Abstract
The effect of autonomic perturbation (AP) on the central nervous system functioning is still largely unknown. Using an automated neck suction device to stimulate the carotid mechanoreceptors in the carotid sinus (parasympathetic pathway), operated synchronously with functional magnetic resonance imaging (fMRI) acquisition, we investigated the effects of AP on the activity of the brain at rest and when engaged in a visuo‐spatial attention task. ECG was always recorded to index changes in autonomic function. At rest, AP induced increased activation in the insula and in the amygdala, which have been previously associated with the autonomic control and emotion processing, as well as in the caudate nucleus and in the medial temporal cortex, both implicated in cognitive functions. Despite a preserved performance during visuo‐spatial attention task, AP induced increased reaction times and a positive modulation on the activation of the right posterior parietal cortex, the occipital cortex, the periaquiductal gray, and nuclei of the brainstem. We speculate that this modulation of brain activity represents, at different anatomical levels, a compensation mechanism to maintain cognitive efficiency under parasympathetic stimulation, which is traditionally considered as the system for energy regain and storage. In conclusion, this study provides the first evidence of a dynamic interaction between AP and higher level functions in humans. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: autonomic nervous system, cognition, emotion, fMRI, parasympathetic stimulation
INTRODUCTION
The autonomic nervous system (ANS) is the division of the nervous system that subserves the maintenance of the homeostatic needs (i.e., blood pressure, heart and breathing rates, body temperature, digestion, metabolism, and other processes), by continuously interacting with other brain functions, including perception, cognition, and emotion [Critchley,2009]. According to the traditional view, ANS operates through two subdivisions, one regulating the “fight and flight” responses, in case of threat or stressful situations (sympathetic system), and the other one controlling our bodily processes during ordinary situations (parasympathetic division), allowing energy regain and storage. The ANS supplies each type of target organ via separate pathways that consist of sets of pre‐ and postganglionic neurones with distinct patterns of reflex activity [Kandel et al.,2000]. Both conscious and unconscious visceral sensations reach brain structures that are known to be implicated or to modulate, different networks involved in cognition and emotion processing [Critchley,2009]. On the other hand, the final visceral output of the ANS is strongly affected by individual cognitive and emotional states [Craig,2002; Damasio,2003; Gray et al.,2010; Porges,2007]. Thus, human mental processes influence the physiological state of the body and changes in the body's physiology influence thoughts, feelings, and motivational behavior. However, in this complex picture, the mechanisms underlying the mutual interaction between the ANS and the higher level functions (cognition and emotion processing) still remain largely unknown.
The aim of the current functional magnetic resonance imaging (fMRI) study was to investigate the effect of the autonomic perturbation (AP), through direct stimulation of carotid baroreceptors (parasympathetic pathway) on the central nervous system (CNS), both at rest and when engaged in a cognitive task. For this purpose, we developed a magnetic resonance compatible neck suction (NS) technique for the direct stimulation of the carotid baroreceptors located at the medial–adventitial border of blood vessels in the carotid sinus bifurcation (Fig. 1). The NS stimulation causes an increase in carotid sinus transmural pressure, which in turn stretches the carotid baroreceptors, thus mimicking the delivery of a hypertensive stimulus. Stimulation of these receptors provokes AP through the afferent parasympathetic pathway (glossopharyngeal nerve and nucleus of the solitary tract) and causes an increase in efferent parasympathetic activity and a decrease in efferent sympathetic activity [Fadel et al.,2003]. These mechanoreceptors function as the sensors in a negative feedback control system that regulates the beat‐to‐beat changes in arterial pressure and heart rate, thus modulating the autonomic neural outflow. In a group of healthy young individuals, we perturbed the ANS using either efficacious or nonefficacious stimuli (control condition), both at rest (experiment 1) and while performing a high‐level cognitive task (experiment 2). ECG recording (to monitor the RR interval series over time) was used to verify whether the ANS stimulation was successful. For the purposes of this exploratory study, we chose to investigate the visuo‐spatial attention function, which represents a basic cognitive function likely to be modulated by ANS perturbation.
Figure 1.
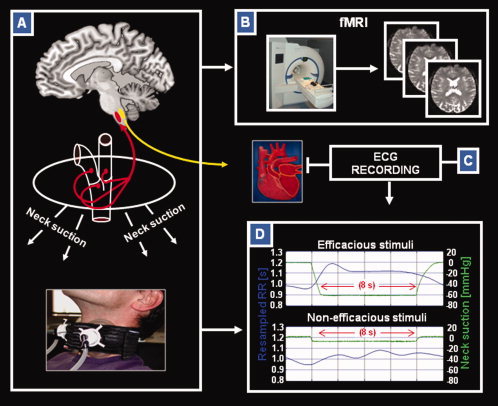
Neck suction (NS) technique used for fMRI investigation. Schematic illustration showing methods and devices used in the study. (A) Representation of the NS technique based on an MR compatible system (bottom); NS induces stimulation of the mechanoreceptors located in the carotid sinus bilaterally, thus producing an autonomic perturbation trough parasympathetic afferents (as detectable by cardiac effects); (B) 3T magnetic resonance scanner equipped for functional MRI (fMRI) experiments; (C) MR‐compliant system for ECG recording; (D) heart‐period stimulus response curve (shown in blue) and NS pressure (shown in green) for efficacious (−60 mm Hg) and nonefficacious (−10 mm Hg) stimuli. In this example, stimuli response curves were collected for one volunteer during experiment 1. Each curve is obtained as an average over 50 NS pulses at −60 mm Hg and 30 NS pulses at −10 mm Hg. Each stimulus has a duration of 8 s. The left axis refers to the heart period values, while the right axis reports the suction pressure applied to the neck. See text for further details.
MATERIALS AND METHODS
Participants
This study involved a group of 15 right‐handed healthy volunteers (all men; mean age = 23.0 years; SD = 3.4; range, 20–25) with no history of medical or psychiatric disorders, autonomic dysfunction, or other major clinical conditions.
They were all recruited from a pool of psychology and engineering students. Local ethical committee approval and written informed consent were obtained from all subjects before study initiation.
MRI‐Compliant NS Technique
Device description
The application of NS increases carotid sinus transmural pressure, which in turn leads to the carotid baroreceptor stretching, thus provoking an AP through the parasympathetic pathway. Various neck chamber devices have been proposed so far. The main difference is between collars that enclose the entire neck [Eckberg and Sleight,1992] and smaller individual cuffs. A review on the historical evolution and the state of the art of the neck suction (NS) technique can be found in Cooper and Hainsworth [2009]. Our NS device uses individual cuffs (Fig. 1A), with appropriate modifications needed to comply with the MRI environment. In this study, the size of the cuffs was chosen according to the size of the neck of each subject. The pressure was set by controlling the aspiration level of a vacuum source, by an analog output line of an acquisition card (NI DaqCard NI USB 6212, National Instrument). This signal served as reference input for a feedback control circuit driving the vacuum source motor. Because the vacuum source can only generate subatmospheric pressure, an air leakage was added to the line. The vacuum source and its controlling unit were placed in the MRI control room. A 5‐m length silicon tube connected the pump with the neck collar, passing through a waveguide of the Faraday cage. In addition, the actual pressure in the neck collar was continuously monitored by a pressure transducer. Because two cuffs were used, the pressure line was split in two parallel arms, and the pressures were monitored independently in both cuffs. All the digital circuitry as well as the electromechanic components were placed in the control room. Pressure transducers were the only electronic components of the NS device included in the cuffs. Their analog conditioning amplifiers were placed in a RF‐shielded box and connected to the electronics in the control room using the patch panel RF filters.
NS stimulation delivery
In both experiments, NS was delivered by 8‐s duration pulses (pressure: −60 mm Hg for efficacious and −10 mm Hg for nonefficacious stimuli), in order to reduce the risk of response accommodation for the mechanoreceptors. It was previously speculated that alteration afferent nerve traffic from the carotid baroreceptors is not maintained during sustained stimuli [Eckberg and Sleight,1992]. Although Ogoh et al. [2003] have demonstrated that significant carotid baroreceptor adaptation to static NS does not occur over 20‐s intervals, this still remains a controversial issue. In experiment 1, we used an event‐related design (see below), characterized by individual NS pulses (duration = 8 s) followed by an average random interval of 4 s (ranging from 3.0 to 5.2 s), in which the cuffs were maintained at atmospheric pressure. In experiment 2, where a block‐size design was adopted, each block included five consecutive NS pulses separated from each other by 4‐s intervals at atmospheric pressure. This experimental procedure, together with the continuous ECG recording, makes us confident about the appropriate delivery of NS stimulation in both fMRI experiments. A preliminary investigation run outside the scanner demonstrated that subjects were unable to distinguish between efficacious and nonefficacious NS stimulation.
Physiological signal acquisition and analysis
For the whole duration of fMRI experiments, acquisition and analog conditioning of ECG, pulseoximetry, and respiration were obtained using an MRI‐compliant system (Biopac Systems Ins, CA). The pressure transducers and the conditioning amplifiers of the NS device were housed in a shielded box, located ∼2‐m apart from the RF coil. ECG, respiration, pulseoximetry, and pressures in the left and right cuffs were monitored using the DaqCard NI USB 6212, located in the MRI control room. Signal acquisition and pulse generation were synchronized to the imaging acquisition, using a RS‐232 trigger from the scanner.
RR interval series were obtained from the R‐mode ECG signal of the Biopac, using a threshold algorithm. Each series was visually searched and manually corrected for artefacts or detection errors. RR intervals from each subject were arranged as a discrete event series (DES), that is, each RR interval was plotted as a function of the R‐wave occurrence time. The DES was interpolated using a piece‐wise polynomial (cubic‐spline) and uniformly resampled at 10 Hz (resampled RR). A cardiac‐response curve was obtained by synchronous averaging of the individual RR response to each stimulation resampled RR, corresponding to each NS pulse. Curves were aligned to the time of 5 mm Hg pressure drop and averaged (Fig. 1D). No motion artefacts were detected when the NS was applied using the two‐cuff device. Physiological monitoring (ECG, pulse oximetry and respiration) and NS delivery did not induce an appreciable increase in radio frequency noise.
Cardiac response was assessed by comparing the RR interval 2 s before the application of each of the NS pulses, to the RR interval during the NS pulse.
fMRI paradigms
Experiment 1
An event‐related design including 80 miniblocks with a duration of 8 s each, randomly administered over 16 min of fMRI acquisition (461 volumes), was used in this experiment (Fig. 2A). Fifty miniblocks consisted of NS pulses with a pressure of −60 mm Hg (efficacious stimulation), and 30 miniblocks consisted of NS pulses with a pressure of −10 mm Hg (nonefficacious stimulation). During the whole experiment, the efficacy and nonefficacy of each miniblock to induce or not AP were monitored by continuous ECG recording. No visual stimulation was delivered, and no active cooperation from studied subjects was required. Subjects were instructed to lie in the scanner, to keep their eyes closed, not to think of anything in particular, and not to fall asleep. Activity of the brain was studied while randomly administering 50 efficacious (suction pressure: −60 mm Hg) and 30 nonefficacious (suction pressure: −10 mm Hg) miniblocks of NS. Cardiac response (i.e., the average increase of the RR interval respect to baseline) was 49 ms (SD = 60) for efficacious and 16 ms (SD = 20) for nonefficacious NS stimuli (P = 0.01). Subjects were unable to distinguish between the two types of stimulation, as assessed in a preliminary study run outside the scanner.
Figure 2.
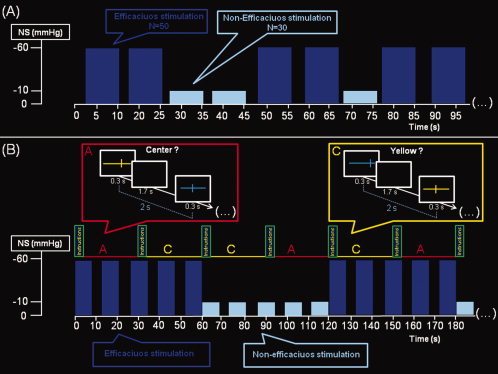
fMRI paradigms. Schematic representation of the fMRI paradigms used to investigate the effect of autonomic perturbation on the brain in subjects at rest (experiment 1; panel A), and when engaged in performing a visuo‐spatial attention task (experiment 2; panel B). In experiment 1, subjects were required to lie still in the scanner, without thinking of anything in particular. Fifty efficacious (−60 mm Hg) and 30 nonefficacious (−10 mm Hg) neck suction (NS) pulses with a duration of 8 s each were randomly administered over 16 min of continuous fMRI data acquisition. Each NS pulse was followed by a random interpulse interval at atmospheric pressure ranging from 3.0 to 5.2 s. In experiment 2 (total fMRI acquisition = 16 min), the visuo‐spatial attention task included 32 blocks, with a duration of 30 s each, half of them requiring the active task (A = active), and half of them requiring a control condition (C = control). Each block started with instructions (duration = 3.2 s, followed by 0.8 s of black screen) and included the presentation of 14 stimuli (duration = 0.3 s each) separated from each other by intervals of black screen (duration = 1.7 s each). The effective intertrial interval was 2 s. The total number of trials was 896, with 224 repetitions for each of four trial‐types (active task under efficacious NS stimulation, active task under nonefficacious NS stimulation, control task under efficacious NS stimulation, and control task under nonefficacious NS stimulation). During the active condition, subjects were requested to press (as soon as possible) a button with the right index for items presenting with a symmetrical bisection of a colored horizontal line by a shorter vertical line and not to press in case of asymmetrical bisection. In the control condition, subjects were requested to press (as soon as possible) the same button when the items were presented in yellow and not to press in any other case. During task performance, NS was delivered in 60 s duration blocks, alternating half of them with a pressure of −60 mm Hg (efficacious stimulation), and the other half with a pressure of −10 mm Hg (nonefficacious stimulation). The active and control 30 s duration blocks were pseudo‐randomly administered in order to obtain half of them under efficacious and half under nonefficacious NS stimulation. Each block of NS consisted of 8 s duration pulses (either −60 or −10 mm Hg) interleaved with 4‐s duration intervals of suction release (cuffs at atmospheric pressure) to avoid the risk of accommodation for the mechanoreceptors. This allowed us to obtain a continuous stimulation of the mechanoreceptors over the entire block of efficacious NS stimulation. See text for further details.
Experiment 2
A block‐size design was used for this second experiment, including AP by NS stimulation overlaid to performance of a visuo‐spatial attention task (Fig. 2B). NS stimulation was delivered in blocks of 60 s, alternating efficacious (−60 mm Hg) and nonefficacious (−10 mm Hg) conditions, as monitored by continuous ECG recording. As mentioned earlier, to avoid the risk of accommodation for the mechanoreceptors in the carotid sinus, each NS block included 8‐s duration pulses (n = 5) of negative pressure (−60 mm Hg for efficacious and −10 mm Hg for nonefficacious stimulations), separated from each other by 4‐s intervals of release to atmospheric pressure. The visuo‐spatial attention task was administered in blocks with a duration of 30 s each. In one half of them, subjects were required to respond by key‐press when presented with items showing a symmetrical bisection of a colored horizontal line by a shorter vertical line and not to press in case of nonsymmetrical bisection (active condition). In the other half, subjects were required to respond by key‐press to items presented in yellow and not to respond to items presented in other colors (control condition). After a 2‐s presentation of written instructions (i.e., “press if centre” or “press if yellow,” according to the condition), each 30‐s block included 14 stimuli with an intertrial interval of 2 s. The total number of trials was 896, with 224 repetitions for each of four trial‐types. The total duration of the fMRI experiment was 16 min.
In both conditions (active and control), the length and position of the horizontal line varied from trial to trial, preventing subjects from using some frame of reference other than the line (e.g., the borders of the screen or retinal position) to perform the task. Active and control blocks were pseudo‐randomly administered in order to obtain half of them under efficacious and half under nonefficacious NS stimulation. In summary, experiment 2 had a 2 × 2 factorial design including the following experimental conditions: (1) active task under efficacious NS stimulation; (2) active task under nonefficacious NS stimulation; (3) control task under efficacious NS stimulation; (4) control task under nonefficacious NS stimulation.
fMRI Acquisition and Analysis
All imaging was obtained using a head‐only 3.0T MR scanner (Siemens Magnetom Allegra, Siemens Medical Solutions, Erlangen, Germany), equipped with a circularly polarized transmit‐receive coil. The maximum gradient strength is 40 mT m−1, with a maximum slew rate of 400 mT m−1 ms−1. Functional images were collected by echo‐planar T2* sequence using blood oxygenation level‐dependent (BOLD) contrast. In both experiments 1 and 2 (see below), each acquired volume consisted of 32 axial slices with a 3‐mm thickness and a 1.5‐mm distance factor in order to cover the entire brain, with an effective repetition time of 2.08 s. Administration of NS stimuli (in both experiments) and visuo‐spatial attention task timing (in experiment 2) was synchronized with fMRI data acquisition. The ratio of interscan to interstimulus interval ensured that slices were sampled at different phases relative to stimulus onset. In both experiments, the first four volumes were discharged to allow for T1 equilibration effects. Data were processed using MATLAB 7.0 (MathWork, Natick, MA) and SPM5 (Statistical Parametrical Mapping, http://www.fil.ion.ucl.ac.uk). In both experiments 1 and 2, the preprocessing included the following steps: (1) realignment of acquired EPI images to the first collected image using the “Realign” routine in SPM5; (2) normalization of the EPI images to a standard echo‐planar image template; (3) smoothing of images with a Gaussian kernel of 8‐mm full‐width half maximum; (4) global scaling of data and high‐pass filtering to 1/128 Hz to remove low‐frequency noise.
In the single subject analysis of experiment 1, both efficacious and nonefficacious NS stimuli were modeled using the onsets of the NS pulses and convolved with the SPM5 hemodynamic response function (HRF). Realignment parameters were entered as covariates of no interest. At group level analysis, the two conditions (efficacious and nonefficacious NS stimuli) were compared using a paired t‐test. Statistical threshold was set to P‐FWE‐corrected <0.05 at cluster level (cluster size defined using an initial voxel‐level threshold P = 0.005 uncorrected).
In experiment 2, single subject analysis considered each of the 30‐s block as onset. All of the four experimental conditions, given by the combination of task (active/control) and NS stimulation (efficacious/nonefficacious), were modeled and convolved with the SPM5 HRF. Again, realignment parameters were entered as covariates of no interest. At group level, the four conditions were modeled using a 2 × 2 within‐subject ANOVA design. We first tested for the main effect of task (active minus control) regardless of NS and then for the interactions between the two factors. Statistical threshold was set to P‐FWE‐corr. <0.05 at cluster level (cluster size defined using an initial voxel‐level threshold P < 0.005 unc.).
RESULTS
One subject was excluded from both analyses (experiments 1 and 2) due to motion artifacts, while another one only participated to the first experiment.
Experiment 1: AP Effect on the Brain at Rest
As shown in Table IA, analyses of the ECG recordings during fMRI acquisition revealed a significant RR increase for efficacious compared to nonefficacious NS stimuli. When comparing the efficacious versus the nonefficacious condition, fMRI analysis revealed a significant increase of brain activation in the right insula, in the right superior temporal gyrus and in the amygdala, in the putamen, in the caudate nucleus, and in the parahippocampal gyrus of the left hemisphere (Fig. 3). The inverse comparison, that is, nonefficacious greater than efficacious NS stimulation, did not reveal any significant change of brain activation.
Table I.
Peripheral recordings of ANS perturbation
| Efficacious NS stimulation | Nonefficacious NS stimulation | P value* | |
|---|---|---|---|
| (A) Brain at rest | |||
| Mean delta RR (SD) across miniblocks | 49 (60) ms | 16 (20) ms | 0.001 |
| (B) Brain engaged in a visuo‐spatial attention task | |||
| Mean delta RR (SD) across blocks | 53(69) ms | 11(28) ms | 0.003 |
Student's T‐test for paired data; ANS, autonomic nervous system; NS, neck suction.
Assessment of RR interval changes (cardiac response due to NS stimuli averaged over the population) to efficacious (NS = −60 mm Hg) and nonefficacious (NS = −10 mm Hg) stimulation. A: Experiment 1 (brain at rest): NS stimuli consisted of 80 miniblocks with an 8‐s duration each (50 of them efficacious and 30 of them nonefficacious), randomly administered in 16 min of fMRI acquisition. B: Experiment 2 (brain engaged in a visuo‐spatial attention task): NS stimuli were continuously delivered in blocks of 60‐s duration, alternating efficacious (NS = −60 mm Hg) and nonefficacious (NS = −10 mm Hg) stimulation. As shown in Figure 2B, to avoid the risk of accommodation for the mechanoreceptors, each 60‐s block consisted of 8‐s pulses of NS separated by 1‐s intervals of release to atmospheric pressure. In both experiments, ECG monitoring revealed a significant increase of RR for efficacious, but not for nonefficacious NS stimulation. See text for further details.
Figure 3.
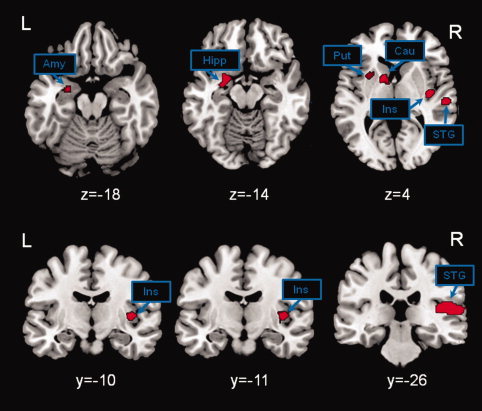
Brain activation due to autonomic perturbation at rest. Brain activation for the comparison of efficacious greater than nonefficacious neck suction (NS) stimulation. Significant activations were observed in the amygdala, in the hippocampus, in the putamen, in the caudate nucleus, in the insula, and in the superior temporal gyrus. The inverse contrast (nonefficacious greater than efficacious NS stimulation) did not reveal any significant activation. Statistical threshold set to p‐FWE‐corr. <0.05 at cluster level. See text for further details. The xyz coordinates refer to MNI standard space. The xyz coordinates refer to MNI standard space. Abbreviations: amy, amygdala; hipp, hippocampus; put, putamen; cau, caudate nucleus; ins, insula; STG, superior temporal gyrus; R, right; L, left.
Experiment 2: AP Effect on the Brain Engaged in a Visuo‐Spatial Attention Task
As shown in Table IB, analyses of the ECG recordings revealed the expected increase in RR during efficacious when compared with nonefficacious NS stimulation, without any further modulation driven by task condition. Thus, in experiment 2, any BOLD activation associated with the interaction between task and AP cannot be explained by AP‐induced changes of heart rate.
As shown in Table II, the analyses of the behavioral task performance revealed that experimental blocks under efficacious compared to those under nonefficacious NS stimulation induced longer reaction times (RTs), irrespective of trial type (active, i.e., visuo‐spatial attention task, or control, i.e., color discrimination). Conversely, the overall task accuracy was not significantly affected by NS stimulation.
Table II.
Behavioral performance during visuo‐spatial attention task
| Efficacious NS stimulation | Nonefficacious NS stimulation | * P value | |
|---|---|---|---|
| Mean (SD) percentage of correct responsesa | |||
| Active task | 93.1 (5.0) | 92.4 (6.8) | n.s. |
| Control task | 99.7 (0.6) | 99.0 (2.5) | n.s. |
| Mean (SD) RTs (ms)b | |||
| Active task | 600.5 (33.2) | 524.9 (121.2) | 0.01 |
| Control task | 455 (30) | 399 (63.8) | 0.01 |
Student's t‐test for paired samples. NS, neck suction; RTs, reaction times.
No significant difference in task performance was observed by comparing blocks under efficacious (−60 mm Hg) and nonefficacious (−10 mm Hg) neck suction (NS) stimulation regardless of the type of task (active or control).
RTs were significantly longer during efficacious as compared to nonefficacious NS simulation in both conditions, active and control task.
fMRI analysis revealed that the visuo‐spatial attention task activated the expected fronto‐parietal network [Corbetta et al.,1998; Fink et al.,2001; Vannini et al.,2007] in both conditions, efficacious and nonefficacious AP (Fig. 4, yellow areas). Consistently, with a previous fMRI study using a paradigm similar to that used here [Fink et al.,2001], the activated network included prefrontal regions (Brodmann areas; BA 8,10), the anterior cingulate cortex (BA 32), the posterior parietal cortex (BA7), the angular gyrus (BA 40), visual areas (BA17,18,19) and the fusiform gyrus (BA 37), the thalamus, the insula, and the cerebellum. As expected, there was an interhemispheric asymmetry, with greater activation in the right hemisphere [Corbetta et al.,1998; Fink et al.,2001; Vannini et al.,2007]. Most importantly, some of these regions showed a significant task‐by‐NS‐stimulation interaction (Fig. 4, red areas). Significant increases of activation for the active condition during efficacious NS were found in the right posterior parietal cortex (BA 7) and in the occipital cortex (BA 17,18) and in the fusiform gyrus (BA 37) bilaterally. All these areas of positive modulation of activation by efficacious NS stimulation are part of the main effect of task. Additionally other areas that are not included in the main effect of task were also activated. These include the parahippocampal gyri, the brainstem (pons and mesencephalon), and the periaqueductal gray. The opposite interaction (i.e., reduction of visuo‐spatial related activity during efficacious NS) did not reveal any significant modulation of brain activation.
Figure 4.
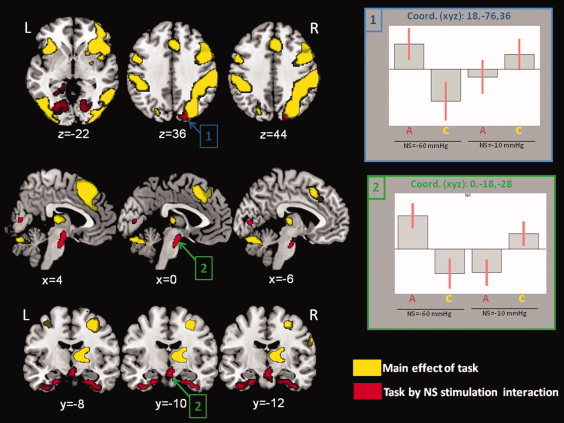
Changes in brain activation in the brain engaged in a visuo‐spatial attention task. Here, we show the main effect of task (yellow color) and the effect of task by neck suction (NS) stimulation interaction (red color). The main effect of task revealed the expected pattern of brain activation (right more than left side), including prefrontal, parietal and occipital cortex, the thalamus, and the cerebellum. The task by NS stimulation interaction revealed a positive modulation of activation in regions included in the main effect of task (posterior parietal cortex and occipital associative cortex) but also in other areas, such as the medial temporal pole bilaterally, the brainstem and the periaquiductal gray. Plots of signal changes across experimental conditions are shown for the cluster including the right posterior parietal cortex (plot 1) and for the cluster including the brainstem and periaquiductal gray (plot 2). Statistical threshold set to p‐FWE‐corr. < 0.05 at cluster level. The xyz coordinates refer to MNI standard space. See text for further details. Abbreviations: R, right; L, left; A = active task; C = control task.
DISCUSSION
In this study, we stimulated the baroreceptors in the carotid sinus to increase the afferent parasympathetic activity synchronously with fMRI acquisition to investigate the effects of AP on brain activity. In both experiments, at rest and during cognitive task, efficacious and nonefficacious NS stimuli were administered and controlled by continuous ECG recording. As expected, peripheral effects on the cardiac rate (i.e., RR increase) were detected during efficacious, but not during nonefficacious stimulation.
At rest, an increased activation was observed, during AP, in brain structures such as the insula and the amygdala, which are known to be involved in ANS regulation [Critchley,2009; Kimmerly et al.,2005]. These brain structures have been consistently associated to interceptive sensitivity [Gray et al.,2007], with the insula being particularly implicated in representations of cardiac physiology. The activation in the insula showed a right‐hemisphere asymmetry, although the NS stimulation was administered bilaterally. Despite a brain mapping of areas involved in autonomic regulation is still largely unknown, such an asymmetry is consistent with the clinical observation of an increased risk for developing complex arrhythmias in stroke patients with a selective damage of the right insula [Colivicchi et al.,2004]. In this view, we might speculate that the lateralization effect found in our first experiment could reflect the prominent control of the right hemisphere on the vagal functions. The insula and amygdala are also known to be implicated in emotion processing [Gray et al.,2009], including disgust (regarded as “visceral emotion”) [Phillips et al., 1997, 1998; Sprengelmeyer et al.,1998], fear, and anger [Davidson and Irwin,1999; Dolan,2002].
Although an exhaustive explanation for the increase of activation in the right superior temporal gyrus remains difficult, this structure has been claimed to play a crucial role in emotional processing and social cognition [Allison et al.,2000; Gallagher and Frith,2003]. However, further studies are needed to address the specific role of this brain area in the relationship between CNS and ANS. Nevertheless, taken altogether, our finding of a direct modulation of activation in these brain structures provides, for the first time, evidence for a strict relationship between autonomic perception and the consequent mental processes for behavioral responses. Additionally, during NS stimulation, we also observed increased activation in other areas, such as the putamen, the caudate nucleus, and the parahippocampal gyrus, which are known to be involved in cognitive functions [Eichenbaumand and Lipton,2008; Grahn et al.,2009]. These results suggest a possible integration between autonomic regulation and cognitive processing, concomitantly with ANS inputs. In a more general perspective, the combination of autonomic, emotional, and cognitive aspects might represent the neurobiological substrate for the processing of “complex emotions” typical of humans, as it was recently suggested by Basile et al. [2011].
In the second experiment, we explored whether ANS perturbation can selectively affect the brain activity during engagement of high‐level cognitive functions. For this purpose, we repeated the ANS perturbation (efficacious vs. nonefficacious NS stimulation) while subjects were engaged in a visuo‐spatial attention task. Attention is a higher level function that is likely to be immediately modulated by ANS perturbation. The ANS regulates the “fight and flight” responses as well as our bodily processes during ordinary situations, for which different levels of alert and attention are required. The analysis of behavioral data revealed that AP did not affect task performance (neither in the active nor in the control task), with subjects maintaining the same level of correct responses during both, efficacious and nonefficacious stimulation. By contrast, the analysis of behavioral data revealed a significant increase of RTs during efficacious NS stimulation in both, the active (i.e., visuo‐spatial attention) and the control task (i.e., color discrimination). This indicates that the AP applied here can affect cognitive efficiency independently from the cognitive demand, which was higher in the active when compared with the control task. Consistent with the high level of performance reported by all subjects, fMRI analysis revealed the expected main effect of task, which induced activation of the fronto‐parietal attention network [Corbetta et al.,1998; Fink et al.,2001; Vannini et al.,2007]. Remarkably, NS stimulation induced an additional positive modulation of activation in specific areas, including the right parietal cortex, the occipital cortex, the medial temporal poles, the brainstem, and the periaquiductal gray. Taken together with the increase of RTs, these findings suggest that a greater activation in these regions might stand for a compensatory mechanism engaged to contrast the reduced efficiency caused by parasympathetic AP. These brain areas, positively responding to the task‐by‐NS‐stimulation interaction, might contribute to compensate for the stronger effort required to correctly accomplish the visuo‐spatial attention task under parasympathetic stimulation. Some of these areas, such as the associative visual and the parietal cortices, are well known to be part of the visuo‐spatial attention network [Fink et al.,2001]. In particular, the right posterior parietal cortex (BA7) is considered as a crucial area for spatial‐attention functions [Corbetta et al.,1998]. On the other hand, positive task‐by‐NS‐stimulation interaction was also observed in brain structures that are not part of the visuo‐spatial attention network, such as the anterior temporal poles (BA 38), the brainstem, and the periaquiductal gray. The medial temporal pole is a complex structure that receives projections from several brain structures [Olson et al.,2007]. Beyond its several cognitive functions, the temporal pole has also been reported to produce changes in heart rate, respiration, and blood pressure [Gloor et al.,1982]. Moreover, the medial temporal pole is connected to the insula, which is believed to play a relevant role in awareness of internal physiological state [Critchley,2004]. We may therefore speculate that the bilateral modulation of activity found in the temporal pole might reflect a relevant role of this structure in the integration/control of internal autonomic inputs and cognitive functioning. A positive task‐by‐NS‐stimulation interaction was also found in the brainstem and in the periaquiductal gray. In a recent fMRI study, Gianaros et al. [2011] suggested (within a more complex network) a critical role of these structures (brainstem and periaquiductal gray) for the central autonomic and cardiovascular control in subjects when performing a multisource interference task. Experiment 2 of this study was based on the performance of a cognitive task under direct manipulation of the baroreflex. Our finding further support the hypothesis that nuclei in the pons and periaquiductal gray, together with the amygdala and the insula, represent important levels for the central control of the ANS and, presumably, for the continuous interfacing between autonomic regulation, and cognition and emotion processing.
In conclusion, this study proposes a novel approach to investigate the effect of a direct stimulation of the ANS on the brain functioning in vivo. This approach seems to be promising for future neurophysiological investigations as well as for potential clinical applications.
Acknowledgements
None of the authors have any financial interest related to the publication of the present manuscript. The study was approved by the local ethics committee before initiation. All subjects gave written informed consent before taking part.
REFERENCES
- Allison T, Puce A, McCarthy G ( 2000): Social perception from visual cues: Role of the STS region. Trends Cogn Sci 4: 267–278. [DOI] [PubMed] [Google Scholar]
- Basile B, Mancini F, Macaluso E, Caltagirone C, Frackowiak RS, Bozzali M ( 2011): Deontological and altruistic guilt: Evidence for distinct neurobiological substrates. Hum Brain Mapp 32: 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colivicchi F, Bassi A, Santini M, Caltagirone C ( 2004): Cardiac autonomic derangement and arrhythmias in right‐sided stroke with insular involvement. Stroke 35: 2094–2098. [DOI] [PubMed] [Google Scholar]
- Cooper VL, Hainsworth R ( 2009): Carotid baroreflex testing using the neck collar device. Clin Auton Res 19: 102–112. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL ( 1998): A common network of functional areas for attention and eye movements. Neuron 21: 761–773. [DOI] [PubMed] [Google Scholar]
- Critchley HD ( 2004): The human cortex responds to an interoceptive challenge. Proc Natl Acad Sci USA 101: 6333–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD ( 2009): Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. Int J Psychophysiol 73: 88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD ( 2002): How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci 3: 655–666. [DOI] [PubMed] [Google Scholar]
- Damasio A ( 2003): Feelings of emotion and the self. Ann NY Acad Sci 1001: 253–261. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W ( 1999): The functional neuroanatomy of emotion and affective style. Trends Cogn Sci 3: 11–21. [DOI] [PubMed] [Google Scholar]
- Dolan RJ ( 2002): Emotion, cognition, and behavior. Science 298: 1191–1194. [DOI] [PubMed] [Google Scholar]
- Eckberg D, Sleight P ( 1992): Human Arterial Baroreceptors. Oxford University Press: Oxford. [Google Scholar]
- Eichenbaum H, Lipton PA ( 2008): Towards a functional organization of the medial temporal lobe memory system: Role of the parahippocampal and medial entorhinal cortical areas. Hippocampus 18: 1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel PJ, Ogoh S, Keller DM, Raven PB ( 2003): Recent insights into carotid baroreflex function in humans using the variable pressure neck chamber. Exp Physiol 88: 671–680. [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Weiss PH, Zilles K ( 2001): The neural basis of vertical and horizontal line bisection judgments: An fMRI study of normal volunteers. Neuroimage 14: S59–S67. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD ( 2003): Functional imaging of ‘theory of mind’. Trends Cogn Sci 7: 77–83. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD (2011): Brain systems for baroreflex suppression during stress in humans. Hum Brain Mapp. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor P, Olivier A, Quesney LF, Andermann F, Horowitz S ( 1982): The role of the limbic system in experiential phenomena of temporal lobe epilepsy. Ann Neurol 12: 129–144. [DOI] [PubMed] [Google Scholar]
- Grahn JA, Parkinson JA, Owen AM ( 2009): The role of the basal ganglia in learning and memory: Neuropsychological studies. Behav Brain Res 199: 53–60. [DOI] [PubMed] [Google Scholar]
- Gray MA, Harrison NA, Wiens S, Critchley HD ( 2007): Modulation of emotional appraisal by false physiological feedback during fMRI. PLoS One 20: e546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Rylander K, Harrison NA, Wallin BG, Critchley HD ( 2009): Following one's heart: Cardiac rhythms gate central initiation of sympathetic reflexes. J Neurosci 11: 1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MA, Minati L, Paletti G, Critchley HD ( 2010): Baroreceptor activation attenuates attentional effects on pain‐evoked potentials. Pain 151: 853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM ( 2000): Principles of Neural Science, 4th ed. McGraw‐Hill: New York. [Google Scholar]
- Kimmerly Ds, O'Leary DD, Menon RS, Gati JS, Shoemaker JK ( 2005): Cortical regions associated with autonomic cardiovascular regulation during lower body negative pressure in humans. J Physiol 569: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoh S, Fadel PJ, Hardisty JM, Wasmund WL, Keller DM, Raven PB, Smith ML ( 2003): Does pulsatile and sustained neck pressure or neck suction produce differential cardiovascular and sympathetic responses in humans? Exp Physiol 88: 595–601. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y ( 2007): The enigmatic temporal pole: A review of findings on social and emotional processing. Brain 130: 1718–1731. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Calder AJ, Rowland D, Brammer M, Bullmore ET, Andrew C, Willimas SCR, Gray J, David AS (1997): A specific neural substrate for perception of facial expressions of disgust. Nature 389:495–498. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Bullmore ET, Howard R, Woodruff PW, Wright IC, Williams SC, Simmons A, Andrew C, Brammer M, David AS (1998): Investigation of facial recognition memory and happy and sad facial expression perception: an fMRI study. Psychiatry Res 83:127–138. [DOI] [PubMed] [Google Scholar]
- Porges SW ( 2007): The polyvagal perspective. Biol Psych 74: 116–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H ( 1998): Neural structures associated with recognition of facial expressions of basic emotions. Proc R Soc Lond Biol Sci 265: 1927–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, Almkvist O, Dierks T, Lehmann C, Wahlund LO ( 2007): Reduced neuronal efficacy in progressive mild cognitive impairment: A prospective fMRI study on visuospatial processing. Psychiatry Res 156: 43–57. [DOI] [PubMed] [Google Scholar]


