Abstract
Writing and drawing are understudied with fMRI, partly for lack of a device that approximates these behaviors well while supporting task feedback and quantitative behavioral logging in the confines of the magnet. Consequently, we developed a tablet based on touchscreen technology that is accurate, reliable, relatively inexpensive, and fMRI compatible. After confirming fMRI compatibility, we conducted preliminary fMRI experiments examining the neural correlates of a widely used pen‐and‐paper neuropsychological assessment, the trail making test. In two subjects, we found left hemisphere frontal lobe activations similar to the major results of a previous group study, and we also noted individual differences mostly in the right hemisphere. These results demonstrate the utility of the new tablet for adaptations of pen‐and‐paper tests and suggest possible uses of the tablet for longitudinal, within‐subjects studies of disease or therapy. We also discuss using the tablet for several other types of tests requiring many, continuous, or two‐dimensional responses that were previously very difficult to perform during fMRI. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, trail making test, computer peripherals, fMRI equipment, fMRI compatibility, input devices, stylus
INTRODUCTION
Behaviors as complex as writing and drawing are difficult to study because demands made in a simplified testing environment differ from demands made in everyday life [Shallice and Burgess,1991]. Unfortunately, tasks that are similar to real life are challenging to implement in fMRI, and such tasks are under‐represented in the existing literature (except perhaps in the area of human spatial navigation [Spiers and Maguire,2007]). In the writing and drawing literature, both Katanoda et al. [2001] and Makuuchi et al. [2003] had subjects write/draw with the right index finger, either in the air or on a fixed surface, during fMRI. Such a configuration is very unlike how people write in everyday life and therefore the generalizability of the reported findings is unclear. Furthermore, without a method to capture user input (i.e., what the user “writes”) it becomes difficult to assess the behavioral performance that underlies the resulting images of brain activity. These issues were partially addressed by Harrington et al. [2007] who had subjects write/draw with a pencil and paper notepad. However, the notepad was not visible to the subject in its position on the lap, and a researcher was required to stand adjacent to the magnet bore to tear pages off the notepad periodically. Methodologically, simply using pencil and paper during fMRI is problematic for many reasons: narrowness and length of the magnet bore; posture while lying on the scanner table; difficulty viewing the page; and task‐related requirements such as timing, changing of pages, and sensory feedback. An fMRI‐compatible tablet system would help to overcome many of these problems.
Touch tablets are computer input peripherals that convert contact position on a touch‐sensitive surface into two or more coordinate values. When interpreted by software, a touch usually translates naturally into an effect at the corresponding projected point on the computer display. The effect may be to mimic a drawing tool or to select an on‐screen control, for example. Tablets come in many forms and sizes, some integrated with other input devices or secondary displays, and there are several different technologies for touch detection. The combination of stylus and tablet has long been exploited in artistic and industrial graphical design applications, and the recent consumer popularity of touch sensitive technologies in general has led to increased support by operating systems and application software in many fields.
Some preliminary work has been conducted to capture pen movements during fMRI using tablet‐like devices. Reithler et al. [2006] built a device in which the pen and drawing path formed an electrical circuit that exhibited resistance changes as the pen was moved. However, the device as reported had several limitations for writing and drawing. First, a single value was output from the device, enabling only one‐dimensional measurement of movement. Second, users were only able to trace predefined paths on the drawing surface, and these required careful machining and calibration. In addition, no visual feedback of performance was provided to the user. Zakzanis et al. [2005] used a stylus incorporating ShapeTape™ (Measureand, Fredericton, New Brunswick, Canada), an fMRI‐compatible position tracking system consisting of a set of fibre‐optic twist and bend sensors embedded in a mylar tape, to record and display lines “drawn” on a tablet surface in realtime. However, their findings came with a proviso due to the nonideal characteristics of the stylus. The spatial accuracy of the position tracking technology within the tablet plane was adequate to capture writing and drawing [Mraz et al.,2003,2004], but users still perceived disparities between their motions with the stylus and what was represented on the display. The imprecise mapping between motions and display may have reduced behavioral performance and arguably shifted some attentional resources away from the task and toward the manipulation of the stylus. Furthermore, the fiber optic tape technology produced position tracking data that were influenced by thermal drift, requiring recalibration every several minutes for best results, and were also dependent on the conformation of the tape even if the tip of the tape was located in the same position on the tablet. Behind many of these issues is the fact that the technology was not specifically designed for writing and/or drawing. On the other hand, computer touchscreen technologies were conceived for this type of application, and careful selection and adaptation of a touchscreen is likely to yield a better performing tablet for the fMRI environment.
In addition to enabling the study of writing and drawing behavior per se, a tablet system with a stylus would facilitate adapting myriad assessment instruments, primarily pen‐and‐paper tests, for use with fMRI. One example of a traditional pen‐and‐paper test is the trail making test [TMT, Lezak et al.,2004], which we describe later. The clinical utility of the TMT and other widely used tests for neuropsychological assessment have been substantiated through rigorous research and clinical trials. However, the exact neurological underpinnings of such tests are often unknown or are based on lesion studies, which have their own inherent confounds [Stuss et al.,2001]. It would be of great benefit to both clinicians and researchers if these tests could be performed together with fMRI to identify the brain regions that are engaged.
To implement fMRI studies using a tablet, technical challenges must be overcome that are associated with introducing any new device to the fMRI environment. Functional MRI is typically conducted in a very strong static magnetic field (>1 T), with accompanying weaker but dynamic spatially varying magnetic fields (∼10 mT/m gradients and ∼100 T/m/μs slew rates), and with stringent constraints regarding radio frequency (RF) electromagnetic interference (EMI). Functional MRI is also very sensitive to head motion. Very subtle head motion on the order of millimeters can significantly degrade image quality. These issues need to be addressed at all stages of device development, including final testing to ensure fMRI compatibility.
To date, no satisfactory system has been available to capture and display drawing and writing movements during fMRI. We addressed this deficit by designing a robust, ergonomic tablet system and then testing its fMRI compatibility and its utility in representative human fMRI experiments based on the TMT.
MATERIALS AND METHODS
fMRI‐Compatible Tablet System
The tablet system (see Fig. 1) included a touch‐sensitive tablet, an elevated support platform, a stylus and a controller box, as well as the necessary cabling and software to record responses and provide task‐related feedback. All equipment residing inside the magnet room was nonferromagnetic, as discussed later.
Figure 1.
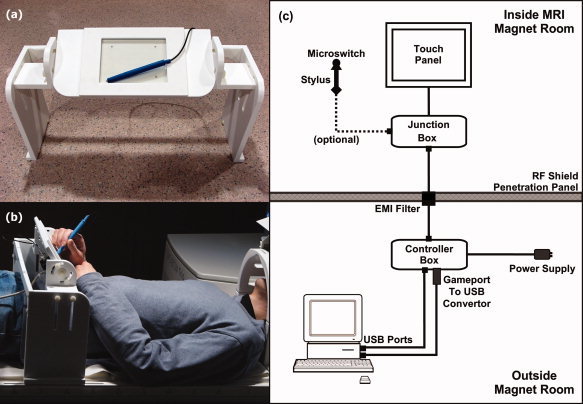
The fMRI‐compatible tablet. (a) Close‐up. (b) In use. A head coil‐mounted mirror was used to view visual stimuli on a rear projection screen (not shown). The platform may be strapped to the table for stability (not shown). (c) Block drawing of hardware setup. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The key component was a transparent sensor panel commonly integrated into commercial touchscreen displays. The prototype device used a 6.4‐in. (16 cm diagonal; 13 cm × 10 cm active area) polyester laminate (PL) resistive four‐wire touchscreen (Microtouch™, Model #RES‐6.4‐PL4, 3M, St. Paul, MN) along with its matching controller board (Microtouch™, Model #SC4, 3M, St. Paul, MN). This panel was chosen for several reasons: (a) the PL coversheet, indium tin oxide resistive coatings and glass substrate were nonferromagnetic and easily attached to shielded and filtered cabling to ensure fMRI compatibility; (b) the resolution (0.13 mm) and report rate (180 reports/s) exceeded our performance criteria; (c) touch operation was feasible with an fMRI‐compatible tool such as a stylus, as well as fingers (gloved or not) or any other reasonable object or body part; (d) ready availability and ease of assembly and system integration; and (e) affordability (less than US$100 for the touchscreen and USB touchscreen controller). Other touchscreen technologies are available (e.g., capacitive or infrared) and could be rendered fMRI compatible. However, none at present have the combination of attractive features indicated earlier.
The support platform was constructed of plastic and featured a tilting, height‐adjustable stage to accommodate users as comfortably as possible within the confines of the magnet bore. The design was intended not only to simulate writing on a desk, but also to reduce interference of (and from) respiratory motion by keeping the writing surface off the torso. Upon the stage, the touch surface was mounted within a raised frame that clearly delimited the sensitive area while offering some protection from unintentional touches and mechanical damage. Beneath the stage, a small junction box (Fig. 1c) provided a receptacle to connect the optional stylus, which was a modified plastic pen barrel with a microswitch on the tip. The junction box also included a connection for a shielded cable leading to the “penetration panel” of the RF shield surrounding the magnet room.
The tablet and stylus signals passed through an EMI filter (56‐705‐005‐LI, Spectrum Control, Fairview, PA) at the penetration panel and went via shielded cables to the tablet controller box (Fig. 1c) in the operator console area. The controller box contained the touchscreen controller board, power conditioner, and receptacles for USB connections to the fMRI stimulus/response computer. Software on the computer interpreted the tablet and/or stylus input to provide task‐related feedback while also recording detailed logs of behavior for subsequent playback or analysis. For example, in the simplest implementation of a pen‐and‐paper task, touching the stylus to the tablet would result in “ink” marks at the analogous locations on the display. This was the interaction scheme adopted in our representative fMRI experiment (see below). Pushing harder with the stylus would activate the microswitch, thus yielding a small amount of tactile feedback and registering a button press. The button input was not used in this experiment, but it is suitable for making discrete selections, for use as a single response button or to provide rudimentary pressure sensitivity.
fMRI Compatibility Testing
Before undertaking fMRI experiments with humans, a series of tests were performed to verify the fMRI compatibility of the tablet. MR images of a 17‐cm diameter spherical water + NiSO4 phantom were acquired with the tablet (a) unplugged and completely removed from the magnet bore (“Baseline”); (b) in the magnet bore (“Inserted”); and (c) in the magnet bore and activated by reaching in to drag the stylus continuously across the tablet (“Activated”). When in the magnet bore, the tablet was positioned 40‐cm away from the head coil, closer than the typical working distance of 50–60 cm, to emphasize the effects of the tablet's presence near the centre of the coil and phantom. In each test iteration, three types of images were acquired under each condition: anatomical (axial 2D FLASH, 4.92 ms TE, 100 ms TR, 16° FA, 25.6 cm × 25.6 cm FoV, 256 × 256 matrix, 2.0 mm thickness, 1.0‐mm gap, 5 slices, 2 measurements), functional (axial gradient echo EPI, 30 ms TE, 2,000 ms TR, 70° FA, 20 cm × 20 cm FoV, 64 × 64 matrix, 5.0 mm thickness, 30 slices, 130 measurements), and magnetic field map (axial 2D FLASH, 5.19 ms TE1, 7.65 ms TE2, 488 ms TR, 60° FA, 24 cm × 24 cm FoV, 64 × 64 matrix, 3.0 mm thickness, 0.75 mm gap, 45 slices). To reduce uncontrolled drift of MRI system hardware over time, and in accordance with common practice for such testing [National Electrical Manufacturers Association,2008], the anatomical scan protocol was shortened compared to our usual human protocol, and the total runtime of the experiment was controlled. Two test iterations were conducted in a single session on a 3‐T MR imager (MAGNETOM Tim Trio, VB15A software; Siemens AG, Erlangen, Germany) using a standard 12 channel phased array head coil. The entire procedure was repeated in two additional sessions 3 and 4 months later, to track any degradation of the system after the launch of a larger, related study using the tablet.
In addition to a visual inspection of all images for artifacts, diagnostic metrics were calculated for each image type using AFNI software [AFNI_2008_07_18_1710, Cox,1996]. A subset of these metrics follows, all calculated on a central 37.5‐mm radius circular region‐of‐interest (ROI) in an axial slice through the centre of the phantom. For anatomical images, a signal‐to‐noise ratio (SNR) was calculated by taking the ROI mean signal across both measurements, dividing by the standard deviation across the same ROI of the difference image formed by subtracting the two measurements, and then multiplying the resulting ratio by √2 [National Electrical Manufacturers Association,2008]. The associated standard deviation was calculated by taking the voxelwise mean signal across both measurements, dividing each voxel value by the standard deviation across the ROI of the difference image formed by subtracting the two measurements, multiplying the resulting ratio in each voxel by √2, and recording the ROI standard deviation of the resulting image. For functional images, a signal‐to‐fluctuation‐noise ratio (SFNR) was calculated by first discarding the initial 10 measurements and forming a “signal image” from the voxelwise time‐series mean. Then a fluctuation noise image was formed by detrending the voxelwise time series with a second‐order Legendre polynomial and computing the voxelwise time‐series standard deviation. The signal image was divided by the noise image, and the ROI mean (and standard deviation) of the resulting SFNR image was recorded as the SFNR metric [Friedman and Glover,2006]. For the magnetic field maps, the ROI mean (and standard deviation) was recorded, to check for subtle magnetic field perturbations potentially arising from the presence of the tablet or from movements associated with writing and drawing.
Trail Making Test
For a preliminary fMRI experiment to test the utility of the new tablet system, we adapted the trail making test (TMT), a commonly used pen‐and‐paper measure of frontal lobe function that engages visuomotor tracking, attention, and cognitive flexibility. The TMT was developed by US Army psychologists for the Army Individual Test Battery [1944] and consists of two parts. In Part A, the subject draws a continuous line, as quickly as possible, to link randomly‐positioned numbers in ascending order (1‐2‐3…). In Part B, the task is similar, but the subject must alternate between numbers (1–13) and letters (A–L) in ascending order (1‐A‐2‐B…). The traditional TMT was modified for fMRI and implemented using Presentation software (12.1, Neurobehavioral Systems, Albany, CA). The number of items to be linked was reduced to 14 from the traditional 25. Considering the reduced resolution and size of the video display and tablet relative to a paper form, having fewer, larger targets was intended to make the test more accessible for future use by older people and stroke patients, who may have poorer visual acuity, reduced motor control, or difficulty maintaining head position for long scanning runs. Parts A and B (see Fig. 2) were each presented in 20‐s task blocks using a different pseudorandom pattern of numbers/letters. Alternating with each TMT task block were 20‐s blocks of a control task, in which the subject drew a line from the centre of the display to a pseudorandomly placed circle and back. A new circle was presented every 2 s. Each 3‐min fMRI run began with a 20‐s static display of brief instructions followed by two repeats of the sequence Part A—Control—Part B—Control.
Figure 2.
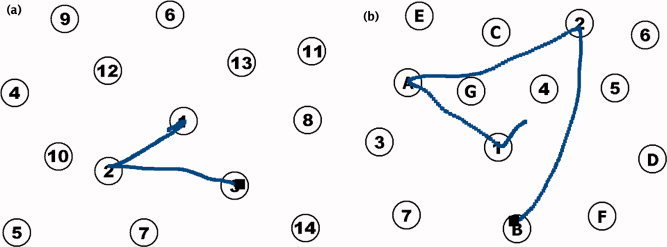
Sample TMT stimuli and illustrative responses. (a) TMT Part A. (b) TMT Part B. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The entire run was performed twice, separated by about 10 min of other neuropsychological assessments as part of a larger test battery (data not reported here). The subject used the fMRI‐compatible tablet as described previously and received visual feedback on a rear projection screen (15° × 12° visual angle) viewed through a mirror mounted on the head coil. Immediately before the fMRI session, the subject was given a 15‐min orientation to the tablet and to each task in an MR simulator system located in a room adjacent to the real MR scanner. During this orientation, the subject completed a shortened practice run (Part A—Control—Part B—Control) consisting of 10‐s blocks and only six items in the TMT displays.
Subjects
Two healthy, right‐handed volunteers (male, 32 years, >10 h documented fMRI participation history; female, 22 years, no fMRI participation history) with no known neurological or psychiatric disease participated in return for reasonable monetary compensation. Subjects gave written informed consent, and the experiment was conducted with the approval of the Baycrest Research Ethics Board.
Image Acquisition and Data Analysis
Functional MRI experiments were conducted in a 3‐T MR imager (MAGNETOM Tim Trio, VB15A software; Siemens AG, Erlangen, Germany) using a standard 12 channel phased array head coil. A high‐resolution anatomical volume was acquired (oblique‐axial 3D MPRAGE, 2.63 ms TE, 2,000 ms TR, 1,100 ms TI, 9° FA, 25.6 cm × 19.2 cm FoV, 256 × 192 matrix, 1.0 mm thickness, 160 slices) prior to blood oxygenation level‐dependent fMRI (oblique‐axial 2D gradient echo EPI, 30 ms TE, 2,000 ms TR, 70° FA, 20 cm × 20 cm FoV, 64 × 64 matrix, 5.0 mm thickness, 30 slices, 92 measurements per run). Functional MRI data were analyzed using AFNI software [AFNI_2008_07_18_1710, Cox,1996]. Before data preprocessing, the first and last time points in the time series for each experimental run were discarded to eliminate the fMRI signal decay associated with magnetization reaching equilibrium, and also to eliminate possible head motion effects inadvertently caused by the onset of scanning or anticipation of the offset of scanning. The remaining fMRI data were corrected for physiological effects using RETROICOR [Glover et al.,2000], temporally interpolated for slice time correction, coregistered to the eleventh time point of the first run for motion correction, and spatially filtered with a 6‐mm FWHM Gaussian kernel. Statistical parameter maps were calculated using a general linear model that included a boxcar waveform for each task convolved with a gamma function, as well as run‐wise third order Legendre polynomials for low frequency detrending and six head motion estimate parameters as baseline covariates. The maps were thresholded using a False Discovery Rate method [Genovese et al.,2002] at q = 0.001 and clusters of activation smaller than 96 μl (<2 voxels) with a connectivity radius of 3.125 mm were removed. Finally, the maps were overlaid on anatomical images aligned with the ICBM 452 T1 atlas in Talairach space (supplied with AFNI).
RESULTS
fMRI Compatibility
The image quality metrics from all three sessions are summarized in Table I, averaged across both test iterations. The pattern of quality metrics did not indicate that the tablet had a noticeable systematic impact on image quality: The small differences between conditions were within normal variability, on the same order as differences between the two iterations of the baseline condition, and less than the spatial variation over the ROI. Upon visual inspection of images and field maps, plots, and AFNI's outlier screening output (3dToutcount), there were no noticeable image artifacts associated with the presence of the tablet. Likewise, no problems with tablet performance were noted in the MRI system.
Table I.
Mean image quality metrics for tablet fMRI compatibility tests with the phantom in three test sessions
| Test session | Condition | Anatomical SNR | Functional SFNR | Field map mean |
|---|---|---|---|---|
| Month 0 | Baseline | 83 (5) | 773 (60) | 2,047 (11) |
| Inserted | 82 (5) | 782 (65) | 2,041 (11) | |
| Activated | 82 (5) | 781 (68) | 2,043 (11) | |
| Baseline difference | 2 | 30 | 9 | |
| Month 3 | Baseline | 72 (5) | 787 (64) | 2,069 (11) |
| Inserted | 73 (5) | 794 (61) | 2,070 (11) | |
| Activated | 74 (5) | 786 (64) | 2,065 (11) | |
| Baseline difference | 1 | 4 | 2 | |
| Month 4 | Baseline | 75 (5) | 782 (57) | 2,061 (17) |
| Inserted | 75 (5) | 780 (55) | 2,063 (17) | |
| Activated | 74 (5) | 778 (55) | 2,060 (19) | |
| Baseline difference | 1 | 1 | 7 |
The voxel standard deviation of the metrics, averaged across the two test iterations in each session, is shown in parentheses. The difference between the two baseline (no tablet) iterations is included for comparison.
Trail Making Test
Both subjects were able to link all 14 TMT items within the allotted time for six out of the eight total TMT blocks, averaging 12 items for the remaining two blocks. The mean times per item, summarized for each subject across all Part A and B blocks in Table II, indicated that the responses were slower for Part B versus Part A.
Table II.
Mean behavioral response times (ms/item)
| TMT condition | Participant 1 | Participant 2 | Average |
|---|---|---|---|
| Part A | 1,292 (800) | 1,239 (497) | 1,265 |
| Part B | 1,355 (871) | 1,379 (757) | 1,367 |
The standard deviation of the per item response times for each stimulus, averaged for Part A and B, is shown in parentheses.
The subjects performed all parts of the experiment with <1 mm or 1° head motion relative to the mean in any of the six motion parameters estimated from the fMRI data during preprocessing, and there were no remarkable motion artifacts in the images or unthresholded statistical parameter maps. The sample size is small, but it is worth noting that the lack of significant head motion, even in Subject 2 who had no prior fMRI experience, is consistent with our experience using an old tablet in larger groups [Ferber et al.,2007; Zakzanis et al.,2005]. Furthermore, in preliminary data from a larger, ongoing study in our laboratory, only 1 out of 15 healthy volunteers (20–32 years; seven having no prior fMRI experience) surpassed the 1 mm or 1° head motion threshold (with a single movement measuring 1.24°) during a similar task using the new tablet.
Thresholded maps of the critical contrast for the TMT, Part B—Part A, are shown in Figure 3. A widely distributed pattern of differential activation was apparent in both subjects. Most notably, both subjects exhibited several areas of increased activation in the left hemisphere, including the middle frontal gyrus, superior frontal gyrus, inferior frontal gyrus, superior temporal gyrus, middle temporal gyrus, and the superior parietal lobule. Also in common were smaller areas with increases in the right middle frontal gyrus and right superior parietal lobule. Most of the substantial differences between the two subjects were in the right hemisphere. Subject 1 had right‐sided increases in the precentral gyrus and superior temporal gyrus, while Subject 2 had right‐sided increases in the superior frontal gyrus, anterior cingulate gyrus, and the medial frontal gyrus, as well as decreased activation in the right inferior frontal gyrus.
Figure 3.
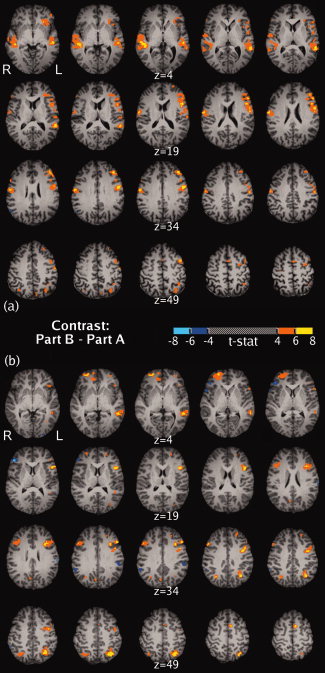
Maps of the Part B to Part A contrast, formatted similar to Figure 3 of Zakzanis et al. [2005]. (a) Subject 1. (b) Subject 2. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Having developed a robust and ergonomic tablet system, first it was important to verify that the device had no noticeable impact on MRI beyond normal variability, through a series of carefully‐conducted phantom tests. The initial result (Month 0 in Table I) was excellent and was obtained with the tablet located closer to the phantom than the typical working distance, indicating that the device has negligible impact on fMRI data quality. In repeated testing 3 and 4 months later, there was no noticeable performance degradation despite regular moves and disconnect–reconnect cycles associated with the launch of a cohort study that used the tablet both for actual fMRI and behavioral testing in a nearby laboratory. Our longer term anecdotal experience, over the time period that the present work has been developed to a scientific publication, is that the device performs consistently and is highly compatible with fMRI experiments.
Second, human experiments were conducted to provide a preliminary illustration of the utility of the tablet for fMRI studies that involve writing or drawing behavior. Our experiments focused on the TMT, as a previous fMRI study characterized a version of this test using an earlier tablet design with reduced performance [Zakzanis et al.,2005]. A detailed comparison of the brain activity observed in Zakzanis et al. [2005] with that obtained using the new tablet would be premature given the small sample size. However, in the context of the present methodological work, it is useful to discuss specific aspects of the TMT and fMRI results relevant to the usefulness of the device and to issues worth considering in future studies.
The behavioral results of the human experiment showed the expected pattern of slower performance during Part B versus Part A of the TMT. This effect is commonly attributed to the increased cognitive resources that are required when performing Part B, including general attention, response set maintenance, and especially cognitive flexibility [Kortte et al.,2002]. The overall mean time per item (Table II) was much lower than attained by Zakzanis et al. [2005] (Part A: 4327 ms, Part B: 5,056 ms approx.), and closer to published median scores for the traditional TMT (Part A: 859 ms, Part B: 1,951 ms) [Stuss et al.,1987,1988], which may be due to the improved usability afforded by the new tablet's high accuracy, sampling rate, and stability. However, the overall average difference between Part B and Part A timings was only 10%. This is slightly lower than the 17% difference found by Zakzanis et al., and far from >100% difference in the traditional TMT (but still plausible given the large variability in the normative data and our small sample size). More likely, the disparity may be related to the portion of the effect in the traditional TMT that can be explained by the 2.4 cm/item longer path traced in Part B [Gaudino et al.,1995], a situation which was not present and even somewhat reversed in our adaptation. In fact the mean length of the paths traced by the subjects was 1.3 cm/item longer in Part A than in Part B of our adaptation and this may account partially for the small observed Part B to Part A difference. While this is speculation based on a very small sample, future studies of the TMT can examine the issue in more detail through the use of a tablet for automated measurement of variables such as path length and response times.
Another consideration is that the response times in our adaptation of the TMT are not directly comparable with other TMT experiments, not only because of the different stimulus and response apparatus, but also because there were only 14 pseudorandomly positioned items to be linked within a 20‐s time limit. The traditional TMT has 25 items and no time limit, and Zakzanis et al. had 25 items in a different configuration and a 45‐s time limit. Our alterations of the TMT procedure were intended to compensate for the apparatus and time constraints, as previously described. Differences in path lengths aside, the demand differences between these three TMT variants probably also affect behavioral results. It is important to note, however, in the present experiment the Part B versus Part A performance drop was consistent across seven of eight total consecutive pairs of task blocks completed by the two subjects. The single exception was due to the especially slow performance during the very first TMT block encountered by Subject 1. Consequently, although our task adaptations likely led to quantitative differences in behavior compared to other versions of the TMT, the pattern of response times still suggest increased effort and engagement of mental resources in Part B compared to Part A for these two subjects.
The corresponding contrast between Part B and Part A in the fMRI data yielded significantly increased activation in the left frontal cortex (large areas mostly in superior and middle gyri) for both subjects. Similar to Zakzanis et al. [2005], these findings agree with existing literature that associate TMT performance with left frontal integrity. Substantial increases were also seen in the left temporal lobe (superior and middle temporal gyri) which as Zakzanis et al. noted may be involved in working memory for numbers and letters in the TMT, and highlight the important role of areas outside the frontal lobe. In addition to matching the major findings of Zakzanis et al. [2005], we also observed increased activity in the superior parietal lobule, an area frequently involved in targeted reaching and pointing tasks, and that may be linked to dorsal (“where”) stream spatial processing and motor guidance [Culham et al.,2006; Husain and Nachev,2007]. Individual differences in activation were apparent predominantly in the right hemisphere, most strikingly the right superior temporal activation in Subject 1 and the right superior frontal activation in Subject 2. It would be premature to draw conclusions given the small sample size, but the robustness (extent and level) of the individual activations in this subtle Part B to Part A contrast suggest opportunities for longitudinal, within‐subjects studies of recovery, disease progression and therapy response, as well as cross‐sectional cohort studies with respect to age and disease state.
Regarding this latter point, work is already underway in our laboratory to extend the present initial findings to a larger group of young, healthy volunteers and then to older and patient groups as part of a broader cognitive test battery. Also of methodological interest will be the characterization of head movement while using the tablet in groups other than young, healthy volunteers. Because response mode may significantly change a task [Jennings et al.,1997], the test battery will use the tablet and/or stylus for most responses to match a collection of clinical tests already in use. More generally, with straightforward software modifications the tablet can be used to study other pen‐and‐paper tests as well as other tasks involving drawing or writing in a way that approximates the original response mode.
Potential use of an fMRI‐compatible tablet extends beyond established neuropsychological assessment and rehabilitation techniques and provides increased flexibility for designing new behavioral tasks. For example, the device could be used as a generic input peripheral, similar to a computer mouse or keyboard but suitable for use within the MRI bore during fMRI. This is important because much of fMRI usage involves (but is also limited by) use of fMRI‐compatible response button pads. Such “response boxes” are sufficient for simple user input, for example choosing one response by button press from a short list based on some previously presented stimuli. However, these devices typically offer only one button per finger, for several fingers. Increasing the complexity of such devices (e.g., more buttons) becomes a “response mapping” problem requiring potentially significant training time for individuals to become comfortable making selections with a nonintuitive interface. Responses on a continuous scale can be implemented using buttons as controllers for a cursor, but this is indirect and also requires training.
A tablet provides a natural way for subjects to make a response at a specific x–y position (on‐screen and in real space) during fMRI, with high precision and accuracy. Several fMRI‐compatible trackballs and joysticks are available, but these are relatively uncommon in everyday use and lead to distinctly poorer behavioral performance than mice or tablets in a range of tasks, including pointing and dragging [MacKenzie et al.,1991] and following a path [Accot and Zhai,1999]. Computer mice have their own inherent limitations because they are not easily handled by some elderly and patient populations whereas tablets may be operated with a tool such as a stylus or simply with one's fingers. A computerized tablet replicates much of the functionality of a mouse and enables manipulation of various graphical user interface elements such as check boxes, scroll bars, dials and buttons as necessary to develop new assessments of human behavior.
The present tablet utilized a resistive touchscreen panel for reasons already outlined. Despite the utility of this device, other touch technologies may be adaptable for fMRI and provide specific desirable features, e.g., better rejection of unwanted touches, pressure sensitivity, or multiple simultaneous touches. Additionally, although unwanted head motion was not a serious problem in the present experiment (or another ongoing study), development of experimental protocols and training regimes to reduce transmission of arm motion to the head may improve the quality of fMRI results. Further mechanical design work could also help reduce motion and make using the tablet more comfortable—more like writing on paper. Finally, future studies involving virtual reality are planned that will simulate more natural environments in the fMRI scanner and will enable the study of both traditional neuropsychological tests and new types of tests in a manner that may be more applicable to real life.
Acknowledgements
Much of the early development of the tablet hardware was done in conjunction with Richard Mraz at Sunnybrook Health Sciences Centre, Toronto, ON, Canada.
REFERENCES
- Accot J, Zhai S ( 1999): Performance evaluation of input devices in trajectory‐based tasks: An application of the steering law Proceedings of the SIGCHI Conference on Human Factors in Computing Systems: The CHI is the Limit Pittsburg: PA; pp 466–472. [Google Scholar]
- Army Individual Test Battery ( 1944): Manual of Directions and Scoring. Washington, DC: War Department, Adjutant General's Office. [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavina‐Pratesi C, Singhal A ( 2006): The role of parietal cortex in visuomotor control: What have we learned from neuroimaging? Neuropsychologia 44: 2668–2684. [DOI] [PubMed] [Google Scholar]
- Ferber S, Mraz R, Baker N, Graham SJ ( 2007): Shared and differential neural substrates of copying versus drawing: A functional magnetic resonance imaging study. NeuroReport 18: 1089–1093. [DOI] [PubMed] [Google Scholar]
- Friedman L, Glover GH ( 2006): Report on a multicenter fMRI quality assurance protocol. J Magn Reson Imaging 23: 827–839. [DOI] [PubMed] [Google Scholar]
- Gaudino EA, Geisler MW, Squires NK ( 1995): Construct validity in the trail making test: What makes Part B harder? J Clin Exp Neuropsychol 17: 529–535. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nicols T ( 2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870–878. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D ( 2000): Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med 44: 162–167. [DOI] [PubMed] [Google Scholar]
- Harrington GS, Farias D, Davis CH, Buonocore MH ( 2007): Comparison of the neural basis for imagined writing and drawing. Hum Brain Mapp 28: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, Nachev P ( 2007): Space and the parietal cortex. Trends Cogn Sci 11: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JM, McIntosh AR, Kapur S, Tulving E, Houle S ( 1997): Cognitive subtractions may not add up: The interaction between semantic processing and response mode. Neuroimage 5: 229–239. [DOI] [PubMed] [Google Scholar]
- Katanoda K, Yoshikawa K, Sugishita M ( 2001): A functional MRI study on the neural substrates for writing. Hum Brain Mapp 13: 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortte KB, Horner MD, Windham WK ( 2002): The trail making test, Part B: Cognitive flexibility or ability to maintain set? Appl Neuropsychol 9: 106–109. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW ( 2004): Neuropsychological Assessment, 4th ed New York: Oxford University Press. [Google Scholar]
- MacKenzie IS, Sellen A, Buxton WAS ( 1991): A comparison of input devices in elemental pointing and dragging tasks Proceedings of the SIGCHI Conference on Human Factors in Computing Systems: Reaching Through Technology. New Orleans: LA; pp 161–166. [Google Scholar]
- Makuuchi M, Kaminaga T, Sugishita M ( 2003): Both parietal lobes are involved in drawing: A functional MRI study and implications for constructional apraxia. Brain Res Cogn Brain Res 16: 338–347. [DOI] [PubMed] [Google Scholar]
- Mraz R, Tam F, McIlroy WE, Staines WR, Black SE, Zakzanis KK, Graham SJ ( 2003): Integration of shape tape (TM) with experiments involving virtual reality and fMRI. Proc Intl Soc Magn Reson Med 11: 1723. [Google Scholar]
- Mraz R, Ferber S, Baker SN, Graham SJ ( 2004): An fMRI‐compatible writing device for investigating the neural substrates of drawing, copying and tracing. Proc Intl Soc Magn Reson Med 12: 1042. [Google Scholar]
- National Electrical Manufacturers Association ( 2008): Determination of signal‐to‐noise ratio (SNR) in diagnostic magnetic resonance imaging. NEMA Standards Publication MS 1–2008. [Google Scholar]
- Reithler J, Reithler H, van den Boogert E, Goebel R, van Mier H ( 2006): Resistance‐based high resolution recording of predefined 2‐dimensional pen trajectories in an fMRI setting. J Neurosci Methods 152: 10–17. [DOI] [PubMed] [Google Scholar]
- Shallice T, Burgess PW ( 1991): Deficits in strategy application following frontal lobe damage in man. Brain 114: 727–741. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA ( 2007): The neuroscience of remote spatial memory: A tale of two cities. Neuroscience 149: 7–27. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Poirier CA ( 1987): Comparison of three tests of attention and rapid information processing across six age groups. Clin Neuropsychol 1: 139–152. [Google Scholar]
- Stuss DT, Stethem LL, Pelchat G ( 1988): Three tests of attention and rapid information processing: An extension. Clin Neuropsychol 2: 246–250. [Google Scholar]
- Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D ( 2001): The trail making test: A study in focal lesion patients. Psychol Assess 13: 230–239. [PubMed] [Google Scholar]
- Zakzanis KK, Mraz R, Graham SJ ( 2005): An fMRI study of the trail making test. Neuropsychologia 43: 1878–1886. [DOI] [PubMed] [Google Scholar]


