Abstract
Viewing other's pain inhibits the excitability of the motor cortex and also modulates the neural activity elicited by a concomitantly delivered nociceptive somatosensory stimulus. As the neural activity elicited by a transient nociceptive stimulus largely reflects non nociceptive‐specific, multimodal neural processes, here we tested, for the first time, whether the observation of other's pain preferentially affects the brain responses elicited by nociceptive stimulation, or instead similarly modulates those elicited by stimuli belonging to a different sensory modality. Using 58‐channel electroencephalography (EEG), we recorded the cortical responses elicited by laser and auditory stimulation during the observation of videoclips showing either noxious or non‐noxious stimulation of a stranger's hand. We found that the observation of other's pain modulated the cortical activity consisting in an event‐related desynchronization in the β band (β ERD), and elicited by nociceptive laser stimuli, but not by auditory stimuli. Using three different source analysis approaches, we provide converging evidence that such modulation affected neural activity in the contralateral primary sensorimotor cortex. The magnitude of this modulation correlated well with a subjective measure of similarity between the model's hand and the onlooker's representation of the hand. Altogether, these findings demonstrate that the observation of other's pain modulates, in a somatosensory‐specific fashion, the cortical responses elicited by nociceptive stimuli in the sensorimotor cortex contralateral to the stimulated hand. Hum Brain Mapp, 2012. © 2012 Wiley Periodicals, Inc.
Keywords: nociception, pain observation, multisensory, EEG, source analysis
INTRODUCTION
Neurophysiological studies indicate that first‐hand experience of painful stimuli and observation of pain in others elicits largely overlapping neural activity [see Hein and Singer, 2008; Jackson et al., 2006]. This overlap between cortical structures responding to both nociceptive pain and the observation of other's pain is not only present in structures usually interpreted as reflecting the affective‐motivational aspects of pain, such as the anterior cingulate cortex (ACC) and the anterior insula [e.g., Lamm et al., 2007; Singer et al., 2004], but also in structures usually interpreted as reflecting the sensory‐discriminative aspects of pain (e.g., SI and SII) [e.g., Bufalari et al., 2007; Cheng et al., 2007, 2008; Lamm et al., 2007; Moriguchi et al., 2007]. Moreover, single pulse transcranial magnetic stimulation (TMS) studies demonstrated that the mere observation of a syringe needle penetrating deeply the hand of a stranger model causes a reduction of the motor cortex excitability. Such reduction is reflected in a decrease of the amplitude of the motor‐evoked potentials (MEPs) recorded from the onlooker's muscles corresponding to those penetrated in the model [Avenanti et al., 2005, 2006, 2009; Fecteau et al., 2008; Minio‐Paluello et al., 2006, 2009], an effect reminiscent of what observed during actual experience of pain [Farina et al., 2001].
Relevant to the current study is the suggestion that even the earliest cortical activity elicited by nociceptive stimuli is inhibited in the onlooker [Valeriani et al., 2008], as indicated by a reduction of the N1 wave of the laser‐evoked potentials (LEPs), a wave thought to reflect the early cortical processing of the ascending nociceptive input [Lee et al., 2009]. It has been recently suggested that the neural activity measured using different functional neuroimaging techniques in response to transient nociceptive stimulation largely reflects non nociceptive‐specific, multimodal cognitive processes [Iannetti and Mouraux, 2010; Legrain et al., 2011; Mouraux and Iannetti, 2009; Mouraux et al., 2011]. Thus, also on the basis of the notion that perception is an inherently multimodal experience [Aglioti and Pazzaglia, 2011], it is surprising that no study to date has examined whether the brain representations elicited by observation of other's pain affects the cortical processing not only of nociceptive information, but also of sensory information transmitted in other sensory modalities.
Here, using high‐density EEG, we tested whether the observation of nociceptive stimuli delivered to the hand of another individual preferentially affects the brain responses elicited by nociceptive stimulation, or instead similarly modulates those elicited by stimuli belonging to a different sensory modality.
METHODS
Subjects
Twelve healthy subjects (six women) aged 22–35 years (25.3 ± 4.2, mean ±SD) participated in the study. All participants gave their written informed consent. This study conformed to the standards required by the Declaration of Helsinki and was approved by the local ethics committee.
Interpersonal Reactivity Trait‐Measures
Before the experimental session, interpersonal reactivity linked to personality traits was assessed by asking the subjects to complete the Interpersonal Reactivity Index questionnaire [IRI; Davis, 1983], The IRI is one of the most widely used self‐report measures of dispositional empathy (i.e., a person's stable personality trait). In particular, the IRI is a 28‐item questionnaire that consists of four subscales that assess (1) self‐oriented aspects of interpersonal reactivity (e.g., the extent to which an individual feels distress as a result of witnessing another's distress, Personal Distress, PD), (2) other‐oriented interpersonal reactivity such as the tendency to experience feelings of sympathy and compassion for others in need (Empathic Concern, EC), (3) the disposition of an individual to adopt the perspective of another (Perspective Taking, PT), and (4) the propensity of an individual to become imaginatively involved with fictional characters and situations (Fantasy Scale, FS).
Nociceptive and Auditory Test Stimulation
Radiant heat stimuli were generated by an infrared neodymium yttrium aluminium perovskite (Nd:YAP) laser with a wavelength of 1.34 μm (Electronical Engineering, Florence, Italy). At this wavelength the laser pulses activate directly the Aδ and C‐fiber nociceptive terminals located in the superficial layers of the skin [Baumgartner et al., 2005]. The laser beam was transmitted via an optic fiber and its diameter was set at ∼8 mm (50 mm2) by focusing lenses. The duration of the laser pulses was 4 ms. Laser pulses were directed at the dorsum of the right hand, on a squared area (5 × 5 cm) defined prior to the beginning of the experimental session. The spot location was automatically controlled by a computer that used two servo‐motors (HS‐422; Hitec RCD; angular speed, 60°/160 ms) to orient the laser beam along two perpendicular axes [see Lee et al., 2009 for details]. To familiarize subjects with the nociceptive stimulus, ten low‐energy laser pulses were delivered to the right‐hand dorsum. The energy of the laser stimulus was then adjusted individually using the method of limits, in order to elicit a clear pricking pain sensation (3.1 ± 0.3 J), related to the activation of Aδ nociceptors [Treede, 1995].
Auditory stimuli were brief, 800 Hz tones (50 ms duration; 5 ms rise and fall times) delivered through a speaker (VE100AO, Audax, France) placed in front of the participant's right hand (∼55 cm from the subject and ∼50 cm from the midline). At the beginning of the experiment the intensity of auditory stimulation was adjusted to match the intensity of the laser stimulation.
The intensity matching procedure consisted in two steps [Gescheider, 1997; Valentini et al., 2011]. First, immediately after setting the intensity of the nociceptive stimulus, a series of tones of increasing loudness was presented to the subjects, to familiarize them with the auditory stimuli. Second, subjects were asked to try and match the perceived intensity of the auditory sensation to the perceived intensity of the nociceptive sensation, by self‐adjusting the intensity of the auditory stimulation.
Laser pulses were delivered shortly before and shortly after the auditory stimulus to make sure that the matching was not related to the order of occurrence of the two stimuli. This matching procedure was repeated at the end of each recording block, to ensure that the perceived intensity of the auditory and nociceptive sensations remained matched throughout the experiment. The average intensity of auditory stimulation was 86 ± 3 dB.
Visual Presentation of Pain and Touch Stimuli
Two types of visual stimuli, extracted from the sample used in Avenanti et al. [2005], were used to induce pain and touch empathic resonance. These stimuli consisted of: (1) “Pain” videoclips showing a needle penetrating the first dorsal interosseous (FDI) muscle at the dorsal surface of the right hand (between the thumb and index finger), from a first person perspective; (2) “Touch” videoclips showing a Q‐tip gently touching the same hand at the level of the FDI muscle. Only videoclips showing the right hand were presented. This allowed us to achieve a complete congruency between the onlooker's stimulated hand and the model's hand penetrated by the needle or touched by the Q‐tip. To minimize habituation effects, nine Pain and nine Touch videoclips where the syringe or the Q‐tip could have one out of three different sizes or colors, were presented in each block. Also, to avoid neural responses related to action observation rather than to the somatosensory event represented in the clip [Avikainen et al., 2002; Rizzolatti et al., 2001], the hand of the person holding the syringe or the Q‐tip was not visible.
Experimental Design and Procedure
A schematic illustration of the experimental design is shown in Figure 1. Event related potentials (ERPs) were collected in a single session. The Pain and Touch videoclips were presented in four blocks of visual stimulation. Only one type of videoclip (either Touch or Pain) was presented in each block. Touch and Pain blocks were alternated, and their order was balanced across subjects. The duration of each block was ∼8 min, and an interval of ∼8 min separated two consecutive blocks. Each stimulation block consisted of 30 trials (15 laser trials and 15 auditory trials). Thus, a total of 120 trials were delivered. Laser and auditory trials were pseudorandomized (no more than three consecutive stimuli belonging to the same modality) and intermixed within each block. Each trial started with a fixation cross presented for 2 s, followed by a 2‐s videoclip. Laser and auditory test stimuli were delivered in a jittered fashion (rectangular distribution) during the second half of the clip (between 1.2 and 1.9 s), only after the effector (either the syringe or the Q‐tip) touched the hand. At the end of the videoclip, a black screen lasting between 6 and 12 s (rectangular distribution) preceded the beginning of the following trial. At the end of the 30 trials composing each block, the subject was asked to rate the intensity, the unpleasantness and the saliency of both the nociceptive and the auditory sensation, using a visual analogue scale (VAS) ranging from 0 (no sensation/not unpleasant/not salient at all) to 100 (as much intense/unpleasant/salient as possible in this context). The order of ratings was counterbalanced across subjects.
Figure 1.
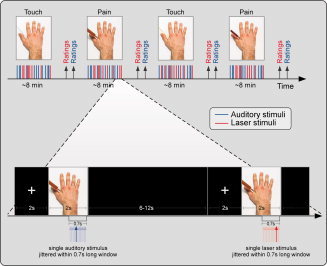
Experimental design. Event‐related potentials (ERPs) were collected in a single session and elicited by either nociceptive somatosensory stimuli delivered to the hand dorsum (in red) or by auditory stimuli delivered in the same area (in blue). ERPs were recorded in four observational blocks, whose order was counterbalanced across subjects. In each block, one of the two possible modulatory visual stimuli, representing either a painful stimulation (infliction of a syringe—Pain—in red) or a tactile stimulation (touch with a Q tip—Touch—in blue) of a model's hand, were presented. In each block, 15 laser and 15 auditory stimuli were delivered in pseudorandom order. Each trial started with a fixation cross presented for 2 s, followed by 2 s movie. After each block, subjects were asked to rate the intensity, saliency, and unpleasantness of both nociceptive and auditory sensation. This procedure allowed us to explore the possible modulation exerted by others' pain observation on the cortical responses elicited by nociceptive and auditory stimuli in the onlooker. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Between each laser pulse, the target of the laser beam was displaced by a motor arm. The direction was balanced in each session following a proximal‐distal spatial displacement. This procedure allowed to minimize the variation in thickness and innervation of the irradiated skin and, consequently, the intensity of the nociceptive stimuli [Schlereth et al., 2001]. Because variations in baseline skin temperature could bias results [Baumgartner et al., 2005], an infrared thermometer was used to ensure that baseline skin temperatures were similar at the beginning of each block.
During the whole procedure, a wooden screen was interposed between the participants and their right hand. Thus, the participants could not see the stimulated hand or the stimulation devices (i.e. the laser motor and the loudspeaker). Participants were instructed to relax and pay attention to the clip, independently of its content and of the sensory modality of the test stimuli.
To obtain information on state reactivity towards the displayed hand, at the end of the experiment, subjects were asked to rate the perceived similarity of the displayed hand with a prototypic human male hand by using a VAS scale, where 0 and 100 corresponded to “not similar at all” and “extremely similar,” respectively. The evaluation was done while the subjects were looking at the picture of the hand alone, i.e. without the syringe or the Q‐tip approaching to it.
EEG Recording
Participants were seated on a comfortable chair in a silent, temperature‐controlled room. They were asked to keep their eyes open and stare at the computer screen in front of them (1 m distance). The electroencephalogram (EEG) was recorded using 58 scalp Ag–AgCl electrodes, placed according the International 10–20 system. The nose was used as reference. The electro‐oculogram (EOG) was recorded from two surface electrodes, one placed over the right lower eyelid, the other placed lateral to the outer canthus of the right eye. Signals were digitized at a sampling rate of 1,024 Hz and a conversion of 12 bit, giving a resolution of 0.195 μV (SD32; Micromed, Treviso, Italy).
EEG Analysis
Preprocessing
EEG data were pre‐processed and analyzed using Letswave (http://amouraux.webnode.com) [Mouraux and Iannetti, 2008] and EEGLAB [Delorme and Makeig, 2004]. EEG data were segmented into epochs using a time window ranging from 1 s pre‐stimulus to 1 s post‐stimulus. Each epoch was baseline corrected using the pre‐stimulus interval from −0.5 to 0 s as reference, and band‐passed from 0.3 to 80 Hz using a fast Fourier transform filter. Although peak latencies and peak amplitudes are prone to high frequency noise, we decided to maintain the band‐pass filter similar to the previous literature [Valeriani et al., 2008] to optimize the comparison with previous results. EOG artifacts were subtracted using a validated method based on independent component analysis [ICA, Jung et al., 2000]. In all datasets, ICs related to eye movements had a large EOG channel contribution and a frontal scalp distribution. After ICA, epochs were baseline corrected once more, using the pre‐stimulus interval from −0.5 to 0 as reference. Epochs with amplitude values exceeding ±65 μV (i.e., epochs likely to be contaminated by an artifact) were excluded from further analyses. These epochs constituted 4% ± 0.7% of the total number of epochs.
Data Extraction in the Time Domain
Epochs belonging to the same experimental condition and the same modality of the test stimulus were averaged time‐locked to the onset of the test stimulus. This procedure yielded four average waveforms (one for each experimental condition: nociceptive/touch, nociceptive/pain, auditory/touch, and auditory/pain) in each subject. Single‐subject waveforms were finally averaged to obtain group‐level average waveforms. For each peak and each subject, the top 10% of time points displaying the highest increase or decrease in amplitude were extracted [Luck, 2005]. N1, N2, and P2 peak amplitudes and latencies were measured as follows. The N2 and P2 waves were measured at the vertex (Cz) referenced to the nose. The N2 wave was defined as the most negative deflection after stimulus onset. The P2 wave was defined as the most positive deflection after stimulus onset. The N1 wave was measured at both the temporal and the central electrode contralateral to the stimulated side (T7 and C3), referenced to Fz [Hu et al., 2010; Tarkka and Treede, 1993]. It was defined as the most negative deflection preceding the N2 wave, which appears as a positive deflection in this montage. For auditory evoked potentials (AEPs), N1 and P2 waves were measured at the vertex (Cz) referenced to the nose. The N1 wave was defined as the most negative deflection after stimulus onset. The P2 wave was defined as the most positive deflection after stimulus onset.
Data Extraction in the Time–Frequency Domain
An estimate of the amplitude of oscillatory activity as a function of time and frequency was obtained for each EEG epoch, according to the modality of the test stimulus (nociceptive or auditory) and the content of the modulatory visual stimulus (Pain or Touch). Because this estimate is a time‐varying expression of oscillation amplitude regardless of its phase, averaging these estimates across trials discloses both phase‐locked and non‐phase‐locked modulations of signal amplitude, provided that these modulations are both time‐locked to the onset of the event and consistent in frequency (i.e., the latency and frequency at which they occur are reproducible across trials). Therefore, we applied a Morlet wavelet of which the initial spread of the Gaussian envelope was set to 2.5/πω0 (ω0 being the central frequency of the wavelet) [Mouraux and Iannetti, 2008; Mouraux et al., 2003]. The transform expressed the oscillation amplitude as a function of time and frequency. Across‐trial averaging of these time–frequency representations produced a spectrogram of the average EEG oscillation amplitude as a function of time and frequency. For each estimated frequency, results were displayed as an event related percentage (ER %) increase or decrease of oscillation amplitude relative to a pre‐stimulus reference interval (−0.5 to −0.1 s before the onset of each test stimulus), according to the following formula: ERt,f % = [A t,f − R f]/R f, where A t,f is the signal amplitude at a given time t and at a given frequency f, and R f is the signal amplitude averaged within the reference interval [Pfurtscheller and Lopes da Silva, 1999].
Source Analysis
Single trial oscillatory activity was further analyzed using Brain Electrical Source Analysis software (BESA 5.3) [Scherg, 1992; Scherg and Berg, 1996]. To minimize the limitations of source analysis approaches, we used three different strategies to estimate the location of the significant differences between the activity recorded during the two experimental conditions (i.e. the observation of others' pain and touch), as implemented in BESA. First, we calculated the significant differences between the activity in the Touch condition and the activity in the Pain condition (see next paragraph for details). Second, we estimated the generators of the significant differences. The locations of the estimated sources are reported in Talairach coordinates (x, y, and z, in mm). Three different approaches were used to estimate the source locations. (1) A minimum norm algorithm to obtain a surface Minimum Norm Image (MNI). This algorithm is commonly used to estimate a distributed electrical current image at each time and frequency sample [Hämäläinen, 1984]. Since the number of sources is much larger than the number of sensors, the inverse problem is highly underdetermined and must be stabilized by a mathematical constraint, the minimum norm. Out of the many possible current distributions that can account for the recorded data, BESA provides the minimum current solution (i.e., the L2 minimum norm). As opposed to the other approaches (which provide 3D images), the surface minimum norm image is not computed on a volumetric MR image, but on the brain surface. Thus, the results of the minimum norm image resemble a classical scalp topography of cortical currents. The source image is expressed as the total root mean square of the regional sources (0% to 100% activity) (2) A spatial scanning method called Multiple Source Beamformer (MSBF). This approach is commonly used to estimate the sources of the neural activity in a user‐defined time–frequency range, time‐locked to a stimulus event. The BESA beamformer is a modified version of the linearly constrained minimum variance vector beamformer in the time–frequency domain, as described by Gross and colleagues [2001]. The algorithm computes complex cross spectral density matrices, resulting in a normalized output power (±q%). (3) A distributed source analysis based on Classical LORETA (low resolution brain electromagnetic tomography) Analysis Recursively Applied (CLARA). CLARA allows an iterative distributed source analysis method, by performing a weighted LORETA with a reduced source space at each iteration. As compared to LORETA [Pascual‐Marqui et al., 1994], this iterative approach reduces the blurring of the estimated sources while keeping the advantage of a predefined distributed source model, thus making it easier to determine the location of the source with maximal activity [Hamalainen et al., 2010]. A default minimum regularization cut‐off was used. Source image is expressed as current density within a standard MRI image (nAm/cm3).
Statistical Analysis
Preliminary assessment of outlier data revealed that one subject had amplitude values above three standard deviations, and was thus excluded from further analysis in both time and time–frequency domains. Differences in the subjective and electrophysiological responses between the observation of pain and touch in the stranger model were assessed using two‐tailed student's paired t test. Results are reported as mean ± standard error of mean (SEM). Statistical differences were considered significant at P < 0.05.
In the time–frequency domain, as we had no a priori hypothesis on where the modulatory differences might take place in time, frequency and space, we explored all the pixels composing the time–frequency matrix in each channel. In other words, we adopted a data‐driven, assumption‐free approach to identify the time, the frequency, and the scalp topography of experimental differences. This approach required particular attention to the statistical correction of multiple comparisons. First, we applied a correction for multiple comparisons by dividing the standard P value for significance (P = 0.05) by the number of pixels in the epoched spectrogram (n = 2,048 pixels on the temporal axis multiplied by 80 pixels on the frequency axis, resulting in an area of 163,840 pixels). The resulting corrected p value (P = 3 × 10−7) was subsequently divided by the number of EEG electrodes (n = 58). This operation provided a final corrected p value of P = 5 × 10−9, corresponding to a t value of 18.3 (similarly to statistical correction in neuroimaging technique) [Frackowiak, 2004].
Spearman rank correlation coefficients were used to compute the correlation between significant differences in electrophysiological activity and subjective and personality data. Statistical correlations were considered significant at P < 0.05.
RESULTS
Personality Measures and Subjective Ratings
The average scores of the four IRI subscales (ranging from 0 to 28) were as follows: EC: 20.0 (±0.13); FS: 21.7 (±0.18); PD: 17.9 (±0.15); PT: 23.7 (±0.14).
Subjective ratings of stimulus intensity (nociceptive stimulation during Pain and Touch: 56 ± 1.20 and 56 ± 1.30; auditory stimulation during Pain and Touch: 48 ± 1.16 and 50 ± 1.12), unpleasantness (nociceptive stimulation during Pain and Touch: 52 ± 1.30 and 57 ± 1.19; auditory stimulation during Pain and Touch: 31 ± 1.39 and 36 ± 1.44) and saliency (nociceptive stimulation during Pain and Touch: 56 ± 1.36 and 58 ± 1.34; auditory stimulation during Pain and Touch: 53 ± 1.18 and 54 ± 1.23) were never different between the two observational conditions (all P values > 0.05).
Nociceptive and Auditory Evoked Potentials
LEP and AEP group average waveforms in each observational condition are shown in the upper and lower panel of Figure 2, respectively. There was no significant effect of observational condition on the peak amplitude of the main waves of both the LEP (N2: t = −1.55; P = 0.15; P2: t = −1.36; P = 0.20) and the AEP (N1: t = 0.66; P = 0.52; P2: t = −1.85; P = 0.09).
Figure 2.
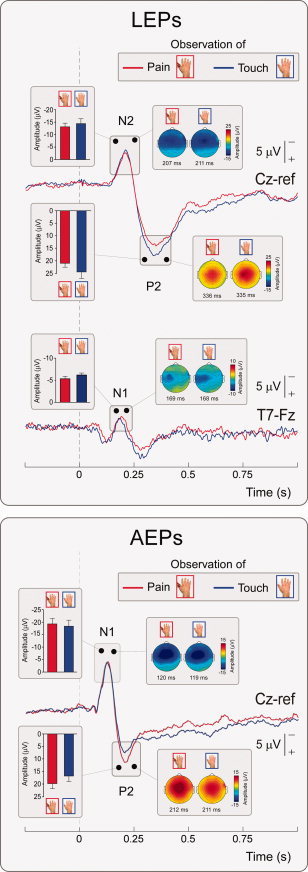
Group‐level average LEP (upper panel) and AEP (lower panel) waveforms in the two experimental conditions. Displayed signals were recorded at electrode Cz referenced to the nose (AEPs; N2–P2 waves of LEPs) and T7 referenced to Fz (N1 wave of LEPs). x axis, time (s); y axis, amplitude (μV). Waveforms extracted during observation of others' pain and touch are represented in red and blue, respectively. The vertical dashed lines mark the onset of the test stimulus. Scalp maps at peak latency and relative peak amplitudes (±SEM) are shown in the insets. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Similarly, there was no significant effect of observational condition on the peak amplitude of the N1 LEP wave, either when measured at electrode C3‐Fz (t = −1.33; P = 0.21) or at T7‐Fz (t = −1.75; P = 0.11) (Fig. 2, bottom upper panel).
Moreover, there was no significant effect of the observational condition on the peak latencies of both the LEP (N1 at C3‐Fz: t = 0.08; P = 0.49; N1 at T7‐Fz: t = 0.03; P = 0.65; N2: t = 0.72; P = 0.49; P2: t = 0.25; P = 0.81) and the AEP (N1: t = 0.76; P = 0.31; P2: t = −0.12; P = 0.90).
Nociceptive and Auditory Related Activity in the Time–Frequency Domain
Group average time–frequency spectrograms of both laser‐ and auditory‐induced modulation of EEG oscillation amplitudes during the observation of Touch and Pain conditions are shown in Figure 3.
Figure 3.
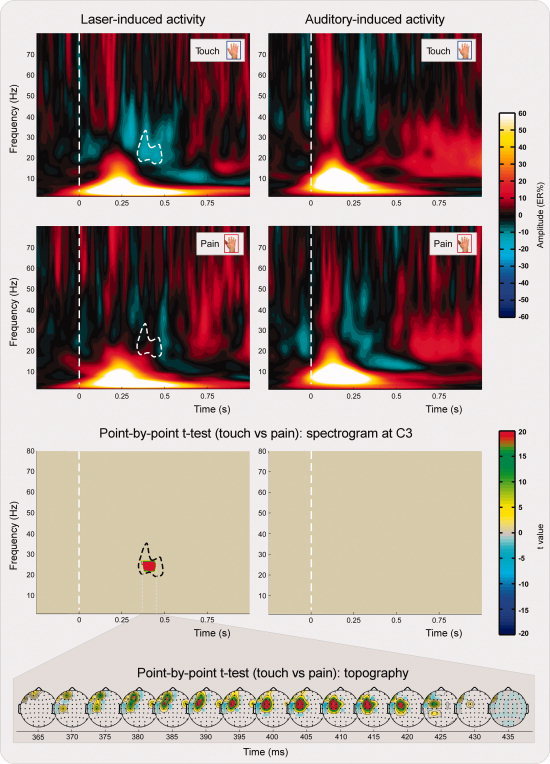
Group‐level average time–frequency representation of nociceptive (left panel) and auditory (right panel) related brain activity in the two experimental conditions. Spectrograms represent the response obtained at electrode C3. x axis, time (s); y axis, frequency (Hz); color scale represents baseline corrected oscillatory amplitude (ER%). The top and middle panels show the group‐level time–frequency response during the observation of touch (blue color, Touch) and pain in others (red color, Pain), respectively. Note that the β ERD observed at 21.5–26.5 Hz and 365–435 ms in the Touch condition is significantly reduced during Pain. The bottom panel shows the results of the point‐by‐point paired t test statistics obtained on the time–frequency representations of nociceptive (left) and auditory (right) related brain activity. The significant difference in the β ERD between Touch and Pain, present only in the time–frequency response elicited by nociceptive stimuli (left), displayed a frontocentral scalp topography, as shown by the time series of scalp maps, plotted every 5 ms. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Although the observational condition had no significant effect on the time–frequency responses induced by auditory stimulation (Fig. 3, right plots), it had a significant effect on the time–frequency responses induced by laser stimulation (Fig. 3, left plots). This effect, localized in a time window between 365 and 435 ms, and in a frequency window between 21.5 and 26.5 Hz, was present on the left central and frontal electrodes contralateral to the stimulated hand (Fig. 3, bottom), and consisted in a reduced ERD activity during the observation of others' pain (t > 18.3; mean ER% difference of 12% ± 2%). This spatial and time–frequency interval corresponds to the sensorimotor β band activity, which is typically found during actual and imagined movement of the contralateral hand [Hari et al., 1998].
Source Analysis
Three different strategies to estimate the location of the significant differences between the electrical activity recorded during other pain and other touch observation were used (Fig. 4). The Minimum norm surface image localized the statistically significant difference at coordinates corresponding to the precentral gyrus (BA 4; x = 28, y = −25, z = 67). The spatial beamforming approach provided a volume image of the statistically significant difference over sensorimotor cortex, with maximal explained variance in the precentral gyrus (BA 6; x = 27, y = −9, z = 67) and in the postcentral gyrus (BA 2; x = 27, y = −34, z = 67). Finally, CLARA distributed source image also localized the difference in the precentral gyrus (BA 4; x = 20, y = −23, z = 54).
Figure 4.
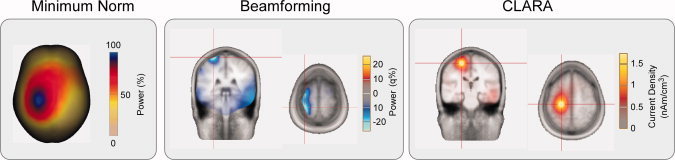
Source localization of the difference between the oscillatory responses during the observation of other's pain and other's touch (β ERD, see Fig. 3). Three different source localization approaches were used: Minimum Norm Image (MNI), Multiple Source Beamformer (MSBF), and Classical LORETA Analysis Recursively Applied (CLARA). The results are projected onto a standard MRI in Talairach space. Left panel: MNI. Maximal activity was located at x = 28, y = −25, z = 67 (corresponding to the precentral gyrus, BA 4). Middle panel, MSBF. Maximal activity was distributed over the sensorimotor area, with two maxima. One located at x = 27, y = −9, z = 67 (corresponding to the precentral gyrus, BA 6) and the other located at x = 27, y = −34, z = 67 (corresponding to the postcentral gyrus, BA 2). Right panel, CLARA. Maximal activity was located at x = 20, y = −23, z = 54 (corresponding to the precentral gyrus, BA 4). Note that all three different source analysis approaches support a source located in the sensorimotor cortex, with a peak of activity corresponding to the precentral gyrus. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Correlation analysis
A positive significant correlation between the mean ER% difference of Pain and Touch conditions and the ratings of similarity of the displayed hand was found (ρ = 0.68; P < 0.05; Fig. 5) indicating that the Pain—Touch ER% difference was higher in the subjects who reported higher similarity between the observed and the “represented” hand (the group average rating of similarity was 6.67 ± 0.12).
Figure 5.
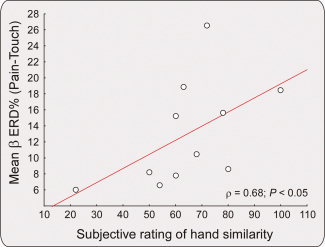
Spearman Rho (ρ) rank correlation between subjective rating of hand similarity (x axis) and significant differences in β oscillatory amplitude (ER%, y axis). The scatterplot shows how the higher the rating of similarity, the higher the difference in β oscillations between pain and touch observational conditions (ρ = 0.68; P < 0.05), with lower β ERD during others' pain observation. In other words, the more the subjects perceived the observed hand as similar to their own representation of a male hand, the higher the sensorimotor inhibition (as indexed by a reduction of β desynchronization). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
There were no significant correlations between the index of electrophysiological activity recorded during the observation of others' pain or touch and the interpersonal reactivity subscales or subjective pain ratings (all P s > 0.5).
DISCUSSION
By recording the laser‐ and auditory‐evoked EEG responses during the observation of videoclips showing others' pain and touch, we demonstrate a modulation of the cortical activity elicited by nociceptive laser stimuli, but not of the cortical activity elicited by auditory stimuli (Fig. 3). The EEG response modulated by the observation of other's pain was an event‐related desynchronization in the beta band (β ERD), with a scalp maximum on the fronto‐central electrodes contralateral to the stimulated hand (Fig. 3, bottom panel). By using three different source analysis approaches, we provide converging evidence that such modulation affects neural activity in the contralateral primary sensorimotor cortex (see Fig. 4). Finally, the magnitude of this specific modulation correlates with a subjective measure of similarity between the model's hand and the onlookers' representation of the hand (see Fig. 5).
Altogether, our findings demonstrate that the observation of others' pain modulates, in a somatosensory‐specific fashion the cortical responses elicited by nociceptive stimuli in the sensorimotor cortex contralateral to the stimulated hand.
Observation of Pain in Others Modulates β Desynchronization in Sensorimotor Regions
Postsynaptic potentials in pyramidal cortical neurons are known to generate patterns of event‐related synchronization/desynchronization (ERS/ERD) of scalp EEG oscillations at different frequencies [Pfurtscheller and Lopes da Silva, 1999]. Suppression of oscillatory amplitude in the 15–35 Hz band (β ERD) has been related to an increased excitability of a thalamocortical gate, which can be “opened” by endogenous or exogenous events [Steriade and Llinas, 1988]. Animal and human studies indicate that a reduction of magnitude of β oscillations (i.e., β ERD) occurs in a large number of brain regions, including the basal ganglia [Alegre et al., 2005; Courtemanche et al., 2003], the thalamus [Paradiso et al., 2004], and the posterior parietal cortex [Brovelli et al., 2004; MacKay and Mendonca, 1995], and is associated to actual or imagined motor activity. It is worth noting that the most prominent source of β ERD is located in the contralateral perirolandic region [Formaggio et al., 2008; Pfurtscheller, 1989; Sanes and Donoghue, 1993; Schnitzler et al., 1997]. Moreover, faster reaction times to target stimuli are associated to low magnitude of ongoing β oscillations over the sensorimotor cortex [Senkowski et al., 2006; Tzagarakis et al., 2010; Zhang et al., 2008]. These findings are consistent with the notion that while β band synchronization (ERS) reflects a state of maintenance of posture, β band desynchronization (ERD) reflects preparation to act [Androulidakis et al., 2007; Gilbertson et al., 2005]. Studies indicate that β ERD over the sensorimotor cortex is present not only during voluntary movement [Stancak and Pfurtscheller, 1995; Toma et al., 2000] and movement imagery [Babiloni et al., 2002; Cochin et al., 1999; Pfurtscheller and Neuper, 1997], but also during the observation of others' movements [Hari et al., 1998; Neuper et al., 2006, 2009].
β ERD has also been found during nociceptive stimulation [Hauck et al., 2007; Mouraux et al., 2003; Ohara et al., 2004; Ploner et al., 2006; Raij et al., 2004] and linked to a possible motor preparatory response to the laser stimulus. Interestingly, the reduction of β ERD during observation of other's pain (Figs. 3 and 4) is opposite to what found during first‐person pain perception and thus suggests a reduced motor preparatory response to nociceptive stimuli received during other's pain observation. It is relevant that three independent source analysis methods indicate the β ERD effect involves neural activity in primary motor and somatosensory cortices. Relevant to the aim of the present study is that the β ERD reduction occurred during nociceptive but not during auditory stimulation, thus showing that the reactivity to others' pain has a specific effect on the brain responses elicited by nociceptive somatosensory stimulation (see Fig. 3).
Importantly, converging evidence from three different source analysis approaches indicates that the somatosensory‐specific modulation is likely to arise from the interplay between primary somatosensory and motor structures in the hemisphere controlateral to the stimulated body district. Indeed, all three source analysis approaches (see Fig. 4) estimated the highest difference between pain and touch observation in the hemisphere contralateral to the stimulated hand, in a region including the primary sensorimotor cortex, the premotor cortex and, possibly, the supplementary motor cortex. In particular, the spatial scanning beamformer showed the presence of a double pre‐ and post‐rolandic source. This observation was confirmed by CLARA iterative analysis, which, by estimating the overall strength of the field instead of its variance (as the beamformer does) [Vrba and Robinson, 2001], indicated that the highest difference between pain and touch observation was arising in a cortical area located in between the two sources estimated with the beamformer approach, and in full agreement with the source image estimated using the Minimum Norm approach (see Fig. 4). Notably, the location of these cortical sources is consistent with previous EEG and magnetoencephalographic (MEG) reports on the origin of the brain oscillations occurring in the β band (∼15–35 Hz) in the sensorimotor region during both the performance and the observation of movements [Hari et al., 1998; Kilner et al., 2009; Neuper and Pfurtscheller, 2001; Pfurtscheller and Berghold, 1989; Salmelin and Hari, 1994].
In keeping with a previous MEG study [Betti et al., 2009] the present study complements and expands TMS [Avenanti et al., 2005, 2009] and somatosensory evoked potential [Bufalari et al., 2008] reports indicating that vision of nociceptive stimuli delivered to others induces changes of neural activity also in the primary sensory and motor cortices. Given that no overt motor reaction was allowed during the experimental task, the reduced sensorimotor reactivity is likely to reflect a inhibitory response to the observed pain in the sensorimotor structures, making them less sensitive to the incoming nociceptive test stimulus.
It is highly likely that nociceptive specific modulation of β ERD entails a strong attentional modulation. Indeed, our subjects were instructed to pay attention to the visual stimuli while they were receiving either a nociceptive laser or a non‐nociceptive auditory stimulus. Thus, an interaction between top‐down (i.e., endogenous) and bottom‐up (i.e., exogenous) attentional processes may result in a specific inhibition of the onlooker motor preparatory response, reflected by the reduction of the β ERD. That is, the exogenous cue represented by the laser stimulus delivered to the onlookers' hand was contrasted by the syringe penetrating the model's hand, which was more effective in keeping onlookers' attention focused on the sensory event taking place on the strangers' hand [Sambo et al., 2009; Simon‐Dack et al., 2009]. The latency of the observed β ERD effect (i.e., 350–450 ms post‐stimulus, Fig. 3) is indeed compatible with the interaction of top‐down and bottom‐up attentional processes, which are well represented by late neural responses (e.g., the P300 wave) evoked by a wide range of sensory stimuli, and classically associated to both involuntary re‐orienting of attention, contextual update and conscious appraisal [Polich, 2007; Valentini et al., 2011]. It is also worth emphasizing that, as indicated by the correlation analysis (see Fig. 5), the β ERD reduction upon others' pain observation was higher in the participants who perceived the models' hand as similar to a typical hand. This observation is in keeping with accounts of empathy highlighting that appraisal of body representation may regulate automatic interpersonal reactivity [Decety and Lamm, 2006; Goubert et al., 2005; Hein and Singer, 2008].
Lack of Influence of Pain Observation on Laser‐Evoked Potentials
The only LEPs study on empathy for pain performed so far [Valeriani et al., 2008] reports that observing pain in others does not cause any modulation of the N2‐P2 wave. Our results (1) confirm this lack of a modulatory effect of observing others' pain on the main LEP vertex complex (i.e., the N2‐P2 biphasic wave), but (2) show a lack of modulation of its early contralateral activity (i.e., the N1 wave) (see Fig. 2). Thus, we cannot confirm the inhibitory effect of observing others' pain reported by Valeriani et al. [2008] on the amplitude of the early‐latency N1 wave of the LEP. Although we do not have an obvious explanation for this discrepancy, an important methodological difference between the two studies has to be noted. Whereas we reported raw LEP amplitudes, the previous study expressed the N1 amplitude measured during others' pain observation as percent change compared to the N1 amplitude measured during the observation of a static hand. It is known that the measurement of the N1 in single subjects often yields near‐to‐zero values [Hu et al., 2010], and that percent changes using near‐to‐zero values are prone to produce extreme values that can bias the results. However, even assuming that the modulation observed by Valeriani et al. [2008] was causally related to the observation of others' pain, the conclusion that it affected cortical activities specifically related to nociceptive processing was not justified, as no control stimulus was employed.
CONCLUSION
Our experiment shows, for the very first time, the specificity of the modulation induced by the observation of others' pain on the neural activity elicited by nociceptive stimuli, while controlling for the modulation of the neural activity elicited by stimuli belonging to a different sensory modality. Though simple, this control comparison had never been performed by researchers claiming the existence of a specific effect on the nociceptive system of the onlooker [Singer et al., 2004; Valeriani et al., 2008]. We thus demonstrate that the experience of others' pain mediated by vision modulates specifically the neural activity in the onlookers' sensorimotor cortex, and that this modulation significantly occurs only in the neural activity elicited by stimuli belonging to the nociceptive, rather than to another sensory modality.
Acknowledgements
The authors thank Dr. Alessio Avenanti for providing the stimuli used in this study. They also thank Dr. Li Hu and Dr. André Mouraux for their helpful comments on the previous version of the manuscript. GDI is grateful to El.En. for its generous support.
REFERENCES
- Aglioti SM, Pazzaglia M ( 2011): Sounds and scents in (social) action. Trends Cogn Sci 15: 47–55. [DOI] [PubMed] [Google Scholar]
- Alegre M, Alonso‐Frech F, Rodriguez‐Oroz MC, Guridi J, Zamarbide I, Valencia M, Manrique M, Obeso JA, Artieda J ( 2005): Movement‐related changes in oscillatory activity in the human subthalamic nucleus: Ipsilateral vs. contralateral movements. Eur J Neurosci 22: 2315–2324. [DOI] [PubMed] [Google Scholar]
- Androulidakis AG, Doyle LM, Yarrow K, Litvak V, Gilbertson TP, Brown P ( 2007): Anticipatory changes in beta synchrony in the human corticospinal system and associated improvements in task performance. Eur J Neurosci 25: 3758–3765. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM ( 2005): Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nat Neurosci 8: 955–960. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Minio‐Paluello I, Bufalari I, Aglioti SM ( 2006): Stimulus‐driven modulation of motor‐evoked potentials during observation of others' pain. Neuroimage 32: 316–324. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Minio‐Paluello I, Sforza A, Aglioti SM ( 2009): Freezing or escaping? Opposite modulations of empathic reactivity to the pain of others. Cortex 45: 1072–1077. [DOI] [PubMed] [Google Scholar]
- Avikainen S, Forss N, Hari R ( 2002): Modulated activation of the human SI and SII cortices during observation of hand actions. Neuroimage 15: 640–646. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Babiloni F, Carducci F, Cincotti F, Cocozza G, Del Percio C, Moretti DV, Rossini PM ( 2002): Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: A high‐resolution EEG study. Neuroimage 17: 559–572. [PubMed] [Google Scholar]
- Baumgartner U, Cruccu G, Iannetti GD, Treede RD ( 2005): Laser guns and hot plates. Pain 116: 1–3. [DOI] [PubMed] [Google Scholar]
- Betti V, Zappasodi F, Rossini PM, Aglioti SM, Tecchio F ( 2009): Synchronous with your feelings: Sensorimotor {gamma} band and empathy for pain. J Neurosci 29: 12384–12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brovelli A, Ding M, Ledberg A, Chen Y, Nakamura R, Bressler SL ( 2004): Beta oscillations in a large‐scale sensorimotor cortical network: Directional influences revealed by Granger causality. Proc Natl Acad Sci USA 101: 9849–9854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM ( 2007): Empathy for pain and touch in the human somatosensory cortex. Cereb Cortex 17: 2553–2561. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lin CP, Liu HL, Hsu YY, Lim KE, Hung D, Decety J ( 2007): Expertise modulates the perception of pain in others. Curr Biol 17: 1708–1713. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Yang CY, Lin CP, Lee PL, Decety J ( 2008): The perception of pain in others suppresses somatosensory oscillations: A magnetoencephalography study. Neuroimage 40: 1833–1840. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Roux S, Martineau J ( 1999): Observation and execution of movement: Similarities demonstrated by quantified electroencephalography. Eur J Neurosci 11: 1839–1842. [DOI] [PubMed] [Google Scholar]
- Courtemanche R, Fujii N, Graybiel AM ( 2003): Synchronous, focally modulated beta‐band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J Neurosci 23: 11741–11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M ( 1983): The effects of dispositional empathy on emotional reactions and helping: A multidimensional approach. J Pers 51: 167–184. [Google Scholar]
- Decety J, Lamm C ( 2006): Human empathy through the lens of social neuroscience. Sci World J 6: 1146–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S ( 2004): EEGLAB: An open source toolbox for analysis of single‐trial EEG dynamics including independent component analysis. J Neurosci Methods 134: 9–21. [DOI] [PubMed] [Google Scholar]
- Farina S, Valeriani M, Rosso T, Aglioti S, Tamburin S, Fiaschi A, Tinazzi M ( 2001): Transient inhibition of the human motor cortex by capsaicin‐induced pain. A study with transcranial magnetic stimulation. Neurosci Lett 314: 97–101. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Pascual‐Leone A, Theoret H ( 2008): Psychopathy and the mirror neuron system: Preliminary findings from a non‐psychiatric sample. Psychiatry Res 160: 137–144. [DOI] [PubMed] [Google Scholar]
- Formaggio E, Storti SF, Avesani M, Cerini R, Milanese F, Gasparini A, Acler M, Pozzi Mucelli R, Fiaschi A, Manganotti P ( 2008): EEG and FMRI coregistration to investigate the cortical oscillatory activities during finger movement. Brain Topogr 21: 100–111. [DOI] [PubMed] [Google Scholar]
- Frackowiak RSJ ( 2004): Human Brain Function. Amsterdam, Boston: Elsevier Academic Press; xvi, 1144 p. [Google Scholar]
- Gescheider G (1997): “Chapter 3: The Classical Psychophysical Methods” Psychophysics: the fundamentals (3rd ed.). Lawrence Erlbaum Associates. [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brown P ( 2005): Existing motor state is favored at the expense of new movement during 13–35 Hz oscillatory synchrony in the human corticospinal system. J Neurosci 25: 7771–7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubert L, Craig KD, Vervoort T, Morley S, Sullivan MJ, de CWAC, Cano A, Crombez G ( 2005): Facing others in pain: The effects of empathy. Pain 118: 285–288. [DOI] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R ( 2001): Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA 98: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamalainen JA, Ortiz‐Mantilla S, Benasich AA ( 2010): Source localization of event‐related potentials to pitch change mapped onto age‐appropriate MRIs at 6 months of age. Neuroimage 54:1910–1918. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M, Ilmoniemi RJ ( 1984): Interpreting Measured Magnetic Fields of the Brain: Estimates of Current Distributions. Espoo: Helsinki University of Technology; p TKK–A559. [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G ( 1998): Activation of human primary motor cortex during action observation: A neuromagnetic study. Proc Natl Acad Sci USA 95: 15061–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck M, Lorenz J, Engel AK ( 2007): Attention to painful stimulation enhances gamma‐band activity and synchronization in human sensorimotor cortex. J Neurosci 27: 9270–9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G, Singer T ( 2008): I feel how you feel but not always: The empathic brain and its modulation. Curr Opin Neurobiol 18: 153–158. [DOI] [PubMed] [Google Scholar]
- Hu L, Mouraux A, Hu Y, Iannetti GD ( 2010): A novel approach for enhancing the signal‐to‐noise ratio and detecting automatically event‐related potentials (ERPs) in single trials. Neuroimage 50: 99–111. [DOI] [PubMed] [Google Scholar]
- Iannetti GD, Mouraux A ( 2010): From the neuromatrix to the pain matrix (and back). Exp Brain Res 205: 1–12. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Brunet E, Meltzoff AN, Decety J ( 2006): Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia 44: 752–761. [DOI] [PubMed] [Google Scholar]
- Jackson PL, Meltzoff AN, Decety J ( 2005): How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage 24: 771–779. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ ( 2000): Removing electroencephalographic artifacts by blind source separation. Psychophysiology 37: 163–178. [PubMed] [Google Scholar]
- Kilner JM, Marchant JL, Frith CD ( 2009): Relationship between activity in human primary motor cortex during action observation and the mirror neuron system. PLoS One 4: e4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J ( 2007): What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One 2: e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Mouraux A, Iannetti GD ( 2009): Characterizing the cortical activity through which pain emerges from nociception. J Neurosci 29: 7909–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A ( 2011): The pain matrix reloaded A salience detection system for the body. Prog Neurobiol 93: 111–124. [DOI] [PubMed] [Google Scholar]
- Luck SJ ( 2005): An Introduction to the Event‐Related Potential Technique. Cambridge, MA: MIT Press. [Google Scholar]
- MacKay WA, Mendonca AJ ( 1995): Field potential oscillatory bursts in parietal cortex before and during reach. Brain Res 704: 167–174. [DOI] [PubMed] [Google Scholar]
- Minio‐Paluello I, Avenanti A, Aglioti SM ( 2006): Left hemisphere dominance in reading the sensory qualities of others' pain? Soc Neurosci 1: 320–333. [DOI] [PubMed] [Google Scholar]
- Minio‐Paluello I, Baron‐Cohen S, Avenanti A, Walsh V, Aglioti SM ( 2009): Absence of embodied empathy during pain observation in Asperger syndrome. Biol Psychiatry 65: 55–62. [DOI] [PubMed] [Google Scholar]
- Moriguchi Y, Decety J, Ohnishi T, Maeda M, Mori T, Nemoto K, Matsuda H, Komaki G ( 2007): Empathy and judging other's pain: An fMRI study of alexithymia. Cereb Cortex 17: 2223–2234. [DOI] [PubMed] [Google Scholar]
- Morrison I, Lloyd D, di Pellegrino G, Roberts N ( 2004): Vicarious responses to pain in anterior cingulate cortex: Is empathy a multisensory issue? Cogn Affect Behav Neurosci 4: 270–278. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD ( 2011): A multisensory investigation of the functional significance of the pain matrix. Neuroimage 54: 2237–2249. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Guerit JM, Plaghki L ( 2003): Non‐phase locked electroencephalogram (EEG) responses to CO2 laser skin stimulations may reflect central interactions between A partial differential‐ and C‐fibre afferent volleys. Clin Neurophysiology 114: 710–722. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD ( 2008): Across‐trial averaging of event‐related EEG responses and beyond. Magn Reson Imaging 26: 1041–1054. [DOI] [PubMed] [Google Scholar]
- Mouraux A, Iannetti GD ( 2009): Nociceptive laser‐evoked brain potentials do not reflect nociceptive‐specific neural activity. J Neurophysiol 101: 3258–3269. [DOI] [PubMed] [Google Scholar]
- Neuper C, Muller‐Putz GR, Scherer R, Pfurtscheller G ( 2006): Motor imagery and EEG‐based control of spelling devices and neuroprostheses. Prog Brain Res 159: 393–409. [DOI] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G ( 2001): Evidence for distinct beta resonance frequencies in human EEG related to specific sensorimotor cortical areas. Clin Neurophysiol 112: 2084–2097. [DOI] [PubMed] [Google Scholar]
- Neuper C, Scherer R, Wriessnegger S, Pfurtscheller G ( 2009): Motor imagery and action observation: Modulation of sensorimotor brain rhythms during mental control of a brain‐computer interface. Clin Neurophysiol 120: 239–247. [DOI] [PubMed] [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz FA ( 2004): Attention to a painful cutaneous laser stimulus modulates electrocorticographic event‐related desynchronization in humans. Clin Neurophysiol 115: 1641–1652. [DOI] [PubMed] [Google Scholar]
- Paradiso G, Cunic D, Saint‐Cyr JA, Hoque T, Lozano AM, Lang AE, Chen R ( 2004): Involvement of human thalamus in the preparation of self‐paced movement. Brain 127 ( Part 12): 2717–2731. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD, Michel CM, Lehmann D ( 1994): Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int J Psychophysiol 18: 49–65. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G ( 1989): Functional topography during sensorimotor activation studied with event‐related desynchronization mapping. J Clin Neurophysiol 6: 75–84. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Berghold A ( 1989): Patterns of cortical activation during planning of voluntary movement. Electroencephalogr Clin Neurophysiol 72: 250–258. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH ( 1999): Event‐related EEG/MEG synchronization and desynchronization: Basic principles. Clin Neurophysiol 110: 1842–1857. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C ( 1997): Motor imagery activates primary sensorimotor area in humans. Neurosci Lett 239: 65–68. [DOI] [PubMed] [Google Scholar]
- Ploner M, Gross J, Timmermann L, Pollok B, Schnitzler A ( 2006): Pain suppresses spontaneous brain rhythms. Cereb Cortex 16: 537–540. [DOI] [PubMed] [Google Scholar]
- Polich J ( 2007): Updating P300: An integrative theory of P3a and P3b. Clin Neurophysiol 118: 2128–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raij TT, Forss N, Stancak A, Hari R ( 2004): Modulation of motor‐cortex oscillatory activity by painful Adelta‐ and C‐fiber stimuli. Neuroimage 23: 569–573. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V ( 2001): Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci 2: 661–670. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R ( 1994): Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. Neuroscience 60: 537–550. [DOI] [PubMed] [Google Scholar]
- Sambo CF, Gillmeister H, Forster B ( 2009): Viewing the body modulates neural mechanisms underlying sustained spatial attention in touch. Eur J Neurosci 30: 143–150. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP ( 1993): Oscillations in local field potentials of the primate motor cortex during voluntary movement. Proc Natl Acad Sci USA 90: 4470–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherg M ( 1992): Functional imaging and localization of electromagnetic brain activity. Brain Topogr 5: 103–111. [DOI] [PubMed] [Google Scholar]
- Scherg M, Berg P ( 1996): New concepts of brain source imaging and localization. Electroencephalogr Clin Neurophysiol Suppl 46: 127–137. [PubMed] [Google Scholar]
- Schlereth T, Magerl W, Treede R ( 2001): Spatial discrimination thresholds for pain and touch in human hairy skin. Pain 92: 187–194. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Salenius S, Salmelin R, Jousmaki V, Hari R ( 1997): Involvement of primary motor cortex in motor imagery: A neuromagnetic study. Neuroimage 6: 201–208. [DOI] [PubMed] [Google Scholar]
- Senkowski D, Molholm S, Gomez‐Ramirez M, Foxe JJ ( 2006): Oscillatory beta activity predicts response speed during a multisensory audiovisual reaction time task: A high‐density electrical mapping study. Cereb Cortex 16: 1556–1565. [DOI] [PubMed] [Google Scholar]
- Simon‐Dack SL, Cummings SE, Reetz DJ, Alvarez‐Vazquez E, Gu H, Teder‐Salejarvi WA ( 2009): Touched by light: Event‐related potentials (ERPs) to visuo‐haptic stimuli in peri‐personal space. Brain Topogr 21: 261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD ( 2004): Empathy for pain involves the affective but not sensory components of pain. Science 303: 1157–1162. [DOI] [PubMed] [Google Scholar]
- Stancak A Jr, Pfurtscheller G ( 1995): Desynchronization and recovery of beta rhythms during brisk and slow self‐paced finger movements in man. Neurosci Lett 196: 21–24. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinas RR ( 1988): The functional states of the thalamus and the associated neuronal interplay. Physiol Rev 68: 649–742. [DOI] [PubMed] [Google Scholar]
- Tarkka IM, Treede RD ( 1993): Equivalent electrical source analysis of pain‐related somatosensory evoked potentials elicited by a CO2 laser. J Clin Neurophysiol 10: 513–519. [DOI] [PubMed] [Google Scholar]
- Toma K, Nagamine T, Yazawa S, Terada K, Ikeda A, Honda M, Oga T, Shibasaki H ( 2000): Desynchronization and synchronization of central 20‐Hz rhythms associated with voluntary muscle relaxation: A magnetoencephalographic study. Exp Brain Res 134: 417–425. [DOI] [PubMed] [Google Scholar]
- Treede RD ( 1995): Peripheral acute pain mechanisms. Ann Med 27: 213–216. [DOI] [PubMed] [Google Scholar]
- Tzagarakis C, Ince NF, Leuthold AC, Pellizzer G ( 2010): Beta‐band activity during motor planning reflects response uncertainty. J Neurosci 30: 11270–11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentini E, Torta DM, Mouraux A, Iannetti GD ( 2011): Dishabituation of laser‐evoked EEG responses: Dissecting the effect of certain and uncertain changes in stimulus modality. J Cogn Neurosci 10:2822–2837. [DOI] [PubMed] [Google Scholar]
- Valeriani M, Betti V, Le Pera D, De Armas L, Miliucci R, Restuccia D, Avenanti A, Aglioti SM ( 2008): Seeing the pain of others while being in pain: A laser‐evoked potentials study. Neuroimage 40: 1419–1428. [DOI] [PubMed] [Google Scholar]
- Vrba J, Robinson SE ( 2001): Signal processing in magnetoencephalography. Methods 25: 249–271. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Bressler SL, Chen Y, Ding M ( 2008): Prestimulus cortical activity is correlated with speed of visuomotor processing. J Cogn Neurosci 20: 1915–1925. [DOI] [PubMed] [Google Scholar]


