Abstract
After prolonged viewing of a continuous periodic motion stimulus at frequencies around 10 Hz, observers experience a fleeting impression of reversed motion: the continuous Wagon Wheel Illusion (c‐WWI). To account for this phenomenon it has been proposed that attentional mechanisms discretely sample motion information. Alternative accounts argue that the illusion relies on the spurious activation of motion detectors, which under the effect of adaptation could trigger a reversed percept. We investigated the neural correlates of the c‐WWI using fMRI (3T). Subjects viewed a vertically bisected ring containing a radial grating unambiguously rotating at 10 Hz; they continuously reported the perceived motion direction within each half of the ring. The two halves always rotated in opposite directions, allowing us to separately explore illusory reversals occurring within each hemifield. Comparing BOLD activity during illusory (c‐WWI) or real perceptual periods revealed systematic differences in right parietal regions, in addition to the right motion complex MT+. This activation pattern did not depend on the side on which the illusion occurred, and could not be accounted for by purely perceptual switch‐related activity—known to encompass parietal regions during other bistable effects. This first characterization of the fMRI correlates of the c‐WWI may have implications for the different theoretical explanations of the phenomenon. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: wagon wheel illusion, fMRI, parietal cortex, attention, bistable perception
INTRODUCTION
Periodic motion in movies sometimes appears to go backward because of the discrete sampling of video cameras (the Wagon Wheel Illusion (WWI)). A similar illusion also occurs under conditions of continuous illumination [Schouten, 1967] (continuous WWI or c‐WWI) leading to the hypothesis that the visual system discretely samples incoming information in much the same way as video cameras do [Purves et al., 1996; VanRullen et al., 2005]. However there are several important differences between the two versions of the illusion—for instance the c‐WWI is a bistable effect that requires some adaptation time. These differences have led other authors to argue that the c‐WWI occurs not because of discrete perception, but is due to the spurious activation of Reichardt‐like motion detectors that might come to dominate perception after periods of adaptation [Holcombe et al., 2005; Kline and Eagleman, 2008; Kline et al., 2004, 2006].
Recently there has been mounting evidence in favor of the high‐level discrete sampling account—even though this is still heavily debated [Andrews and Purves, 2005; Andrews et al., 2005; Holcombe and Seizova‐Cajic, 2008; Holcombe et al., 2005; Kline and Eagleman, 2008; Kline et al., 2004, 2006; Rojas et al., 2006]. The c‐WWI has been shown to depend on attentional and object‐based mechanisms [VanRullen, 2006; VanRullen et al., 2005] and can occur for both first order (luminance defined) and second order (contrast defined) motion stimuli [VanRullen et al., 2005]. In addition, the illusion is not directly related to the amount of adaptation [VanRullen, 2007]. Furthermore, electro‐encephalographic (EEG) recordings have suggested a right parietal correlate for the c‐WWI, in a frequency band compatible with the discrete sampling, snapshot‐based hypothesis [VanRullen et al., 2006]. This finding was recently confirmed with a repetitive transcranial magnetic stimulation (r‐TMS) study in which disruption of right (but not left) parietal areas by r‐TMS weakened the c‐WWI [VanRullen et al., 2008]. The involvement of right parietal cortex in the illusion would be consistent with its role in attentional processes and the temporal perception of the visual world [Battelli et al., 2007, 2008]. However, direct evidence for the involvement of parietal regions in the c‐WWI is lacking: EEG topographies cannot be directly interpreted in terms of neural sources, and the r‐TMS‐induced decrease of c‐WWI could simply imply that right parietal regions normally feed into the area(s) responsible for generating the illusion. Surprisingly, this phenomenon has not yet been investigated using fMRI. Thus the purpose of the present study was to characterize the regions whose BOLD activation correlate with the occurrence of the illusion. Since fMRI studies can only provide correlational evidence, we do not expect to unequivocally distinguish between the theories accounting for the cause of the illusion. Rather we hope that the current results may shed some light on the mechanisms responsible for the effect, and thus contribute to an eventual resolution of the different existing theories.
METHODS
Subjects
One author (RV) and 13 naive subjects (age range: 18–33 years, eight male, all right‐handed) with normal or corrected‐to‐normal vision were scanned in one fMRI imaging session each at Neurospin (Saclay, France). Informed consent was obtained from each participant according to procedures approved by the ethics committee at the CNRS. Prior to the scanning session, all naïve subjects were briefly familiarized with the stimulus and the required behavioral responses for between 2 and 5 min. After this initial practice period 14 out of 15 subjects consistently perceived the c‐WWI for longer than 1 s (see below) and were thus included in the study.
Stimulus and Task
The stimulus was a radial grating (24 cycles) at maximum contrast displayed in an annulus of radius 5° and width 1°, centered at fixation (see Fig. 1). The vertical midline of the annulus was occluded by a vertical grey band (width 2°) such that the stimulus appeared to consist of two half rings. One of the half‐rings rotated clockwise and the other counterclockwise (i.e., the two halves either both moved up or down). The temporal frequency of the rotation was 10 Hz in both halves of the stimulus, which is known to maximize the illusion [VanRullen et al., 2005]. A central fixation point was presented throughout the experiment and subjects were instructed to fixate and refrain from making eye movements. We used a projector with a 60‐Hz refresh rate. Because our motion stimuli were all shown at a frequency of 10 Hz, the refresh rate of the projector was well above the corresponding Nyquist frequency (20 Hz), implying that temporal sampling artifacts from the display were unlikely to affect the motion percepts [Burr et al., 1986]. In fact, six frames were displayed for each period of the motion stimulus, whose direction was thus unambiguous.
Figure 1.
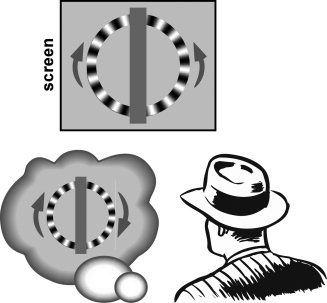
The c‐WWI effect. Periodic motion around 10 Hz viewed under continuous illumination (or using a monitor with a fast enough refresh rate) can result in an illusion of reversed motion. Here observers viewed two half‐rings moving nonambiguously, either upward or downward at 10 Hz. Perceptual reversals often occur in one half of the ring, giving rise to an illusion of the entire ring moving coherently in either the clockwise or anticlockwise direction. Observers constantly reported the perceived motion direction in each half of the ring using key‐presses.
Each fMRI run consisted of four visual stimulation trials (76‐s each) preceded and followed by five fixation intervals (16‐s each). Thus each fMRI run lasted 384 s. Subjects performed between 10 and 12 runs in the scanner. Within a given run, the direction of motion of the two half rings (i.e., clockwise/counterclockwise) changed on every 76‐s visual stimulation trial. Across two successive runs, the order was reversed (i.e., if one run was ABAB the next was BABA). The direction on the first trial of the entire experimental session was counter‐balanced across subjects. Note that during each 76‐s trial, subjects would report several perceptual periods corresponding to real and illusory motion. These perceptual periods were included in an event‐related analysis (see fMRI analysis section below).
Subjects held the button box in both hands, and used the left hand and right hand (with the finger of their choice) to press the left and right buttons, respectively. To indicate the perceived direction of motion of each half‐ring, half of the subjects were instructed to hold down the left and/or right buttons of the button box when motion (real or illusory) in the corresponding half‐ring was perceived upward, and to release the buttons for downward motion; the other half of the subjects held down the buttons to indicate downward motion, and released them for upward motion. Therefore, motor response (i.e., holding or releasing) was not consistently associated with real or illusory motion perception, and motor activity would thus be unlikely to contaminate our contrasts.
fMRI Data Acquisition and Analysis
Echo planar images were collected during the 384‐s runs in a 3T Siemens scanner with a 12‐channel head coil (3 × 3 × 3 mm3 voxels, 38 interleaved axial slices, TR = 2.0 s (i.e., 192 acquisitions per run), TE = 30.0 ms, 64 × 64 slice matrix). In addition to the functional scans, a high resolution T1 MPRAGE anatomical scan (1 × 1 × 1 mm3) was also collected in the same session. The total scan time was 1.5 h for each subject.
Data analysis was performed using FS‐FAST and FreeSurfer v.3.0.5. Data were motion‐corrected (using AFNI with standard parameters), intensity normalized, and smoothed with a 5‐mm full width at half maximum Gaussian kernel. Lower levels of volume smoothing (including no smoothing) were also applied to the data in separate analyses and yielded qualitatively similar results (data not shown). Average signal intensity maps were then computed for each voxel using FS‐FAST. For each subject based on behavioral responses, we created an event‐related design matrix with real motion perception, illusory percepts (further separated as left and right illusions), and blank periods. Only the perception periods lasting longer than 1 s were considered for analysis; the first and last percept of each trial was also rejected from this analysis. The predictor for each stimulus condition (0 or 1 at each time point) was convolved with a gamma function, and the general linear model was used to compute the response of each voxel in each condition. This response was expressed as the percent signal change, i.e., the response in each condition minus the response in the fixation condition, normalized by the mean signal in each voxel. To determine the voxels that were significantly more activated during the illusion periods we contrasted event‐related brain activations during perceptual events corresponding to illusory reversed motion versus real motion (see Fig. 2).
Figure 2.
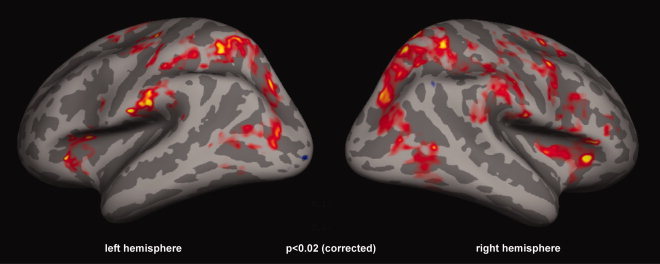
Results of the group analysis for the contrast of periods of c‐WWI versus real motion percepts. A widespread bilateral network of parietal and frontal areas was activated during the c‐WWI. However, this activation could simply reflect a correlate of switching mechanisms during bistable perception because of unbalanced distributions of perceptual durations in each condition (see main text).
As discussed in detail in the Results section and Figure 3, for the main analysis (see Fig. 4) we resampled the distributions of perceptual events corresponding to real and illusory motion percepts: for each subject we attempted to balance the distributions by randomly rejecting perceptual periods in excess of the intersection of both distributions. Thus, the interval since the previous and before the next perceptual switch was comparable on average across the two perceptual conditions. In the fMRI analysis based on these resampled distributions we created an event‐related design matrix as before (i.e., blank, real, and illusory percepts, further separated as left and right illusions), but with additional regressors corresponding to the rejected perceptual periods (i.e., those that lay outside the intersection of distributions). Furthermore, to make sure that the main results did not depend on the specific selection of trials in the equalized distribution, we repeated this entire analysis three additional times, with a different random selection of trials on each iteration (Supporting Information Fig. S1).
Figure 3.
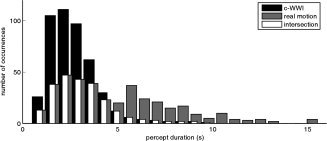
Distribution of perceptual periods. The distributions of real and illusory motion percepts for one representative subject are shown in grey and black, respectively. Note that, because the duration of real motion percepts was longer, the distribution corresponding to the c‐WWI periods is, on average, closer to the time of perceptual switches (t = 0 s) than the distribution for real motion percepts. In other words, the c‐WWI distribution is associated with more switch‐related activity. To minimize the unequal contribution of switching events to the contrast of c‐WWI versus real motion periods, the two distributions were made comparable to each other by randomly rejecting events that lay outside the intersection of the two distributions (white bars).
Figure 4.
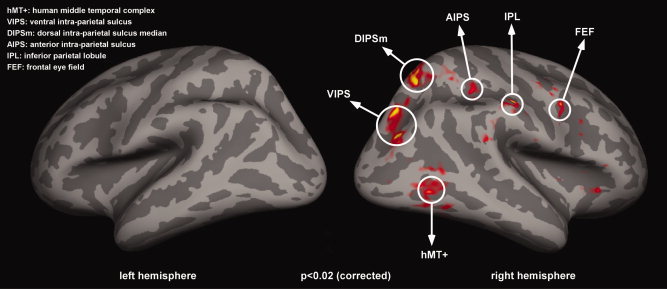
Correlates of the c‐WWI. The results shown here reflect a random effects analysis of the contrast c‐WWI versus real motion periods, over the group of 14 subjects. The c‐WWI mainly activated a right lateralized parietal network, involving areas VIPS, DIPSm, AIPS, and IPL. In addition, significant activations were also observed in the right FEF and right hMT+ (P < 0.02; FDR corrected). No significant activations were found in the left hemisphere (all P > 0.05, corrected) and no significant activation was observed for the opposite contrast.
FreeSurfer was used to reconstruct the original surface for each participant from the high‐resolution anatomical scan. To perform a group analysis, individual brains were aligned to each other with FreeSurfer by spatially normalizing the cortical surfaces to a spherical surface template using an automated procedure to align the major sulci and gyri [Fischl et al., 1999]. The functional data were smoothed with a 5‐mm kernel on the surface. Lower levels of surface smoothing (down to a 3‐mm kernel) were also applied to the data in separate analyses and yielded qualitatively similar results (data not shown). Random effects analyses were computed across the group of subjects and the statistics were sampled onto the average FreeSurfer target brain. Statistical activation maps were corrected for multiple comparisons using the false discovery rate (Figs. 2, 4, and Supporting Information S1). To estimate the significance of this group analysis in a nonparametric way, we used a bootstrap procedure over 1,000 iterations. Specifically, for each subject we first computed the surrogate “illusory versus real” contrast 10 times, after randomly shuffling the “illusory” and “real” labels on each iteration. We then recomputed the group analysis 1,000 times, each time randomly drawing one of the 10 analysis for each subject to be included in the group analysis. This procedure allowed us to estimate a distribution of the expected number of significant voxels under the null hypothesis.
RESULTS
The stimulus was an annulus split vertically in the middle, and each half contained a radial luminance grating that rotated at 10 Hz. The left and right halves of the annulus rotated in opposite directions, creating an inconsistent global motion pattern that was resolved when one of the two halves reversed (see Fig. 1).
On each trial, subjects saw the stimulus for 76 s during which time they reported periods of illusory motion by holding down or releasing one of two predetermined keys. The mean duration of the perceptual periods in the scanner was 2.04 ± 0.3 s for illusory reversed motion, and 6.59 ± 0.93 s for real motion. The reversals occurred with comparable frequencies in the left (49.5%) and right (50.5%) hemifields (n.s., t(13) = 0.14; P = 0.89), and were virtually never reported simultaneously for both hemifields.
To determine which areas were more active during the illusion, event‐related brain activation during perceptual periods (>1 s) corresponding to illusory reversed motion was contrasted with periods corresponding to real motion over the group of 14 subjects. The first and last percepts of each trial were not included in the analysis. This contrast resulted in a widespread bilateral network of parietal and frontal areas during the c‐WWI (see Fig. 2). However this network is known to be generally involved during switches of perception associated with bistable stimuli [Britz et al., 2009; Kleinschmidt et al., 1998, 2002; Lumer et al., 1998; Sterzer and Kleinschmidt, 2007; Sterzer et al., 2002; Williams et al., 2003]. Therefore, the observed activations in Figure 2 may not reflect the c‐WWI per se, but could instead be due to a general switching mechanism. Indeed, for all subjects real motion percepts tended to last longer than the illusory motion percepts (see Fig. 3), meaning that, on average, any given c‐WWI period was closer in time to the preceding switching event. These unbalanced distributions of perceptual durations could thus lead to unequal contributions of switching events to the contrast of c‐WWI versus real motion periods. To minimize the effects of the unbalanced distributions, we attempted to balance the distributions for each subject by randomly rejecting perceptual periods in excess of the intersection of both distributions so that, for each perceptual condition, the interval since the previous and before the next perceptual switch was comparable. Figure 3 details this procedure and the resulting resampled distributions for one representative subject.
With this balanced design we performed a random effects group analysis over the 14 subjects to investigate regions that were significantly more activated (P < 0.02; FDR corrected) during perceptual periods of illusory versus real motion. This analysis revealed activations in a right‐lateralized network (Fig. 4, Table I) that included the inferior parietal lobule (IPL) and several regions along the intraparietal sulcus (IPS), as well as MT+, and the frontal eye field (FEF). Specifically, the right parietal activations were observed in several regions along the IPS—in more ventral regions along the occipital portion of the IPS (VIPS), in the dorsal segment in a posterior region (that might correspond to DIPSm (dorsal IPS medial) in [Orban et al., 2003]), and an anterior region (aIPS) at the junction with the postcentral sulcus (and might be similar to DIPSA (dorsal IPS anterior) in [Orban et al., 2003]). Consistent with an attentional account of the c‐WWI, a number of previous studies have implicated these right parietal regions in high‐level motion perception [Claeys et al., 2003; Orban et al., 2003, 2006], and attentional modulation [Corbetta et al., 1998, 2000; Culham and Kanwisher, 2001; Culham et al., 2001; Kastner et al., 1999; Wojciulik and Kanwisher, 1999].
Table I.
For each region of interest in the right hemisphere (identified in Fig. 3), the table reports Talairach coordinates, number of 3 × 3 × 3 mm3 voxels (mean ± S.E.M. across subjects), and number of subjects for whom the ROI could be identified based on a threshold of P < 0.05
| Regions | x | y | z | No. of voxels | No. of subjects |
|---|---|---|---|---|---|
| VIPS | 31 | −70 | 21 | 99.7 ± 26.5 | 12 |
| DIPSM | 16 | −63 | 59 | 82.4 ± 26.5 | 10 |
| AIPS | 32 | −35 | 42 | 52.9 ± 10.2 | 11 |
| IPL | 54 | −26 | 39 | 66.1 ± 13.0 | 11 |
| FEF | 46 | 2 | 37 | 69.9 ± 12.4 | 7 |
| MT+ | 42 | −66 | 2 | 97.1 ± 26.5 | 10 |
To verify that the results in Figure 4 did not depend on the specific selection of trials, we repeated this group analysis three additional times, each time with a different random selection of trials in the equalizing procedure. In all cases we observed a similar pattern of activations in a right lateralized parietal network (Supporting Information Fig. S1).
To determine the extent of BOLD activations expected by chance in our group analysis, we applied a bootstrapping procedure with 1,000 random reshufflings of the “real” and “illusory” event labels (see Methods). We found no significantly activated voxels in either hemisphere in any of these 1,000 iterations (using the same threshold as applied in Fig. 4). In contrast, in the main analysis we had found 6,927 significantly activated voxels in the right hemisphere (and this number varied from 6,390 to 13,823 across the repetitions displayed in Supporting Information Fig. S1), and 0 voxels in the left hemisphere (in all repetitions of the analysis). This nonparametric test thus implies that our results were highly unlikely to occur by chance (P < 0.001). Note that these results do not indicate that the left hemisphere had no activation whatsoever during periods of the illusion. Indeed, as shown in Supporting Information Figures S2 and S3, left hemisphere activation was observed, but at more liberal statistical thresholds (P < 0.05; uncorrected).
Since illusory reversals were uniquely associated with either the left or the right visual field, we could separately examine the contribution of the ipsilateral and contralateral hemispheres to the illusion. To this end, we separated the left and the right‐visual field illusory events for each subject and repeated the random effects group analysis (illusory versus real contrast). Supporting Information Figure S4 shows the results of this analysis: the pattern of activation described above was not affected by the laterality of the illusory motion percept. Regardless of whether the c‐WWI occurred in the left or right visual field, a consistent network of right parietal areas was activated, with no significant activation in the left hemisphere. Note that as mentioned above the reversals occurred with comparable frequencies in the left (49.5%) and right (50.5%) visual fields (n.s., t(13) = 0.14; P = 0.89), so this pattern of results is not an artifact of unequal numbers of reversals in the two analyses. A more direct contrast of left versus right visual field illusory events revealed no differential activation within the regions of interest (all P > 0.05, corrected; data not shown) suggesting again that the same network was involved regardless of the laterality of the illusion.
DISCUSSION
The current study investigated, for the first time, the neural correlates of the c‐WWI with fMRI. Using a novel technique that allowed us to make the distributions of perceptual durations comparable, and thus avoid confounds due to perceptual switching, we found significantly higher BOLD activation in right parietal areas, right MT+, and right FEF during periods of illusory versus real motion. The strong lateralization may seem surprising, since the illusion occurred equally in the left and right visual hemifields. Thus, we also measured the incidence and the strength of this lateralization for each subject, and found that it was consistent over the group, i.e., it cannot be explained by the presence of outliers in our group analysis (Supporting Information Fig. S3). On the other hand, the predominant right parietal involvement in the c‐WWI is compatible with both a previous EEG study that linked this illusion to 13 Hz activity over right parietal electrodes [VanRullen et al., 2006], as well as with a more recent r‐TMS study that demonstrated a causal role of right (but not left) parietal deactivation in the disruption of the c‐WWI [VanRullen et al., 2008]. Note that the illusory and real conditions corresponded to different global motion percepts; in the former case the percept was of global clockwise or counterclockwise motion, whereas in the latter the motion was either globally upward or downward. This difference in global motion percepts between the two conditions could account for the greater level of hMT+ activation that is observed during periods of illusory percepts.1
Right parietal regions including VIPS, DIPSm, and aIPS are known to be sensitive to diverse forms of motion, such as random dot translation and three‐dimensional structure from motion [Orban et al., 2003, 2006]. Higher order, saliency‐based or attention‐dependent motion is specifically linked to activity in the IPL [Claeys et al., 2003], which also supports subjective motion judgments during ambiguous perception [Williams et al., 2003]. Parietal regions are actually part of a well‐known attentional network: aIPS, regions in the IPL, and at the junction of the intraparietal and transverse occipital sulci (that might correspond to DIPSm) are involved in a wide variety of attention tasks [Corbetta et al., 2000; Culham and Kanwisher, 2001; Culham et al., 2001; Kastner et al., 1999; Wojciulik and Kanwisher, 1999; Yantis et al., 2002]. In addition, neurons in certain parietal areas in monkeys (e.g., area 7a) tend to have large, bilateral receptive fields that can sometimes encompass the entire visual field [Motter et al., 1987]. In fact, lesion studies in humans have revealed that the right parietal cortex contributes to transient visual attention and higher‐level motion processing in a bilateral manner [Battelli et al., 2001, 2003]. These findings converge to indicate that motion processing in parietal cortex is a high‐level, not purely local or retinotopic, but attention‐based process. Thus, at first sight the present results may seem to support the high‐level, attentional sampling account of the c‐WWI.
On the other hand, the very nature of fMRI is such that it may be premature to embrace this conclusion. First, the correlation of BOLD signals to the c‐WWI percept in a wide network of right‐hemispheric regions does not allow us to precisely pinpoint the source of the effect; it might well be that all of the highlighted regions contribute to the generation of the illusion, or even that it is caused by specific interactions between the different regions. Furthermore, the absence of significant BOLD activations in lower‐tier visual areas (e.g., V1) is not sufficient to rule out a participation of these regions in the c‐WWI. Indeed, the fine columnar organization of early retinotopic cortex could mask any differential activation, because the same voxel would comprise several cortical columns supporting both the real and the illusory motion directions. This is an important point, since the c‐WWI has also been proposed to result from the spurious activation of Reichardt motion detectors2 in early retinotopic cortex, coupled with adaptation [Kline et al., 2004, 2006]. For all these reasons, we think it is better to remain cautious in our interpretation of the present results: while they undoubtedly demonstrate an involvement of right parietal regions, further work may be needed to completely distinguish between low‐level and higher‐level accounts of the c‐WWI, perhaps using multivoxel decoding techniques that could reveal the encoded motion direction at a subvoxel resolution [Kamitani and Tong, 2006].
Finally, the current set of results is consistent with the known involvement of a fronto‐parietal network in the perception of bistable stimuli. Particularly relevant to our findings are studies of bistable perception of ambiguous motion stimuli that show switch‐related activity in these regions [Sterzer et al., 2002]. More recently, it has been shown that the activation observed in frontal areas occurs earlier than in hMT+ during spontaneous versus stimulus‐driven perceptual changes of motion [Sterzer and Kleinschmidt, 2007] suggesting a top‐down control in the switching between different perceptual states. However, beyond suggesting a general involvement of parietal regions during perceptual switches, our results indicate that the activity in these regions can also be more strongly correlated with a particular perceptual state (i.e., in our stimulus, the illusory direction of motion). By employing a novel procedure for balancing the distributions of perceptual duration periods, we have been able to distinguish the neural correlates of competing perceptual representations from the mechanisms involved in the switching of perception. These results are therefore likely to have implications for future studies of bistable perception.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figures.
Acknowledgements
The authors thank D. Fize and J.B. Durand for discussions about the data, D. Fize for comments on the manuscript, and J. Swisher for discussions about Freesurfer.
On the other hand, this account would not directly explain the strong lateralization of hMT+; one possibility could be that an ipsilateral interaction with the right parietal network specifically enhances hMT+ activity in the right hemisphere.
This type of automatic detector computes motion information using an intrinsic spatial sampling interval and time sampling delay [Reichardt, 1961], which at certain temporal frequencies of motion can result in erroneous or “aliased” outputs [Adelson and Bergen, 1985; van Santen and Sperling, 1985]. It has been argued that the c‐WWI could be due to these spurious signals, which would come to dominate perception after sufficient adaptation time—a sort of motion after effect that would take place even during stimulus presentation, i.e. a “motion during‐effect” [Holcombe and Seizova‐Cajic, 2008; Kline and Eagleman, 2008].
REFERENCES
- Adelson EH, Bergen JR ( 1985): Spatiotemporal energy models for the perception of motion. J Opt Soc Am A 2: 284–299. [DOI] [PubMed] [Google Scholar]
- Andrews T, Purves D ( 2005): The wagon‐wheel illusion in continuous light. Trends Cogn Sci 9: 261–263. [DOI] [PubMed] [Google Scholar]
- Andrews T, Purves D, Simpson WA, VanRullen R ( 2005): The wheel keeps turning: Reply to Holcombe et al. Trends Cogn Sci 9: 561. [Google Scholar]
- Battelli L, Cavanagh P, Intriligator J, Tramo MJ, Henaff MA, Michel F, Barton JJ ( 2001): Unilateral right parietal damage leads to bilateral deficit for high‐level motion. Neuron 32: 985–995. [DOI] [PubMed] [Google Scholar]
- Battelli L, Cavanagh P, Martini P, Barton JJ ( 2003): Bilateral deficits of transient visual attention in right parietal patients. Brain 126 ( Part 10): 2164–2174. [DOI] [PubMed] [Google Scholar]
- Battelli L, Pascual‐Leone A, Cavanagh P ( 2007): The “when” pathway of the right parietal lobe. Trends Cogn Sci 11: 204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelli L, Walsh V, Pascual‐Leone A, Cavanagh P ( 2008): The “when” parietal pathway explored by lesion studies. Curr Opin Neurobiol 18: 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz J, Landis T, Michel CM ( 2009): Right parietal brain activity precedes perceptual alternation of bistable stimuli. Cerebral Cortex 19: 55–65. [DOI] [PubMed] [Google Scholar]
- Burr DC, Ross J, Morone MC ( 1986): Smooth and sampled motion. Vision Res 26: 643–652. [DOI] [PubMed] [Google Scholar]
- Claeys KG, Lindsey DT, De Schutter E, Orban GA ( 2003): A higher order motion region in human inferior parietal lobule: Evidence from fMRI. Neuron 40: 631–642. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, et al. ( 1998): A common network of functional areas for attention and eye movements. Neuron 21: 761–773. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL ( 2000): Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3: 292–297. [DOI] [PubMed] [Google Scholar]
- Culham JC, Kanwisher NG ( 2001): Neuroimaging of cognitive functions in human parietal cortex. Curr Opin Neurobiol 11: 157–163. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavanagh P, Kanwisher NG ( 2001): Attention response functions: Characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron 32: 737–745. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM ( 1999): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8: 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcombe AO, Seizova‐Cajic T ( 2008): Illusory motion reversals from unambiguous motion with visual, proprioceptive, and tactile stimuli. Vision Res 48: 1743–1757. [DOI] [PubMed] [Google Scholar]
- Holcombe AO, Clifford CW, Eagleman DM, Pakarian P ( 2005): Illusory motion reversal in tune with motion detectors. Trends Cogn Sci 9: 559–560. [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Tong F. ( 2006): Decoding seen and attended motion directions from activity in the human visual cortex. Curr Biol 16: 1096–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG ( 1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22: 751–761. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Buchel C, Zeki S, Frackowiak RS ( 1998): Human brain activity during spontaneously reversing perception of ambiguous figures. Proc Biol Sci 265: 2427–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt A, Thilo KV, Buchel C, Gresty MA, Bronstein AM, Frackowiak RS ( 2002): Neural correlates of visual‐motion perception as object‐ or self‐motion. Neuroimage 16: 873–882. [DOI] [PubMed] [Google Scholar]
- Kline KA, Eagleman DM ( 2008): Evidence against the temporal subsampling account of illusory motion reversal. J Vis 8: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline K, Holcombe AO, Eagleman DM ( 2004): Illusory motion reversal is caused by rivalry, not by perceptual snapshots of the visual field. Vision Res 44: 2653–2658. [DOI] [PubMed] [Google Scholar]
- Kline K, Holcombe AO, Eagleman DM ( 2006): Illusory motion reversal does not imply discrete processing: Reply to Rojas et al. Vis Res 46: 1158–1159. [Google Scholar]
- Lumer ED, Friston KJ, Rees G ( 1998): Neural correlates of perceptual rivalry in the human brain. Science 280: 1930–1934. [DOI] [PubMed] [Google Scholar]
- Motter BC, Steinmetz MA, Duffy CJ, Mountcastle VB ( 1987): Functional properties of parietal visual neurons: Mechanisms of directionality along a single axis. J Neurosci 7: 154–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Fize D, Peuskens H, Denys K, Nelissen K, Sunaert S, Todd J, Vanduffel W. ( 2003): Similarities and differences in motion processing between the human and macaque brain: Evidence from fMRI. Neuropsychologia 41: 1757–1768. [DOI] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W ( 2006): Mapping the parietal cortex of human and non‐human primates. Neuropsychologia 44: 2647–2667. [DOI] [PubMed] [Google Scholar]
- Purves D, Paydarfar JA, Andrews TJ ( 1996): The wagon wheel illusion in movies and reality. Proc Natl Acad Sci USA 93: 3693–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt W ( 1961): Autocorrelation, a principle for the evaluation of sensory information by the central nervous system In: Rosenblith WA, editor. Sensory Communication. Cambridge, MA: MIT Press; pp 300–321. [Google Scholar]
- Rojas D, Carmona‐Fontaine C, Lopez‐Calderon J, Aboitiz F ( 2006): Do discreteness and rivalry coexist in illusory motion reversals? Vis Res 46: 1155–1157. [DOI] [PubMed] [Google Scholar]
- Schouten JF ( 1967): Subjective stroboscopy and a model of visual movement detectors In: Wathen‐Dunn I, editor. Models for the Perception of Speech and Visual Form. Cambridge, MA: MIT Press; pp 44–45. [Google Scholar]
- Sterzer P, Kleinschmidt A ( 2007): A neural basis for inference in perceptual ambiguity. Proc Natl Acad Sci USA 104: 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterzer P, Russ MO, Preibisch C, Kleinschmidt A ( 2002): Neural correlates of spontaneous direction reversals in ambiguous apparent visual motion. Neuroimage 15: 908–916. [DOI] [PubMed] [Google Scholar]
- VanRullen R ( 2006): The continuous wagon wheel illusion is object‐based. Vis Res 46: 4091–4095. [DOI] [PubMed] [Google Scholar]
- VanRullen R ( 2007): The continuous wagon wheel illusion depends on, but is not identical to neuronal adaptation. Vis Res 47: 2143–2149. [DOI] [PubMed] [Google Scholar]
- VanRullen R, Reddy L, Koch C ( 2005): Attention‐driven discrete sampling of motion perception. Proc Natl Acad Sci USA 102: 5291–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R, Reddy L, Koch C ( 2006): The continuous wagon wheel illusion is associated with changes in electroencephalogram power at approximately 13 Hz. J Neurosci 26: 502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRullen R, Pascual‐Leone A, Battelli L ( 2008): The continuous wagon wheel illusion and the “when” pathway of the right parietal lobe: A repetitive transcranial magnetic stimulation study. PLoS ONE 3: e2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Santen JP, Sperling G ( 1985): Elaborated Reichardt detectors. J Opt Soc Am A 2: 300–321. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Elfar JC, Eskandar EN, Toth LJ, Assad JA ( 2003): Parietal activity and the perceived direction of ambiguous apparent motion. Nat Neurosci 6: 616–623. [DOI] [PubMed] [Google Scholar]
- Wojciulik E, Kanwisher N ( 1999): The generality of parietal involvement in visual attention. Neuron 23: 747–764. [DOI] [PubMed] [Google Scholar]
- Yantis S, Schwarzbach J, Serences JT, Carlson RL, Steinmetz MA, Pekar JJ, Courtney SM. ( 2002): Transient neural activity in human parietal cortex during spatial attention shifts. Nat Neurosci 5: 995–1002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Figures.


