Abstract
Because tool naming activates motor‐related areas in the posterior parietal cortex, it has been suggested that conceptual knowledge of tools relies on their unique manipulation patterns. However, this view is questioned by the finding that some patients impaired in retrieving manipulation knowledge of man‐made objects are still able to perform conceptual judgments on them. To address this issue, we used repetitive transcranial magnetic stimulation (rTMS) to interfere with the functioning of the anterior part of the right or left supramarginalis gyrus (SMG), a region critically involved in object‐directed actions. rTMS was delivered in healthy participants performing four judgment tasks designed to explore different aspects of manipulation and conceptual knowledge of man‐made objects. The two manipulation judgment tasks consisted in determining whether (1) two objects displayed on a computer screen are normally used by adopting a comparable hand posture, or (2) a given hand posture is appropriate to use an object. In the two conceptual judgment tasks, subjects had to decide whether (1) two objects displayed on the computer screen are normally used in the same context or (2) they are functionally related. We found that virtual lesions of left SMG interfere only with the performance of the manipulation judgment task in which subjects had to decide whether two different objects are used by adopting the same hand posture, all the other tasks being unaltered. rTMS applied over the right SMG had no effect. These results challenge the assumption that conceptual knowledge of tools is grounded upon motor representations. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: parietal, transcranial magnetic stimulation, apraxia, conceptual processing, mirror neurons, sensorimotor theories
INTRODUCTION
Since the contributions of early philosophers, the independence between conceptual processing and body experience is still a matter of debate [Barsalou,1999]. Embodied, or sensorimotor, theories of cognition postulate that the organization of conceptual knowledge of man‐made objects evolves from the motor and sensory experiences associated with their use [Warrington and Shallice,1984]. Some authors even proposed that retrieving tool conceptual knowledge requires one to simulate mentally their utilization by reactivating these sensorimotor representations [Barsalou,2008]. For example, according to this view, retrieving the concept of a hammer would entail accessing the visual features and action‐related properties of this tool via the reactivation of the sensorimotor experience associated with its use. The most radical versions of sensorimotor theories predict that conceptual knowledge of manipulable man‐made objects is fully represented, or embodied, in the sensorimotor system [Gallese and Lakoff,2005].
In monkeys, canonical neurons in the anterior intraparietal area (AIP) have been regarded as a possible component of the neural system responsible for mental simulation of object‐directed action [Gallese and Lakoff,2005]. Indeed, these canonical neurons discharge both when the monkey performs an action directed to a manipulable object, and when the monkey observes the object without acting on it. Reversible inactivation of AIP with muscimol has been shown to cause a visuomotor deficit in grasping movements, despite a preserved ability to move hands and fingers [Gallese et al.,1994]. The activity of some canonical neurons is correlated with the general purpose of actions (e.g., grasping and tearing apart), whereas others are tuned with the hand posture used to grasp the object (e.g., precision grip vs. power grip). For example, canonical neurons that respond to the execution of a precision grip will respond maximally during the observation of small or oblong objects [Murata et al.,2000; Sakata and Taira,1994]. Canonical neurons therefore fire for a given manipulation pattern as well as for the observation of an object that, if grasped, would require the same hand configuration, suggesting that the motor system is endowed with a mechanism allowing the reactivation of the associated action while viewing an object [Gallese and Lakoff,2005].
In humans, recent studies have indicated that the parietal network underlying tool use involves a phylogenetically recent area, that is, the supramarginal gyrus (SMG) [Peeters et al.,2009]. Left SMG damage leads to apraxia, an impairment of object utilization that cannot be explained by difficulties in visual recognition or low‐level motor skills [Goldenberg and Spatt,2009; Heilman and Rothi,1985; Mahon et al.,2007]. The involvement of the left SMG in object use is also supported by functional magnetic resonance imaging (fMRI) studies showing its activation during the planning and execution of object‐oriented actions [Johnson‐Frey et al.,2005]. In line with the behavior of canonical neurons, increased activation in left SMG has also been reported during tool naming [Chao and Martin,2000], tool observation [Grezes et al.,2003a; Vingerhoets,2008], and conceptual judgment on tool use [Boronat et al.,2005; Canessa et al.,2008; Kellenbach et al.,2003]. The finding that both the use and observation of tools lead to an increased SMG activation suggests that action representations may be reactivated in the absence of actual movements, providing support for embodied cognition [Gallese and Lakoff,2005]. It is unclear, however, whether the reactivation of object‐directed action mediates explicit judgments about their use.
Neuropsychological data indicate that conceptual processing of manipulable objects is independent of tool manipulation knowledge [Buxbaum et al.,2000; Caramazza and Mahon,2003]. Indeed, semantic dementia can yield deficits in conceptual knowledge of objects while leaving unaffected the ability to use them appropriately [Buxbaum,1997; Hodges et al.,2000; Negri et al.,2007a]. In some apraxic patients, conceptual knowledge of manipulable objects has been shown to be preserved, suggesting that the integrity of manipulation knowledge representations is not a prerequisite to perform conceptual judgments [Moreaud et al.,1998; Negri et al.,2007b; Rapcsak et al.,1995]. This double dissociation found in patients indicates that the activation of SMG when looking at an object may not reflect its contribution to conceptual processing.
Because fMRI studies provide correlation data, which are inadequate to address the causal relationship between SMG activation and tool processing, here, we used repetitive transcranial magnetic stimulation (rTMS) to perform a virtual lesion of left or right SMG in healthy subjects performing conceptual and manipulation judgments on man‐made manipulable objects. These tasks started with the display of a pair of pictures on a computer screen and consisted of determining whether (1) two different objects are normally used by adopting a comparable hand posture, (2) a given hand posture is suitable for using a particular object, (3) two objects are normally used in the same context, or (4) two objects are functionally related. Because left SMG is known to play a crucial role in selecting the appropriate hand posture to use a given object, we predicted that tasks 1 and 2, because they rely on manipulation judgments, should be affected by left SMG virtual lesion [Buxbaum et al.,2003,2006; Grezes et al.,2003b; Tunik et al.,2008]. Additionally, according to the most radical version of the sensorimotor theories [Gallese and Lakoff,2005; Rizzolatti et al.,2001], if SMG virtual lesions impair manipulation knowledge, the retrieval of tool conceptual knowledge should also be affected, which should result in degraded performance during tasks 3 and 4.
MATERIALS AND METHODS
Participants
Sixteen volunteers (mean age ± SD: 26.1 ± 5.4 years) without any history of neurological problems participated in the experiment. They were all right handed according to the Edinburgh handedness inventory [Oldfield,1971]. The experimental procedure was approved by the Ethics Committee of the Université catholique de Louvain, and all subjects gave their written informed consent.
Tasks Description
The experiment was performed in a dimly illuminated room. Subjects sat comfortably in an armchair, 60 cm in front of a computer screen, with their elbows flexed, and their hands half‐pronated in a relaxed position. They were instructed to remain as relaxed as possible throughout the experiment. They wore earplugs to attenuate the sound of the TMS.
Stimuli consisted of a pair of grayscale pictures (maximum 5° of visual angle) vertically arranged on a light gray background and displayed on a computer screen. Twenty‐two pairs of stimuli were selected for each task from a set of 101 pictures of familiar and manipulable man‐made objects and 22 pictures of right hands in different postures. A given picture was never presented more than once in the same task but could be used again in another task, so that, on average, 33% of pictures overlapped across tasks.
Subjects performed two manipulation and two conceptual judgment tasks. The two manipulation judgment tasks required subjects to access their manipulation knowledge of objects to determine the appropriate hand posture normally adopted to use them. More specifically, in the Hand Configuration task (Task 1), the subjects had to decide whether the same hand posture is normally adopted to use the two objects displayed on the computer screen (e.g., a saw and pitcher). In the Object–Hand task (Task 2), an object was presented on the screen together with the picture of a hand in a given posture, and the subjects had to decide whether the hand posture was compatible with using the object. It is noteworthy that the manipulation judgment tasks concerned the relative position of the fingers and not the global hand posture, defined by some authors as the relative position of the hand with respect to the body [Goldenberg,1999]. Moreover, the hand posture appropriate for the use of two distinct objects was never exactly the same but could be very similar. These two points were emphasized in the instructions given to the subjects, and they received feedback about their response accuracy during a preliminary training session (see below). Our prediction was that these two manipulation judgment tasks should be impaired by a lesion of left SMG, known to be involved in manipulation knowledge underlying object use.
The two conceptual judgment tasks related either to the contextual or the functional relationship between the two objects displayed on the screen. In the Contextual task (Task 3), subjects had to decide whether the two objects displayed on the computer screen are normally used in the same context (e.g., a wing compass and a set square), whereas the Functional task (Task 4) consisted in deciding whether the two objects are used together to achieve a common goal (e.g., a knife and a fork, see Fig. 1A). By definition, all pairs of objects associated to a common goal in the Functional task also shared the same context of use. However, distinct instructions were given to participants to test different levels of conceptual processing. In the Contextual task, participants were asked to judge the co‐occurrence of two objects in the same activity without considering their functional relationship, whereas, in the functional task, they had to determine whether the two objects are used together and simultaneously to achieve a common goal. In the Hand Configuration task, the objects of each pair were carefully selected to avoid a possible contextual or functional relationship between them. Likewise, none of the objects constituting the pairs in the Contextual and Functional tasks had the same manipulatory pattern (for a list of the stimuli used in this study, see Supporting Information).
Figure 1.
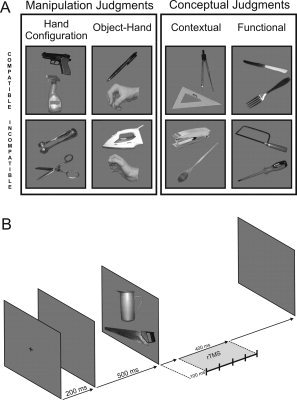
A: Examples of compatible and incompatible trials for the motor (Hand Configuration; Object–Hand) and conceptual (Contextual; Functional) tasks. B: Time course of the stimulus display and the TMS train in the main experiment.
To avoid any possible ambiguity in the responses that the subjects had to provide in the different tasks, a pilot study was performed in which we asked 10 participants to perform a compatibility judgment for the 44 pairs (22 compatible and 22 incompatible pairs) of objects/hands initially created for all four tasks following the same procedure as for the TMS experiment; only the pairs judged correctly by at least 80% of the participants were selected for the main TMS experiment. Because this criterion was met for 22 pairs (11 compatible and 11 incompatible pairs) in the Hand Configuration task and for more in other tasks, we selected the 22 pairs (22 compatible and 22 incompatible pairs) with the largest rate of intersubject agreement in the Object–Hand, the Contextual, and the Functional tasks. The frequency of use and the familiarity of the objects was also assessed by two other groups of 15 naive subjects by using a Likert scale [frequency of use: (1) never used; (5) used every day; familiarity: (1) unknown; (5) very familiar]. The different objects used in the four tasks did not differ in frequency of use (Hand Configuration task: 2.55 ± 0.25; Object–Hand task: 2.63 ± 0.36; Contextual task: 2.51 ± 0.32; Functional task: 2.72 ± 0.3, Friedman ANOVA, F(3, 42) = 1.568; P > 0.211) or in familiarity (Hand Configuration task: 4.69 ± 0.44; Object–Hand task: 4.71 ± 0.42; Contextual task: 4.67 ± 0.48; Functional task: 4.67 ± 0.41, Friedman ANOVA, F(3,42) = 0.677; P > 0.571).
Experimental Procedure
None of the subjects who participated in the pilot studies was included in the TMS experiment. Before the TMS experiment, each participant had to perform a naming task on all objects displayed during the training and TMS sessions to familiarize subjects with those objects. Then, before the first block of each task, subjects performed 10 training trials on pairs of pictures distinct from those used in the TMS experiment. The name as well as a brief reminder of the instructions of each task was given for each experimental block.
The TMS experiment consisted of 12 blocks resulting from the combination of the four tasks and three TMS sites (left SMG, right SMG, and vertex, see below). Each task was repeated three times, once per stimulation site. The order of the four tasks was counterbalanced across subjects. For each task repetition, the same set of 22 pairs was used, including the same number of compatible (11) and incompatible (11) trials; the order of the 22 trials was different in each task repetition and was pseudo‐randomized, so that the same response (“yes” or “no”) never occurred more than three times in a row.
Each trial began by displaying a cross on the screen centre for 200 ms, followed by a 500‐ms blank screen. Stimuli were then displayed in pairs, vertically, apart from the screen center, until the subject gave a response (see Fig. 1B). Subjects were asked to respond as quickly as possible, by “oui” (yes) or “non” (no). A microphone was used to detect verbal responses, and the RT was measured online by using E‐Prime V1.0 [Psychological Software Tools, 2002]. Verbal responses were preferred over manual responses to prevent possible TMS interference in‐hand response programming [Johnson‐Frey et al.,2005]. The experimenter made a note of all errors.
Transcranial Magnetic Stimulation
rTMS (10 Hz, 5 pulses, and 400 ms) was delivered using Rapid Magstim model 200 stimulator (Magstim Company, Whitland, UK) through a 35‐mm inner diameter figure‐of‐eight coil. The coil was held tangentially to the skull with the handle pointing laterally and backward and located either over left or right SMG; the vertex was used as a control site. The TMS intensity was set arbitrarily to 65% of the stimulator output, and rTMS trains were separated by at least 6 s. In each trial, the rTMS was delivered 100 ms after the picture display on the computer screen. This delay was chosen so that TMS would interfere optimally with the conceptual and manipulation processing. Indeed, regarding the conceptual processing, it has been shown to start as soon as 160 ms after the stimuli presentation, as indicated by the timing of interference or facilitation induced by action verbs on reaching movements [Boulenger et al.,2006]. An indication of the timing of the manipulation judgement is provided by a MEG study showing that the activation of the inferior parietal lobule may be observed as soon as 130 ms after the stimuli presentation during a hand laterality judgment [Kawamichi et al.,1998]. Congruently, in a previous study, a similar TMS protocol was used successfully to interfere with a motor imagery task requiring a verbal response [Pelgrims et al.,2009].
During the whole experiment, the subjects wore a closely fitting EEG cap used to mark the different stimulation sites located by means of an online neuronavigation technique using individual anatomical magnetic resonance images (MRI) [Noirhomme et al.,2004]. We targeted the anterosuperior part of SMG based on previous functional imaging studies showing an increased activation in this region during manipulation judgments [Canessa et al.,2008; Johnson‐Frey et al.,2005; Mahon et al.,2007]. After the experiment, individual coordinates of the TMS sites were normalized with respect to the Montreal Neurological Institute (MNI) brain atlas [Andres et al.,2005; Collignon et al.,2008; Davare et al.,2006]. The mean‐normalized MNI coordinates were 60 ± 4, −28 ± 7, and 47 ± 6 mm (x, y, z ± SD) for the right SMG site and −59 ± 3, −32 ± 11, and 46 ± 6 mm for the left SMG site (see Fig. 2); those coordinates are comparable to the spots of activations observed in SMG during manipulation judgments [Canessa et al.,2008; Johnson‐Frey et al.,2005; Mahon et al.,2007].
Figure 2.
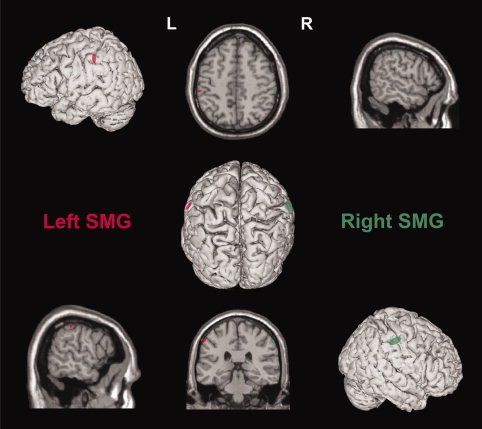
Coregistration of the stimulation sites over the right (green) and left (red) SMG. Each ellipse is centred on the mean MNI coordinates (x, y, z, mean ± SD; n = 16) of the stimulation sites in the right (60 ± 4, −28 ± 7, and 47 ± 6 mm) and left (−59 ± 3, −32 ± 11, and 46 ± 6 mm) hemisphere. The ellipse surface shows the 95% confidence interval of the normalized coordinates calculated for each subject.
Data Analysis
Statistical analyses were performed on both error rates and RT and defined as the delay between the picture presentation and the onset of verbal responses. For the RT analysis, all error trials (4.7%) and trials with an RT falling outside a 500–3500‐ms range (0.6%) were discarded. The mean RT and SE were calculated on the remaining trials (more than 93% of trials in each task, all subjects taken into account) for each subject and condition. The effects of rTMS on error rate and RT were analyzed by means of a repeated measure (RM) ANOVA with TASK (Hand Configuration, Object–Hand, Contextual, and Functional) and SITE (left SMG, right SMG, and vertex) as within‐subject factors. Post hoc comparisons were performed using Tukey's paired t‐tests. Table I gives the mean RT gathered during each TMS session for each task.
Table I.
Mean RTs and SE for each TMS condition, as a function of the task
| Site/task | Hand configuration (ms) | Object– Hand (ms) | Contextual (ms) | Functional (ms) |
|---|---|---|---|---|
| Vertex | 1052 ± 54 | 976 ± 52 | 1017 ± 41 | 1024 ± 60 |
| Left SMG | 1329 ± 91 | 943 ± 23 | 1062 ± 38 | 1012 ± 43 |
| Right SMG | 1130 ± 62 | 972 ± 45 | 1061 ± 53 | 1011 ± 61 |
RESULTS
The error rate was comparable in all four tasks, and TMS had no effects on this parameter. Indeed, in the Hand Configuration task, the percentage of errors was 5.3% and 3.8% in the Object–Hand task; it was 3.8 and 6.2% in the Contextual and Functional tasks, respectively. Neither the TASK [RM ANOVA, F(3,45) = 2.1; P > 0.1] nor the SITE [F(2,30) = 1.8; P > 0.1] had a main effect on error rate, and no interaction was found between these two factors [F(6,90) = 0.98; P > 0.1].
As far as the RT is concerned, an RM ANOVA showed a main effect of the TASK [F(3,45) = 17.161; P < 0.001] on RT and a significant two‐way interaction between TASK and SITE [F(6,90) = 5.879, P < 0.001]. The decomposition of this interaction by the TASK revealed an effect of TMS only during the Hand Configuration task [F(2,30) = 7.17; P < 0.003]. As illustrated in Figure 3, in the Hand Configuration task, the RT increased significantly following virtual lesions of left SMG (1329 ± 91 ms, mean ± SE) when compared with the vertex [1052 ± 54 ms; t(15) = 5.197, P < 0.003] and with the right SMG conditions [1130 ± 62 ms; t(15) = 3.729, P < 0.034]. No RT increase was found in the Hand Configuration task following a right SMG virtual lesion when compared with the control condition [vertex, t(15) < 1; see Table I]. In contrast to our predictions, TMS failed to impair the Object–Hand task. Indeed, the mean RT observed in the left (943 ± 23 ms), right SMG (972 ± 45 ms), and vertex (976 ± 52 ms) conditions did not differ significantly [F(2,30) = 0.335; P > 0.1] (see Fig. 3).
Figure 3.
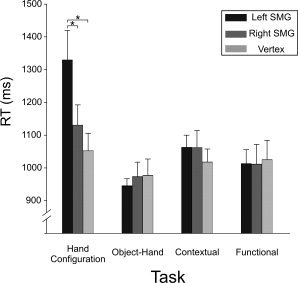
Mean RT and SE for each task and for different TMS conditions. Asterisks indicate a significant difference between two conditions (P < 0.05).
Furthermore, post hoc analysis showed that the difficulty of the four tasks was identical. Indeed, we found no significant difference between the RT gathered in the four tasks in the control condition, that is, when TMS was applied over the vertex [Hand Configuration: 1052 ± 54 ms; Object–Hand: 976 ± 52 ms; Contextual: 1017 ± 41 ms; Functional: 1024 ± 60 ms, F(3,15) = 1.581, P > 0.1].
Finally, we also performed an RM ANOVA with SITE (left vs. right SMG) and TASK as within‐subject factors on the RT difference (dRT) relative to the control condition (vertex TMS). The results showed a significant two‐way interaction [F(3,45) = 4.945, P < 0.004] that corroborated the specific effect of left SMG virtual lesions on the Hand Configuration task [F(3,45) = 10.798, P < 0.001]. Indeed, we confirmed that in the left SMG condition, the dRT was larger in the Hand Configuration task than in the three other tasks (all P < 0.001). These additional analyses also confirm the absence of effects of right SMG virtual lesions on the performance of any of the four tasks [F(3,45) = 1.201, P > 0.1].
DISCUSSION
The aim of this study was to test the prediction made by some sensorimotor theories that motor representations, which underlie the selection of the appropriate hand posture(s) to use tools, participate in their conceptual knowledge [Barsalou,2008; Gallese and Lakoff,2005]. To address this issue, we used rTMS to interfere with the function of SMG, an area known to be critically involved in object manipulation knowledge [Buxbaum et al.,2003,2006; Grezes et al.,2003b; Hamilton and Grafton,2008; Tunik et al.,2008]. Virtual lesions of SMG were induced in healthy subjects performing manipulation judgments about the hand posture required to use a given object and conceptual judgments relying on the retrieval of conceptual knowledge of object use. The finding that left SMG virtual lesions impaired the Hand Configuration task corroborated the key role played by this region in manipulation knowledge. However, the absence of a deficit in the conceptual tasks following left SMG lesions indicates that manipulation knowledge processed by this area, if any, is not a prerequisite for retrieving contextual or functional knowledge of tool use. In addition, the absence of effects of the right SMG virtual lesion confirms the left hemisphere dominance in manipulation judgments [Johnson‐Frey et al.,2005], a finding consistent with the well‐known left dominance for motor control [Sabate et al.,2004].
Although the role of SMG in tool use is widely accepted [Buxbaum et al.,2003,2006; Tunik et al.,2008], the exact processes performed by this area remain unknown. Given its distinct position in the dorsal visual stream, it is unlikely that the left SMG is involved in low‐level visual processes dedicated to action [Kroliczak et al.,2008]. This hypothesis is further ruled out by the absence of TMS interference in the conceptual judgment tasks, which used the same display as the Hand Configuration task. A more plausible hypothesis is that left SMG determines the appropriate hand posture to handle and uses tools based on their extrinsic (orientation) and intrinsic (size and shape) visual properties. This mechanism, known as the “affordance,” is probably automatic and refers to all actions that are physically possible with the object.
Interestingly, affordances have been shown to be influenced by the context in which an action takes place [Ellis and Tucker,2000; Gibson,1977,1979]. For example, subjects are slower to respond to the presentation of an object with a hand movement when this movement is incompatible with the posture adopted to use it (e.g., a power grip in response to the display of a small object). In a functional neuroimaging study using a comparable task, the left SMG activation was found larger in incompatible trials than in compatible ones, suggesting that that region may play a role in detecting and/or orienting attention to object affordances when the hand movement initially programmed is incompatible with the visual properties of the object [Grezes et al.,2003a]. However, one limitation of this interpretation is the finding that left SMG is also activated when an action is performed in the absence of object vision, for example, in response to its name [Johnson‐Frey et al.,2005]. The hypothesis that left SMG processes the object affordances is also insufficient to account for the recent finding that this brain region is more active when observing familiar than unfamiliar objects with similar affordance [Vingerhoets,2008]. On the basis of a comprehensive analysis of the cognitive processes underlying apraxia, some authors have proposed the existence of a memory of learned gestures whose representation captures the interactions between the objects and hand [Heilman et al.,1982; Rothi et al.,1985]. Because of the correlation between the deficits in producing or discriminating gestures oriented to object use and left SMG lesion [Buxbaum et al.,2003; Heilman et al.,1982] or activation [Buxbaum et al.,2003,2006], left SMG could underlie those representations. According to this view, the deficit observed in the Hand Configuration task after a virtual lesion of left SMG would reflect a transient inability to access, in memory, the repertoire of gestures underlying tool use.
One puzzling aspect of the results is that, in contrast to our predictions, virtual lesions of left SMG only altered the Hand Configuration task, leaving the Object–Hand task unaffected. This absence of effect of left SMG lesion on the Object–Hand task is even more surprising that we have already shown that its performance leads to increased corticospinal excitability, strongly suggesting an involvement in motor imagery [Pelgrims et al.,2005]. One possible explanation for this apparent discrepancy is that the Object–Hand task, because of the display of a hand picture, could be performed by using visual imagery when manipulation knowledge is impaired. For instance, in a series of neuropsychological studies, Tomasino and colleagues [Tomasino and Rumiati,2004; Tomasino et al.,2004] have shown that patients with left hemispheric lesions experience difficulties in doing mental object rotations when perceived as a result of their own manual activity, whereas they are still able to perform the task when the instructions emphasize the use of visual imagery. Accordingly, we recently demonstrated that visual imagery relies on distinct brain areas in the superior parietal lobule [Pelgrims et al.,2009].
Critically, our results are at variance with some clinical studies reporting a relationship between the capacity to use objects and the ability to retrieve conceptual information related to the same objects (e.g. [Coccia et al.,2004; Hodges et al.,2000; Mahon et al.,2007]). In some studies, the interpretation of this association was made difficult, because different objects or gestures were used in recognition and production tasks (e.g. [Pazzaglia et al.,2008]). However, if such a relationship between the capacity to use objects and the ability to retrieve their conceptual knowledge actually exists, it may reflect the inability for the patients to use action simulation as a compensatory strategy for recognizing objects or gestures. That strategy would rely on the interactions between motor and conceptual knowledge, so that the mental simulation of actions would help to activate the corresponding conceptual representation in memory. Accordingly, the performance in naming objects was found positively correlated with the ability to use the same objects only in patients whose temporal lesions, responsible for the conceptual impairment, extended to SMG [Mahon et al.,2007].
The view that, in healthy subjects, conceptual knowledge depends on intact manipulation knowledge is challenged by the present result that a left SMG virtual lesion alters access to motor knowledge but not conceptual processes. This finding questions the interpretation of the SMG activations reported by many functional neuroimaging studies in tasks involving conceptual processing (e.g. [Boronat et al.,2005; Chao and Martin,2000; Vingerhoets,2008]. We propose that, instead of unveiling an embodied representation of tool concept, the recruitment of manipulation knowledge during conceptual judgments on object use could be interpreted in the light of a pragmatic rather than a semantic theory of tool knowledge [Mahon and Caramazza,2009]. Therefore, the activation of motor‐related areas consequent to object observation could reflect a strategy used by the nervous system to be ready to interact with this object as soon as possible and in any circumstances [Negri et al.,2007b].
Because we performed lesions of either left or right SMG, a last issue raised by our results is the lateralization of manipulation knowledge. Indeed, the finding that only left SMG virtual lesions altered the motor judgment task may seem at odds with some studies showing an equal contribution of both hemispheres in mental rotation of hand postures [Pelgrims et al.,2009]. However, except perhaps for complex movements such as sequential movements involving multiple fingers [Haaland et al.,2004; Verstynen et al.,2005], an involvement of the two hemispheres seems to be specific to intransitive gestures and may explain the higher resistance of those movements to unilateral brain damage [Johnson‐Frey et al.,2005]. In contrast, processes involving transitive gestures performed on familiar and novel tools have been recurrently assigned to the left hemisphere and in particular to a left temporo‐fronto‐parietal network [Frey et al.,2005; Goldenberg and Hagmann,1998; Johnson‐Frey,2004], whatever the handedness and the hand used [Frey et al.,2005]. The left dominance for tool use has also been demonstrated during movement planning [Johnson‐Frey et al.,2005]. These data are consistent with the classical observation that ideomotor apraxia, characterized by a deficit in pantomiming tool use and, to some extent, in using tools [Leiguarda and Marsden,2000], typically results from a left brain damage [Zadikoff and Lang,2005]. Altogether, this suggests that the lateralization of motor processes in SMG would depend on the type of movements performed: SMG would be involved bilaterally to perform or simulate intransitive gestures, whereas only the left SMG would be concerned with object–hand interactions.
A central question in contemporary neuroscience is the role of sensorimotor experience in the representations of conceptual knowledge of actions and of manipulable objects. Our results showed that manipulation knowledge as supported by left SMG is not a prerequisite to access contextual and functional information about object use, suggesting that some features of actions performed on objects can be retrieved without relying on their manipulation knowledge. Although it cannot be excluded that the contribution of manipulation knowledge to conceptual processing could take place at a different level and/or in a different cortical area, such an assumption encourages sensorimotor theories to make more precise hypotheses about processes shared by manipulation abilities and conceptual knowledge.
Acknowledgements
M.A. is a Postdoctoral Researcher at the Fonds de la Recherche Scientifique (FRS‐FNRS). B.P. is a Research Fellow at the Fonds de la Recherche Scientifique (FRS‐FNRS).
REFERENCES
- Andres M, Seron X, Olivier E ( 2005): Hemispheric lateralization of number comparison. Brain Res Cogn Brain Res 25: 283–290. [DOI] [PubMed] [Google Scholar]
- Barsalou LW ( 1999): Perceptual symbol systems. Behav Brain Sci 22: 577–609; discussion 610–660. [DOI] [PubMed] [Google Scholar]
- Barsalou LW ( 2008): Grounded cognition. Annu Rev Psychol 59: 617–645. [DOI] [PubMed] [Google Scholar]
- Boronat CB, Buxbaum LJ, Coslett HB, Tang K, Saffran EM, Kimberg DY, Detre JA ( 2005): Distinctions between manipulation and function knowledge of objects: Evidence from functional magnetic resonance imaging. Brain Res Cogn Brain Res 23: 361–373. [DOI] [PubMed] [Google Scholar]
- Boulenger V, Roy AC, Paulignan Y, Deprez V, Jeannerod M, Nazir TA ( 2006): Cross‐talk between language processes and overt motor behavior in the first 200 msec of processing. J Cogn Neurosci 18: 1607–1615. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ ( 1997): The role of semantic memory in object use. Cogn Neuropsychol 14: 219–254. [Google Scholar]
- Buxbaum LJ, Veramonti T, Schwartz MF ( 2000): Function and manipulation tool knowledge in apraxia: Knowing ‘what for’ but not ‘how.’ Neurocase 6: 83–97. [Google Scholar]
- Buxbaum LJ, Sirigu A, Schwartz MF, Klatzky R ( 2003): Cognitive representations of hand posture in ideomotor apraxia. Neuropsychologia 41: 1091–1113. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Tang K, Detre JA ( 2006): Neural substrates of knowledge of hand postures for object grasping and functional object use: Evidence from fMRI. Brain Res 1117: 175–185. [DOI] [PubMed] [Google Scholar]
- Canessa N, Borgo F, Cappa SF, Perani D, Falini A, Buccino G, Tettamanti M, Shallice T ( 2008): The different neural correlates of action and functional knowledge in semantic memory: An FMRI study. Cereb Cortex 18: 740–751. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Mahon BZ ( 2003): The organization of conceptual knowledge: The evidence from category‐specific semantic deficits. Trends Cogn Sci 7: 354–361. [DOI] [PubMed] [Google Scholar]
- Chao LL, Martin A ( 2000): Representation of manipulable man‐made objects in the dorsal stream. Neuroimage 12: 478–484. [DOI] [PubMed] [Google Scholar]
- Coccia M, Bartolini M, Luzzi S, Provinciali L, Lambon Ralph MA ( 2004): Semantic memory is an amodal, dynamic system: Evidence from the interaction of naming and object use in semantic dementia. Cogn Neuropsychol 21: 513–527. [DOI] [PubMed] [Google Scholar]
- Collignon O, Davare M, De Volder AG, Poirier C, Olivier E, Veraart C ( 2008): Time‐course of posterior parietal and occipital cortex contribution to sound localization. J Cogn Neurosci 20: 1454–1463. [DOI] [PubMed] [Google Scholar]
- Davare M, Andres M, Cosnard G, Thonnard JL, Olivier E ( 2006): Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J Neurosci 26: 2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Tucker M ( 2000): Micro‐affordance: The potentiation of components of action by seen objects. Br J Psychol 91 ( Pt 4): 451–471. [DOI] [PubMed] [Google Scholar]
- Frey SH, Funnell MG, Gerry VE, Gazzaniga MS ( 2005): A dissociation between the representation of tool‐use skills and hand dominance: Insights from left‐ and right‐handed callosotomy patients. J Cogn Neurosci 17: 262–272. [DOI] [PubMed] [Google Scholar]
- Gallese V, Lakoff G ( 2005): The brain's concepts: The role of the sensory‐motor system in conceptual knowledge. Cogn Neuropsychol 22: 455–479. [DOI] [PubMed] [Google Scholar]
- Gallese V, Murata A, Kaseda M, Niki N, Sakata H ( 1994): Deficit of hand preshaping after muscimol injection in monkey parietal cortex. Neuroreport 5: 1525–1529. [DOI] [PubMed] [Google Scholar]
- Gibson JJ ( 1977): The theory of affordances perceiving, acting, and knowing: Toward an eclological psychology. Hillsdate, NJ: Lawrence Erlbaum. [Google Scholar]
- Gibson JJ ( 1979): The Ecological Approach to Visual Perception. Boston: Houghton Mifflin. [Google Scholar]
- Goldenberg G ( 1999): Matching and imitation of hand and finger postures in patients with damage in the left or right hemispheres. Neuropsychologia 37: 559–566. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Hagmann S ( 1998): Tool use and mechanical problem solving in apraxia. Neuropsychologia 36: 581–589. [DOI] [PubMed] [Google Scholar]
- Goldenberg G, Spatt J ( 2009): The neural basis of tool use. Brain 132( Pt 6): 1645–1655. [DOI] [PubMed] [Google Scholar]
- Grezes J, Armony JL, Rowe J, Passingham RE ( 2003a): Activations related to “mirror” and “canonical” neurones in the human brain: An fMRI study. Neuroimage 18: 928–937. [DOI] [PubMed] [Google Scholar]
- Grezes J, Tucker M, Armony J, Ellis R, Passingham RE ( 2003b): Objects automatically potentiate action: An fMRI study of implicit processing. Eur J Neurosci 17: 2735–2740. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Elsinger CL, Mayer AR, Durgerian S, Rao SM ( 2004): Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J Cogn Neurosci 16: 621–636. [DOI] [PubMed] [Google Scholar]
- Hamilton AF, Grafton ST ( 2008): Repetition suppression for performed hand gestures revealed by fMRI. Hum Brain Mapp 30: 2898–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Rothi LJG. 1985. Apraxia In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. New York: Oxford University Press; pp 131–150. [Google Scholar]
- Heilman KM, Rothi LJ, Valenstein E ( 1982): Two forms of ideomotor apraxia. Neurology 32: 342–346. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Bozeat S, Lambon Ralph MA, Patterson K, Spatt J ( 2000): The role of conceptual knowledge in object use evidence from semantic dementia. Brain 123 ( Pt 9): 1913–1925. [DOI] [PubMed] [Google Scholar]
- Johnson‐Frey SH ( 2004): The neural bases of complex tool use in humans. Trends Cogn Sci 8: 71–78. [DOI] [PubMed] [Google Scholar]
- Johnson‐Frey SH, Newman‐Norlund R, Grafton ST ( 2005): A distributed left hemisphere network active during planning of everyday tool use skills. Cereb Cortex 15: 681–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamichi H, Kikuchi Y, Endo H, Takeda T, Yoshizawa S ( 1998): Temporal structure of implicit motor imagery in visual hand‐shape discrimination as revealed by MEG. Neuroreport 9: 1127–1132. [DOI] [PubMed] [Google Scholar]
- Kellenbach ML, Brett M, Patterson K ( 2003): Actions speak louder than functions: The importance of manipulability and action in tool representation. J Cogn Neurosci 15: 30–46. [DOI] [PubMed] [Google Scholar]
- Kroliczak G, McAdam TD, Quinlan DJ, Culham JC ( 2008): The human dorsal stream adapts to real actions and 3D shape processing: A functional magnetic resonance imaging study. J Neurophysiol 100: 2627–2639. [DOI] [PubMed] [Google Scholar]
- Leiguarda RC, Marsden CD ( 2000): Limb apraxias: Higher‐order disorders of sensorimotor integration. Brain 123 ( Pt 5): 860–879. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A ( 2009): Concepts and categories: A cognitive neuropsychological perspective. Annu Rev Psychol 60: 27–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Milleville SC, Negri GA, Rumiati RI, Caramazza A, Martin A ( 2007): Action‐related properties shape object representations in the ventral stream. Neuron 55: 507–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreaud O, Charnallet A, Pellat J ( 1998): Identification without manipulation: A study of the relations between object use and semantic memory. Neuropsychologia 36: 1295–1301. [DOI] [PubMed] [Google Scholar]
- Murata A, Gallese V, Luppino G, Kaseda M, Sakata H ( 2000): Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J Neurophysiol 83: 2580–2601. [DOI] [PubMed] [Google Scholar]
- Negri GA, Lunardelli A, Reverberi C, Gigli GL, Rumiati RI ( 2007a): Degraded semantic knowledge and accurate object use. Cortex 43: 376–388. [DOI] [PubMed] [Google Scholar]
- Negri GA, Rumiati RI, Zadini A, Ukmar M, Mahon BZ, Caramazza A ( 2007b): What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cogn Neuropsychol 24: 795–816. [DOI] [PubMed] [Google Scholar]
- Noirhomme Q, Ferrant M, Vandermeeren Y, Olivier E, Macq B, Cuisenaire O ( 2004): Registration and real‐time visualization of transcranial magnetic stimulation with 3‐D MR images. IEEE Trans Biomed Eng 51: 1994–2005. [DOI] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pazzaglia M, Smania N, Corato E, Aglioti SM ( 2008): Neural underpinnings of gesture discrimination in patients with limb apraxia. J Neurosci 28: 3030–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters R, Simone L, Nelissen K, Fabbri‐Destro M, Vanduffel W, Rizzolatti G, Orban GA ( 2009): The representation of tool use in humans and monkeys: Common and uniquely human features. J Neurosci 29: 11523–11539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelgrims B, Andres M, Olivier E ( 2005): Motor imagery while judging object‐hand interactions. Neuroreport 16: 1193–1196. [DOI] [PubMed] [Google Scholar]
- Pelgrims B, Andres M, Olivier E ( 2009): Double dissociation between motor and visual imagery in the posterior parietal cortex. Cereb Cortex 19: 2298–2307. [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Ochipa C, Anderson KC, Poizner H ( 1995): Progressive ideomotor apraxia: Evidence for a selective impairment of the action production system. Brain Cogn 27: 213–236. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V ( 2001): Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661–670. [DOI] [PubMed] [Google Scholar]
- Rothi LJ, Heilman KM, Watson RT ( 1985): Pantomime comprehension and ideomotor apraxia. J Neurol Neurosurg Psychiatry 48: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabate M, Gonzalez B, Rodriguez M ( 2004): Brain lateralization of motor imagery: Motor planning asymmetry as a cause of movement lateralization. Neuropsychologia 42: 1041–1049. [DOI] [PubMed] [Google Scholar]
- Sakata H, Taira M ( 1994): Parietal control of hand action. Curr Opin Neurobiol 4: 847–856. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Rumiati RI ( 2004): Effects of strategies on mental rotation and hemispheric lateralization: Neuropsychological evidence. J Cogn Neurosci 16: 878–888. [DOI] [PubMed] [Google Scholar]
- Tomasino B, Vorano L, Skrap M, Gigli G, Rumiati RI ( 2004): Effects of strategies of mental rotation performed by unilateral brain damaged patients. Cortex 40: 197–199. [DOI] [PubMed] [Google Scholar]
- Tunik E, Lo OY, Adamovich SV ( 2008): Transcranial magnetic stimulation to the frontal operculum and supramarginal gyrus disrupts planning of outcome‐based hand‐object interactions. J Neurosci 28: 14422–14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstynen T, Diedrichsen J, Albert N, Aparicio P, Ivry RB ( 2005): Ipsilateral motor cortex activity during unimanual hand movements relates to task complexity. J Neurophysiol 93: 1209–1222. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G ( 2008): Knowing about tools: Neural correlates of tool familiarity and experience. Neuroimage 40: 1380–1391. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T ( 1984): Category specific semantic impairments. Brain 107 ( Pt 3): 829–854. [DOI] [PubMed] [Google Scholar]
- Zadikoff C, Lang AE ( 2005): Apraxia in movement disorders. Brain 128( Pt 7): 1480–1497. [DOI] [PubMed] [Google Scholar]


