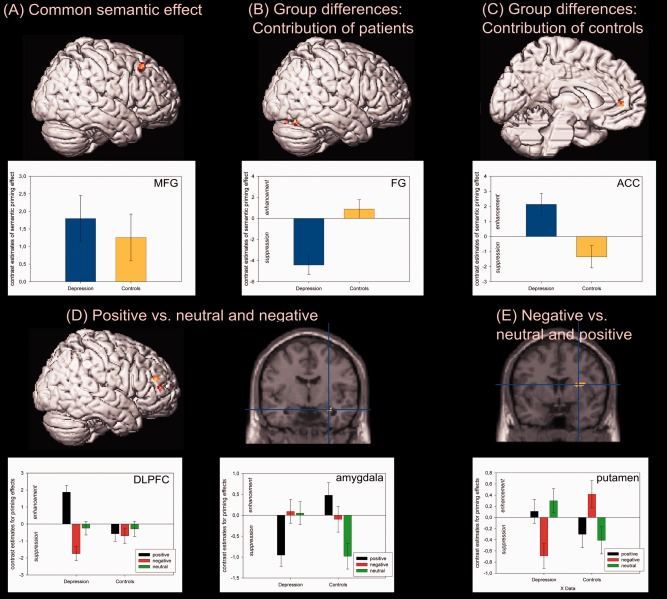
Neural correlates of the relation and valence effect. (A–C) Significant activations for the comparison of related and unrelated word pairs. The plots depict the size of the priming effect with response enhancement (upper panel) and suppression (lower panel) with common activation in the right middle frontal gyrus (MFG), differences for patients in the right fusiform gyrus (FG), and for the controls within the left anterior cingulate cortex (ACC). (D and E) The comparison of positive > neutral and negative valence revealed signal changes within the dorsolateral prefrontal cortex (DLPFC) and the amygdala while for negative > positive and neutral valence differences within the putamen were found. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Valence effect
The group differences for emotionally valenced priming effects, revealed right‐hemispheric activation within the DLPFC, amygdala and left cerebellum for positive as compared with neutral and negative stimuli (see Fig. 2D). The post hoc analysis showed that both cluster of the DLPFC showed response enhancement for positive stimuli in patients. For the amygdale cluster it was shown that patients showed response suppression and controls response enhancement.
The comparison of negative versus neutral and positive stimuli showed significant differences between conditions within the right putamen and left cerebellum (see Fig. 2E and Table 3). In detail, while patients showed significant response suppression for negative stimuli, controls showed response enhancement.
Correlation of current mood and regions of interest
The patients showed for the amygdala cluster [30, −6, −20] significant correlations between positive priming effect and positive mood (r = 0.73, P < 0.01), negative mood (r = −0.51, P < 0.05), and BDI (r = −0.59, P < 0.01); the lower the positive mood and the higher the negative mood, the smaller the positive priming effect. Both clusters in the DLPFC (BA 46, 10) revealed negative correlations between negative mood and positive priming effect (BA 10: r = −0.59, P < 0.05; BA 46: r = −0.53, P < 0.05) and between BDI and negative priming effect (BA 10: r = −0.55, P < 0.05; BA 46: r = −0.65, P < 0.01). For the ACC, no significant correlations were found. Healthy controls showed no significant correlations (see Fig. 3).
Figure 3.
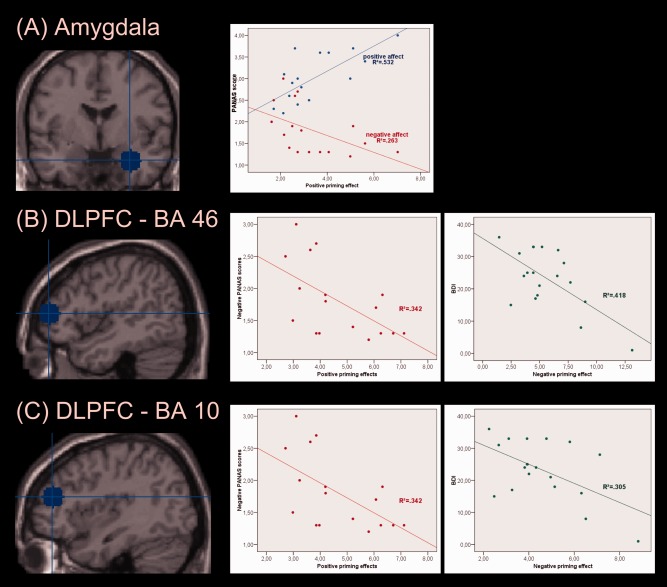
Modulation of neural activation through current mood in depressed patients. (A) For the amygdala, there were correlations between positive (blue) and negative (red) PANAS scores and the positive neural priming effect. (B) The DLPFC (BA 46) showed negative correlations between the negative PANAS scores and the positive priming effect (left plot) as well as between the BDI scores and the negative priming effect (right plot). (C) For the second DLPFC cluster (BA 10) also negative correlations between negative PANAS and positive priming effect as well as BDI and negative priming effect were found. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
The current study investigated the influence of emotional valence on neural correlates of semantic priming in major depression. At the behavioral level, we found similar semantic relation effects for positive and neutral information in depressed patients and healthy controls. In contrast, negative information induced no priming effect in controls but an inhibition effect in depression (slower RT for related in comparison with unrelated word pairs). The neural correlates highlighted a right‐hemispheric frontotemporal focus (MFG/BA8, ACC, fusiform gyrus) for the semantic relation effect while group differences for the valence effects were located within the DLPFC and amygdala (positive valence) and within the right putamen (negative valence).
Behavioral Results
Positive and neutral stimuli showed similar priming effects; the negative information caused either no effect (controls) or against our hypothesis of potentiated negative processing an inhibition effect (patients). First, the behavioral results replicate the finding of differentially organized emotional material in memory where positive information might be better elaborated and interconnected than negative ones [Ashby et al., 1999; Bower, 1981; Sass et al., 2012]. Second, the inhibition effect of negative information in depression might be caused by enhanced attention on negative valence information. In other words, even if subjects do not require the negative information to make a lexical decision, the patients were not able to ignore that information which interferes with a fast response. The correlation analysis of current mood (PANAS) and priming effects revealed a relationship between mood and positive priming effect: in controls, the higher the positive mood, the larger the priming effect while for patients, the more negative the current mood, the smaller the priming effect. These data highlight the interaction of semantic information processing and current mood. However, as this was only true for positive information it also contradicts the predictions of the negativity bias in depression. Hence, based on the behavioral results depressed patients showed reduced priming effects and enhanced attention on (negative) emotional information rather than facilitated negative information processing as suggested by the negativity bias. Thus, in depression the processing of emotions and the (behavioral) reactivity to emotional stimuli is reduced as it is suggested by the hypothesis of emotional context insensitivity [ECI; Rottenberg et al., 2005].
Neural Correlates of Semantic Relation Processing in Patients and Controls
Common neural activation in both groups was present in the right MFG/BA8. This area is associated with retrieval effort during semantic processing and more efficient stimulus processing [Sachs et al., 2008]. However, based on earlier results on semantic priming, “classical” areas of semantic processing were expected, like middle temporal and inferior frontal regions [Binder et al., 2009]. But according to our earlier study on emotional influence on semantic processing, participants might be “biased toward emotional aspects of word meaning because the majority of our stimuli had either a positive or negative emotional connotation” [Sass et al., 2012]. Subjects experience an automatic emotion‐induced bias [Kuchinke et al., 2005] leading to enhanced activation of right‐lateralized areas that are more influenced by valence than left hemispheric regions [Buchanan, 2007].
Differences between groups were found within the right fusiform gyrus (suppression: patients > controls) and the left ACC (patients enhancement vs. controls suppression). The bilateral fusiform gyrus is involved in object/word imagery [Wheatley et al., 2005] and processing of semantic information [Kuniecki et al., 2003]. During emotional processing the right fusiform gyrus reflects an attention bias toward negative information [Leung et al., 2009] and as patients might show enhanced attention toward negative information a greater response suppression might be found reflecting the ease to retrieve primed targets in comparison to controls. The ACC effect replicates earlier findings [Sass et al., 2012] reflecting an automatic attentional system during semantic processing, i.e., response suppression for controls reflects less routine and higher response competition for unpriming words [Mummery et al., 1999; Sass et al., 2012; Wible et al., 2006]. In contrast, enhanced activity for depressed patients might be based on general hyperactivity in this region [Hamilton and Gotlib, 2008; Harvey, 2005; Kober et al., 2008] due to increased sensitivity for emotional conflict and enhanced attention, i.e., patients might not be able to ignore the emotional content and therefore, enhanced attention is necessary to successfully accomplish the task. To conclude, semantic processing regions were recruited by both groups while differences were based on the kind of signal changes (suppression vs. enhancement) rather than on different localizations in the brain. The right‐lateralized focus might reflect an influence of emotional valence that interacts with the nonemotional semantic information.
Neural Correlates of the Influence of Valence on Semantic Processing
The semantic relation effect indicated that there is a strong influence of valence. This suggestion is supported by the corresponding neural correlates: our data highlight a right‐hemispheric dominance for semantic processing most probably induced by the inclusion of valence addressing a wider and broader semantic field relying on valence as well as semantic information. Support arises by the suggestion that the right hemisphere might mediate the emotional influences on semantic processing [Atchley et al., 1996] through a semantic network with inherent emotional items as salient semantic features, and emotional experience alters the structural organization of the right‐lateralized network (“emotion”‐network [Bower, 1981].
The group comparison for the positive valence information exhibit differences within the DLPFC (BA 10/46; patients enhancement vs. controls small suppression) and the amygdala. The activation of the DLPFC correlates with stimulus valence, associate learning, emotion regulation, attention, cognitive control as well as memory retrieval influenced by affect [Baayen et al., 1993; Buchanan, 2007; Klumpp and Deldin, 2010]. It is also known that this area shows abnormally enhanced brain responses in depression that might reflect increased sensitivity for affective conflict and enhanced attention on emotional stimuli [Grimm et al., 2008; Wagner et al., 2006]. According to our hypotheses, we assume that in depression positive stimuli induce response enhancement due to higher attention and cognitive load and require more effort to be processed because of incongruency with the current mood. The second neural cluster for positive information was within the amygdala (patients suppression vs. controls enhancement). These results replicate the findings of Canli et al. 2004 who found the same pattern of results for positive information. The amygdala is a crucial node in the emotion network implicated in a variety of emotional functions [Hamilton and Gotlib, 2008]. In depression, this region shows structural [decreased volume; Hamilton and Gotlib, 2008] and functional abnormalities [increased/sustained activity; Siegle et al., 2007]. In controls, enhanced activation might reflect the generation of emotional feeling states, processing of emotion and evaluation of emotional significance [Canli et al., 2004; Hamilton and Gotlib, 2008].
The negative information revealed group differences within the putamen (controls enhancement vs. patients suppression). The putamen is involved in controlled processes of expectancy generation, semantic matching [Sheline et al., 2001] and might be modulated by emotional processes of positive or negative valence [Surguladze et al., 2003]. Hence, we assume that the putamen reflects the effortful processing of negative valence that is related to larger priming effects for controls. For patients it is easier to retrieve negative information—therefore, response suppression was found.
Correlation of Mood, Severity of Depression, and Three ROIs in Patients With Depression
Within the DLPFC, the size of the neural priming effects declined with increasing negative mood and more severe depression. In other words, both positive and negative priming effects became smaller. These correlations again contradict our hypothesis of a negativity bias but support the assumption of a emotional context insensitivity [Canli et al., 2004; Hamilton and Gotlib, 2008; Rottenberg et al., 2005]. The same pattern of result was found for the amygdala: activity was correlated with PANAS and BDI values demonstrating that the more severe the depression, the smaller the positive priming effect. Hence, we suggest that the hypoactivation within the DLPFC and the amygdala in correlation with mood and severity of depression reflects the emotional insensitivity of depressed patients [Canli et al., 2004; Hamilton and Gotlib, 2008; Rottenberg et al., 2005].
Conclusion and Theoretical Implications
Beside emotional deficits, cognitive dysfunctions are a prominent symptom in depression. Interestingly, semantic processing per se seems to be preserved. On a behavioral level, positive and neutral information induced the same effects in both groups while negative valence led to no effect and inhibition, respectively. Therefore, positive and negative information seem to exhibit asymmetric effects [Isen, 1987] that might be caused by a different organization of emotional material in memory [Ashby et al., 1999; Bower, 1981].
The neural correlates highlighted a right hemispheric focus for semantic processing influenced by valence underlining the assumption of a boarder and wider semantic network that interacts with an emotional association network [Atchley et al., 1996; Bower, 1981]. Similar brain responses were found within the right MFG/BA8 indicating that cognitive deficits in depression (especially semantic disturbances) might be explained by an interaction of cognition and valence rather than deficits in the cognitive domain itself. Our assumption is supported by the fact that there were correlations between current mood of patients and priming effects on behavioral and neural level. Mainly the variation of negative mood influenced the signal changes (lower neural effects correlated with higher negative mood). Hence, there might be an interaction between behavior, mood, and neural correlates that leads to the specific cognitive symptoms in depression. Here, the DLPFC as “cognitive” region and the amygdala as emotion‐related area seem to play an important role.
The current findings contradict the negative potentiation hypothesis [Cohen et al., 2005] because diminished emotional reactivity in depressive patients was found. Our findings rather support the emotional‐context insensitivity hypothesis [Rottenberg et al., 2005] that suggests disrupted emotional reactions and minimal emotional regulation in depression, i.e., patients show lower responses to negative and positive emotional information in correlation with the current mood.
In summary, we found an influence of emotionally valenced information on automatic semantic processing in patients with depression and healthy controls. The semantic deficits in depression seem to be linked to valence information and current status of mood and this interaction influences not only behavior but also the neural correlates in (right hemispheric) cognitive and limbic areas.
Supporting information
Supporting Information
Acknowledgments
The authors thank Freddie Steiner, Sonja Eskens, and Isabelle Reinhardt for their help during the development of the design and the stimuli and Franziska Kintzel as well as Juliane Mühlhaus for their support during the data collection.
REFERENCES
- Ashby FG, Isen AM, Turken U (1999): A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev 106:529–550. [DOI] [PubMed] [Google Scholar]
- Atchley RA, Burgess C, Audet C, Arambel S (1996): Timecourse, context effects, and the processing of lexical ambiguity in the cerebral hemispheres. Brain Cognit 30:257–434.8812005 [Google Scholar]
- Baayen RH, Piepenbrock R, Rijn HV (1993): The CELEX Lexical Database [CD‐ROM]. Version Release 1. Philadelphia: Linguistic Data Consortium, University of Pennsylvania. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W (1996): Comparison of Beck Depression Inventories‐IA and ‐II in psychiatric outpatients. J Pers Assess 67:588–597. [DOI] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL (2009): Where Is the semantic system? A critical review and meta‐analysis of 120 functional neuroimaging studies. Cereb Cortex 19:2767–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower GH (1981): Mood and memory. Am Psychol 36:129–148. [DOI] [PubMed] [Google Scholar]
- Bower GH, Forgas JP (2001): Mood and social memory In: Forgas JP, editor. Handbook of Affect and Social Cognition. Mahwaw, NJ: Lawrence Erlbaum; pp 95–120. [Google Scholar]
- Bradley B, Mogg K, Williams R (1995): Implicit and explicit memory for emotion‐congruent information in clinical depression and anxiety. Behav Res Ther 33:755–770. [DOI] [PubMed] [Google Scholar]
- Buchanan TW (2007): Retrieval of emotional memories. Psychol Bull 133:761–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Sivers H, Thomason ME, Whitfield‐Gabrieli S, Gabrieli JD, Gotlib IH (2004): Brain activation to emotional words in depressed vs healthy subjects. Neuroreport 15:2585–2588. [DOI] [PubMed] [Google Scholar]
- Cohen LH, Gunthert KC, Butler AC, O'Neill SC, Tolpin LH (2005): Daily affective reactivity as a prospective predictor of depressive symptoms. J Pers 73:1687–1713. [DOI] [PubMed] [Google Scholar]
- Davidson R, Pizzagalli D, Nitschke J, Putnam K (2002): Depression: Perspectives from affective neuroscience. Annu Rev Psychol 53:545–574. [DOI] [PubMed] [Google Scholar]
- Dietrich DE, Emrich HM, Waller C, Wieringa BM, Johannes S, Munte TF (2000): Emotion/cognition‐coupling in word recognition memory of depressive patients: An event‐related potential study. Psychiatry Res 96:15–29. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K (2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G (2008): Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: An fMRI study in severe major depressive disorder. Biol Psychiatry 63:369–376. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Gotlib IH (2008): Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biol Psychiatry 63:1155–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Fossati P, Pochon JB, Levy R, Lebastard G, Lehéricy S, Allilaire JF, Dubois B (2005): Cognitive control and brain resources in major depression: An fMRI study using the n‐back task. Neuroimage 26:860–869. [DOI] [PubMed] [Google Scholar]
- Henson R (2003): Neuroimaging studies of priming. Prog Neurobiol 70:53–81. [DOI] [PubMed] [Google Scholar]
- Ingram RE (1984): Toward an information‐processing analysis of depression. Cognit Ther Res 8:443–477. [Google Scholar]
- Isen AM (1987): Positive affect, cognitive‐processes, and social‐behavior. Adv Exp Soc Psychol 20:203–253. [Google Scholar]
- Joormann J, Gotlib IH (2010): Emotion regulation in depression: relation to cognitive inhibition. Cogn Emot 24:281–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, Deldin P (2010): Review of brain functioning in depression for semantic processing and verbal fluency. Int J Psychophysiol 75:77–85. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss‐Moreau E, Lindquist K, Wager TD (2008): Functional grouping and cortical‐subcortical interactions in emotion: A meta‐analysis of neuroimaging studies. Neuroimage 42:998–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD (2002): Modulation of the lexical‐semantic network by auditory semantic priming: An event‐related functional MRI study. Neuroimage 17:1761–1772. [DOI] [PubMed] [Google Scholar]
- Kuchinke L, Jacobs AM, Grubich C, Vo MLH, Conrad M, Herrmann M (2005): Incidental effects of emotional valence in single word processing: An fMRI study. Neuroimage 28:1022–1032. [DOI] [PubMed] [Google Scholar]
- Kuniecki M, Urbanik A, Sobiecka B, Kozub J, Binder M (2003): Central control of heart rate changes during visual affective processing as revealed by fMRI. Acta Neurobiol Exp (Wars) 63:39–48. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas‐Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT (2007): Bias between MNI and Talairach coordinates analyzed using the ICBM‐152 brain template. Hum Brain Mapp 28:1194–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KK, Lee TM, Yip P, Li LS, Wong MM (2009): Selective attention biases of people with depression: positive and negative priming of depression‐related information. Psychiatry Res 165:241–251. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E (2003): Spatiotemporal dynamics of modality‐specific and supramodal word processing. Neuron 38:487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G, Southall A (1991): depression and the processing of emotional stimuli—A study of semantic priming. Cognit Ther Res 15:283–302. [Google Scholar]
- Mummery CJ, Shallice T, Price CJ (1999): Dual‐process model in semantic priming: A functional imaging perspective. Neuroimage 9:516–525. [DOI] [PubMed] [Google Scholar]
- Neely JH (1991): Semantic priming effects in visual word recognition: A selective review of current findings and theories In: Besner D, Humphreys GW, editors. Basic Processes in Reading: Visual Word Recognition. Hillsdale, NJ: Lawrence Erlbaum; pp 264–336. [Google Scholar]
- Northoff G, Heinzel A, Bermpohl F, Niese R, Pfennig A, Pascual‐Leone A, Schlaug G (2004): Reciprocal modulation and attenuation in the prefrontal cortex: An fMRI study on emotional‐cognitive interaction. Hum Brain Mapp 21:202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power M, Cameron C, Dalgleish T (1996): Emotional priming in clinically depressed subjects. J Affect Disord 38:1–11. [DOI] [PubMed] [Google Scholar]
- Raposo A, Moss HE, Stamatakis EA, Tyler LK (2006): Repetition suppression and semantic enhancement: An investigation of the neural correlates of priming. Neuropsychologia 44:2284–2295. [DOI] [PubMed] [Google Scholar]
- Rissman J, Eliassen JC, Blumstein SE (2003): An event‐related FMRI investigation of implicit semantic priming. J Cognit Neurosci 15:1160–1175. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Gross JJ, Gotlib IH (2005): Emotion context insensitivity in major depressive disorder. J Abnorm Psychol 114:627–639. [DOI] [PubMed] [Google Scholar]
- Sachs O, Weis S, Zellagui N, Huber W, Zvyagintsev M, Mathiak K, Kircher T (2008): Automatic processing of semantic relations in fMRI: Neural activation during semantic priming of taxonomic and thematic categories. Brain Res 1218:194–205. [DOI] [PubMed] [Google Scholar]
- Sass K, Habel U, Sachs O, Huber W, Gauggel S, Kircher T (2012): The influence of emotional associations on the neural correlates of semantic priming. Hum Brain Mapp 33:676–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sass K, Krach S, Sachs O, Kircher T (2009): Lion–tiger–stripes: Neural correlates of indirect semantic priming across processing modalities. Neuroimage 45:224–236. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA (2001): Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry 50:651–658. [DOI] [PubMed] [Google Scholar]
- Siegle G, Thompson W, Carter C, Steinhauer S, Thase M (2007): Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry 61:198–209. [DOI] [PubMed] [Google Scholar]
- Slotnick SD (2003): Model fitting in (n+1) dimensions. Behav Res Methods Instrum Comput 35:322–324. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Brammer MJ, Young AW, Andrew C, Travis MJ, Williams SC, Phillips ML (2003): A preferential increase in the extrastriate response to signals of danger. Neuroimage 19:1317–1328. [DOI] [PubMed] [Google Scholar]
- Wagner G, Sinsel E, Sobanski T, Kohler S, Marinou V, Mentzel HJ, Sauer H, Schlosser RG (2006): Cortical inefficiency in patients with unipolar depression: An event‐related FMRI study with the Stroop task. Biol Psychiatry 59:958–965. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Telegen A (1988): Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54:1063–1070. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A (2005): Automatic priming of semantically related words reduces activity in the fusiform gyrus. J Cognit Neurosci 17:1871–1885. [DOI] [PubMed] [Google Scholar]
- Wible CG, Han SD, Spencer MH, Kubicki M, Niznikiewicz MH, Jolesz FA, McCarley RW, Nestor P (2006): Connectivity among semantic associates: An fMRI study of semantic priming. Brain Lang 97:294–305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


