Abstract
This study was undertaken to investigate the reciprocity effect between postural and suprapostural performances and its underlying neural mechanisms wherein subjects executed a perceptual‐motor suprapostural task and maintained steady upright postures. Fourteen healthy individuals conducted force‐matching maneuvers (static vs. dynamic) under two stance conditions (bipedal stance vs. unipedal stance); meanwhile, force‐matching error, center of pressure dynamics, event‐related potentials (ERPs), and the movement‐related potential (MRP) were monitored. The behavioral results showed that force‐matching error and postural sway were differently modulated by variations in stance pattern and force‐matching version. Increase in postural challenge undermined the precision of static force‐matching but facilitated a dynamic force‐matching task. Both static and dynamic force‐matching tasks improved postural control of unipedal stance but not of bipedal stance, in reference to the control conditions. ERP results revealed a stance‐dependent N1 response, which was greater around the parietal cortex in the unipedal stance conditions. Instead, P2 was modulated by the effect of the suprapostural motor task, with a smaller P2 in the right parietal cortex for dynamic force‐matching. Spatiotemporal evolution of the MRP commenced at the left frontal‐central area and spread bilaterally over the frontal‐central and parietal cortex. MRP onset was subject to an analogous interaction effect on force‐matching performance. Our findings suggest postural prioritization and a structural alternation effect of stance pattern on postural performance, relevant to implicit expansion and selective allocation of central resources for relative task‐loads of a postural‐suprapostural task. Hum Brain Mapp, 2013. © 2011 Wiley Periodicals, Inc.
Keywords: attentional resource, event‐related potential, movement‐related potential, postural balance, suprapostural task
INTRODUCTION
Human stance is felt to be an automatic task without higher‐level cortical processing, as large‐scale stance synergies are well established. Recently, however, the evidence has indicated that stance equilibrium is a complex physical task in need of continuing attentional resources [Swan et al.,2007; Woollacott and Shumway‐Cook,2002]. When a suprapostural task is performed on upright stance (postural task), it requires further central resources for posture control and achievement of suprapostural goal. The outcome performance of parallel loading seems to be a trade‐off result [Maki and McIlroy,1996; Pellecchia,2003], and demand compatibility centrally determines the reciprocity effect for competing or sharing central resources on the two response programs of postural and suprapostural tasks [Sigman and Dehaene,2008]. For upright stance concurrent with a working memory task, standing with the feet lined up heel‐to‐toe placed greater postural demands on central resources than wide stance [Reilly et al.,2008]. Alternatively, an auditory‐spatial task introduced a greater postural sway as compared with the conditions employing auditory‐object and visual‐object tasks [Woollacott and Velde,2008].
Abbreviations.
- ACoP
absolute CoP
- AFE
absolute force error
- CoP
center‐of‐pressure
- EEG
electroencephalogram
- EMG
electromyogram
- ERP
event‐related potential
- FDI
first dorsal interosseous
- MRP
movement‐related potential
- MVC
maximal voluntary contraction
- NCoP
normalized CoP
- NFE
normalized force error
- RT
reaction time
- RQA
recurrence quantification analysis
The current theoretical framework used to illustrate operation of postural control with a suprapostural goal tends to gravitate towards the binding of a non‐perceptual cognitive or a verbal task with stance condition [Andersson et al.,2002; Fraizer and Mitra,2008]. As a result of competition between postural and suprapostural activities for a limited‐capacity of central resources, suprapostural performance and/or postural stability is sacrificed. However, considering the degree of functional overlap in resource pools and demands compatibility, resource allocation for a postural task with a suprapostural perceptual‐motor goal (such as juggling on stance) may differ from that for the classic posture‐cognition setups [Klingberg,2000]. Postural and suprapostural motor performances can both be degraded by the rather intensive resource competition between the two motor subsystems with reciprocally‐related dynamics. Alternatively, stance and perceptual‐motor suprapostural tasking can be functionally integrated as a perception‐action unit [Stoffregen et al.,2007], such that addition of suprapostural task does not necessarily impose a more competitive command nor undermine response outcomes. In fact, behavioral experiments suggested a variety of reciprocity results. Accompanied motor activity may destabilize upright stance or benefits from reduction in postural sway conditional to stance pattern [Wulf et al.,2004; Huang et al.2010].
Movement‐related potential (MRP) and event‐related potential (ERP) consist of several subcomponents in different temporal windows. They are valuable to exploration of time‐dependent attentional states and information scheduling for a dual tasking (or postural‐suprapostural tasking) with postural and motor subtasks. Preceding movement onset by approximately 1.5 seconds, a negative MRP with subsequent negative slope is functionally related to preparation of a voluntary motor act. The MRPs are organized with task complexity. A complex sequential task is associated with earlier and higher negativity in the midcentral and parietal recordings than a simple movement [Lang et al.,1989; Simonetta et al.,1991]. The amplitude of earlier ERP components (N1, 80‐150 ms post‐stimulus and P2, 150‐240 ms post‐stimulus) is subject to the level of attention or arousal [Hillyard et al.,1973; Woldorff and Hillyard,1991]. In particular, being time‐locked to stance disturbance, N1 response is considered to be a physiological indicator of attentive control over posture stability [Quant et al.,2004; Adkin et al.,2008]. A later component of P3 wave (roughly 300 ms post‐stimulus), typically measured by electrodes covering the parietal lobe, is a metric of cognitive function in decision making [Başar‐Eroglu et al.,2001]. The topography and timing of ERP can vary with task approach (or modality pairing) designed for a dual‐task experiment. For classic dual‐task setups using a secondary perceptual‐cognitive or visuospatial task, cognitive load leads to P3 wave modulation in the frontoparietal networks [Gontier et al.,2007; Sigman and Dehaene,2008], indexing additional central costs for response‐related interference. Surprisingly, so far, no ERP has been used to assess central cost and information scheduling for upright stance with a perceptual‐motor suprapostural task, although behavioral approaches lend limited insight into underlying cortical processes.
Behavior phenomena of a postural‐suprapostural task are compromised by task difficulty [McNevin and Wulf,2002; Vuillerme and Nougier,2004], since a relative task‐load biases the resource allocation and attentional prioritization in one or alternative postural‐suprapostural components [Huang et al.,2010; Swan et al.,2007]. For a postural‐suprapostural task, an increase in task‐load may intensify the reciprocity effect between the postural and suprapostural motor components. This study first examined the neuroprocessing level of steady stance with a perceptual‐motor suprapostural task by manipulation of the task load. The reciprocity effect, task order coordination, and attentional function for postural and suprapostural components were characterized with behavioral data, ERP, and MRP. It was hypothesized that (1) behavioral performance of postural and suprapostural motor tasks varied with the level of stance stability and difficulty of suprapostural motor task, (2) the ERP and MRP components were dissociable, functionally specific to information processing of stance control and suprapostural task pattern, and (3) task prioritization and resource allocation could be flexibly modulated in accordance with the task‐load of the postural‐suprapostural tasking. The theoretical implications of a reciprocity effect on postural and suprapostural motor components are discussed in view of load‐dependent variations in ERP, MRP components, and behavior measures.
MATERIALS AND METHODS
Subjects
The study was conducted with 14 healthy right‐handed volunteers (10 males, 4 females; mean age: 25.4 ± 2.0 years) without past neurological or neuromuscular impairment. These subjects were recruited to perform a static or dynamic force‐matching task (a suprapostural motor task) upon signaling with auditory cues in two different stance conditions (unipedal and bipedal stances). They gave informed consent to participate according to a protocol approved by the local Institutional Review Board to protect the rights of human subjects.
System Set‐Up and Data Recording
The behavioral data of postural and suprapostural motor components were measured at the same time (Fig. 1a). Postural sway, the center‐of‐pressure (CoP) displacement in anterior‐posterior and mediolateral directions, was recorded using a customized force platform (Model 9286AA, Kistler, Switzerland). The presentation of auditory stimuli to conduct additional force‐matching on stances was controlled by a personal computer running “Presentation” software (Neurobehavioral Systems, Albany, CA). The level of force‐matching was recorded with a load cell (15‐mm diameter ×10‐mm thickness, net weight = 7 grams; Model: LCS, Nippon Tokushu Sokki, Japan) mounted on the right thumb. The load cell was connected to a distribution box via a thin and flexible wire so that the grip force apparatus would not act as a stable support in steady stance by providing a mechanical effect. To determine the reaction time (RT) of force‐matching, the initial activation of the first dorsal interosseous (FDI) muscle was recorded with surface electromyogram (EMG) in a bipolar arrangement (Ag/AgCl, 1.1 cm in diameter, Model: F‐E9‐40‐5, GRASS) and an AC amplifier (gain: 500, cut‐off frequency: 5 and 450 Hz; Model: P511 series, GRASS). The target signal for the force‐matching was generated by a functional generator (Model: AFG3000, Tektronix), and then displayed on an oscilloscope (5.7″ wide LCD screen; Model: TDS2002, Tektronix) 50 cm in front of the subjects at eye level.
Figure 1.
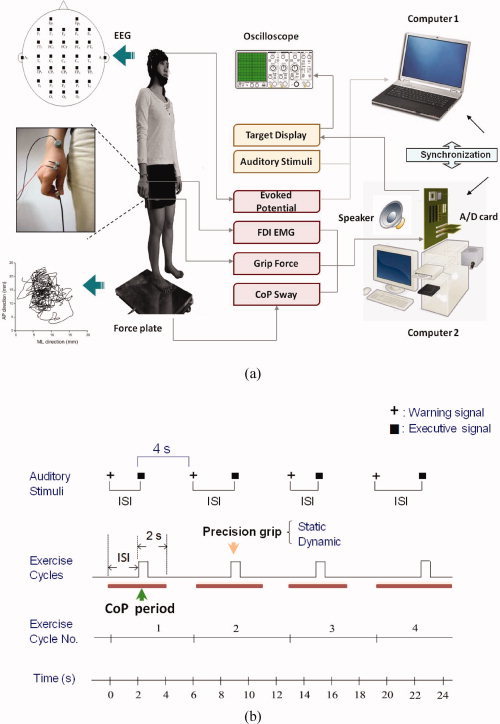
(a) Diagram of experimental setup and recordings of physiological data for precision grip force, EMG of the FDI, EEG, and postural sway. (b) A schematic illustration of the auditory stimulus paradigm for force‐matching tasks. Warning signals (+) to catch the subject's attention were presented before executive signals (▪), at which the subjects started a precision grip for force‐matching. The interval between the warning and the executive signals, or interstimulus interval (ISI), was randomized. A fixed interval of 4 seconds was assigned between the executive signal and the next warming signal. The CoP period is the interval between the start of the warning signal and 2 seconds after the executive signal, during which average CoP velocity was determined. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The electrophysiological recording of cortical excitability was made with a Quick‐Cap Electrode Helmet with 32 electrodes and an electroencephalogram (EEG) amplifier system (NeuroScan, EI Paso, TX). The EEG electrodes were positioned according to the 10‐20 International System. The montage included the following scalp positions: Fp1, Fp2, Fz, F3, F4, F7, F8, FT7, FT8, FCz, FC3, FC4, FC7, FC8, Cz, C3, C4, CPz, CP3, CP4, Pz, P3, P4, T3, T4, T5, T6, TP7, TP8, Oz, O1, and O2. Eye movements and blinks were monitored by electrodes attached above the arch of the left eyebrow and below the eye. The ground electrode was placed along the midline behind Fz. The impedances of all the electrodes were below 5 kΩ, and referenced to linked mastoids of both sides. Data were recorded with a band‐pass filter set at 0.1‐100 Hz. Target force, precision grip force, postural sway, EMG of the FDI muscle, and EEG data were synchronized and digitized at a sample rate of 1 kHz.
Experimental Conditions and Procedures
Manipulation of the task‐load of the postural and suprapostural motor tasks allowed two independent variables in this study, including: (1) target pattern of force‐matching (static versus dynamic), and (2) stance pattern for the postural task (bipedal versus unipedal). Combinations of the two task components were (1) static force‐matching in bipedal stance (BS_static), (2) dynamic force‐matching in bipedal stance (BS_dynamic), (3) static force‐matching in unipedal stance (US_static), and (4) dynamic‐force matching in unipedal stance (US_dynamic). In the control conditions (BS_control and US_control), postural sway was recorded when the subjects held the force‐grip apparatus but did not exert any gripping force or receive visual input of a force target during quiet bipedal and unipedal stances. The standard position of bipedal stance was that the subjects stood with both heels mediolaterally parallel, separated by a distance equivalent to the individual's bipedal shoulder width. Their arms hung by the sides of the trunk in a relaxed manner. In the unipedal stance condition, the subjects stood on the dominant (right) foot, slightly elevating the left side of the pelvis to keep the left leg straight with the left foot off the ground (hip: 0 degrees of flexion; knee: 180 degrees of extension; ankle: plantarflexion 0 degrees) (Fig. 1a). The standard position for the unipedal stance was similar to that for the bipedal stance. The subjects performed static/dynamic force‐matching maneuvers on the force platform in both stance conditions.
The visual target for force‐matching was presented in one of two forms: static (a fixed level of 50% maximal voluntary contraction (MVC) of the thumb‐index precision grip) or dynamic (0.25 Hz sinusoidal force varying within the range of 30–70% MVC; mean force level = 50% MVC). The subjects were instructed to stand upright and respond to the auditory cues of a given force‐matching task. The auditory cues consisted of 65‐second sequences of tone pips, with a total of nine warning‐executive signal pairs (Fig. 1b). To minimize prediction of the force target, a warning tone (an 800 Hz tone lasting for 100 ms) was randomly presented at the intervals of 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 3.25, or 3.5 seconds before an executive tone (a 500 Hz tone lasting for 100 ms). The interval between the end of the executive tone and the beginning of the next warning tone was exactly 4 seconds. Once the subjects heard the executive tone, they started a quick thumb‐index precision grip to match the peak precision force instantly with a fluctuating or a fixed target on the display, defined as a dynamic or static force‐matching task, respectively. The duration of a force impulse was generally less than 0.5 sec. There were eight trials in an experimental condition; therefore, each condition contained a total of 72 force‐matching events (nine precision grip × eight trials). Each subject was tested in a random order across experimental conditions.
Data Analyses
The analysis of behavioral data focused on task performance of suprapostural motor and postural components. The RT of force‐matching was the elapsed time between the presentation of the executive tone and the subsequent EMG onset of the FDI. RT was recorded to measure the duration of mental operations to perform the force‐matching task. The force‐matching performance was represented with absolute force error (AFE) (|PGF − ITF|) and normalized force error (NFE) (
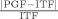 × 100%) (PGF: peak precision grip force, ITF: instantaneous target force). Average values of the NFE and RT of an individual were obtained in each experimental condition.
× 100%) (PGF: peak precision grip force, ITF: instantaneous target force). Average values of the NFE and RT of an individual were obtained in each experimental condition.
The CoP trajectory in the transverse plane was conditioned (second‐order low‐pass Butterworth filter, cut‐off frequency of 6 Hz). The absolute CoP velocity (ACoP, unit: mm/s) in the period between the warning signal and 2 seconds after the executive signal was used to quantify postural sway in this study. The ACoP was obtained by dividing the total sway‐path length of the period with the duration equivalent to the ISI plus 2 seconds, or the CoP period (Fig. 1b). For a short period of time, we consider ACoP to be a major sway measure because of its high test‐retest reliability [Lafond et al.,2004] and to be the most informative parameter in comparison with other CoP measures [Raymakers et al.,2005]. In reference to the ACoP of the control condition, normalized CoP velocity (NCoP) was formulated as
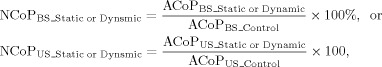 |
The applied normalization procedure provided a scale‐independent measure of the amount of twisting and turning of the ACoP velocity. Also, NCoP is recommended for comparison of postural sway changes among differing dual‐tasking conditions on a relatively equal basis for added motor tasks [Fraizer and Mitra,2008]. The second set of postural assessment employed recurrence quantification analysis (RQA) to evaluate temporal dynamics of sway velocity during the CoP period [Webber and Zbilut,1994]. RQA has demonstrated sensitivity to alterations in postural sway dynamics due to suprapostural behavioral constraints [Balasubramaniam et al.,2000]. To preclude possible deterministic dynamic patterns, RQA results were analyzed after down‐sampling the CoP velocity data to 200 Hz and random shuffling. The final parameter settings to construct a recurrent plot of CoP velocity data were: embedding dimension = 5, time‐delay = 1 sample, radius = 10% of the mean Euclidean distance separating points in the reconstructed phase space, and number of successive points defining a line segment = 2 [Riley et al.,1999]. Although selection of proper input parameters for RQA could be challenging, the parameters used in this study resulted in sparse recurrence plots sufficient to compute the RQA‐dependent variables. The degree of sway velocity repeating itself over time was represented by absolute %RECUR (%ARECUR) with characterizing the percentage of darkened pixels (out of all possible coordinate pairs) in the recurrent plot. A higher %ARECUR means a better regularity of the sway velocity data. The normalized %RECUR (%NRECUR) was the %ARECUR of a given postural‐suprapostural condition relative to that of the control condition.
ERPs were averaged off‐line according to task pattern. To visualize ERPs for subsequent analysis, the recorded EEG data were edited using NeuroScan's 4.3 software (NeuroScan). The DC shift of each channel was compensated for off‐line analysis using a third‐order trend correction over the entire set of recorded data. The continuous EEG data were digitally filtered with a low pass filter of 70 Hz and 12 dB roll‐off. Stimulus‐locked ERPs were epoched over 700 ms, commencing 100 ms before executive stimulus onset. Proper responses were all baseline‐corrected at the prestimulus interval. Each epoch was visually inspected and those with artifacts (such as excessive drift, eye movements, or blinks) were removed. Only epochs with proper responses were averaged. At least 65 trials were averaged for each experimental condition, and the ERP data were also grouped according to a two‐factor design (suprapostural task: static and dynamic force‐matching; postural task: bipedal and unipedal stances). In accordance with previous ERP studies [Kotchoubey,2006; Näätänen,1992], the N1 and P2 were analyzed to characterize attention for sensorimotor performance in the present postural‐suprapostural setups. The N1 and P2 amplitudes were quantified across all cortical electrodes, as the mean amplitude in two separate time windows (80‐150 ms, 150‐240 ms after stimulus onset). Baseline to peak amplitude was determined from the grand mean, averaged, and individually derived within the particular time windows. After N1 and P2, a consistent slow‐rising negative cortical potential (MRP) was noted with maximum negativity preceding the EMG onset of the FDI muscle. The beginning of an MRP was determined as the first point in a time frame from which the next 50 ms of the negative waveform exceeded the 2.5 times standard deviations of the baseline interval (100 ms before executive stimulus onset). MRP onset has been reported to be linked to task complexity [Simonetta et al.,1991].
Statistical Analysis
The effects of stance pattern (posture effect) and target force version (supraposture effect) on behavioral and electrophysiological parameters, including AFE, NFE, RT, ACoP, NCoP, %ARECUR, %NRECUR, ERP amplitudes of the N1 and P2 components, and the onset of MRP, were examined with the repeated‐measures analysis of variance (ANOVA) and post‐hoc comparisons. The level of significance was set at P = 0.05. Signal processing of behavior data and statistical analyses were completed with Matlab v. 7.4 (Mathworks, Natick, MA) and the statistical package for Social Sciences for Windows v. 15.0 (SPSS, Chicago, IL).
RESULTS
Behavioral Data
The results of ANOVA showed that RT of force‐matching did not vary with either force‐matching version or stance pattern (RT BS_static: 375.2 ± 18.1 ms, RT BS_dynamic: 383.9 ± 15.8 ms, RT US_static: 383.8 ± 20.4 ms; RT US_dynamic: 375.9 ± 18.7 ms) (supraposture effect: F 1,13 = 0.003, P = 0.96; posture effect: F 1,13 = 0.004, P = 0.95; supraposture × posture: F 1,13 = 3.38, P = 0.09). Figure 2a shows typical time histories for the two force‐matching tasks. Figures 2b, c are typical CoP displacement trajectory and recurrent plots of the CoP velocity data in the bipedal/unipedal conditions, respectively. Figure 3a summarizes the means and standard errors of AFE and NFE chosen to represent force‐matching precision for all postural‐suprapostural setups. The results of ANOVA test of posture and supraposture effects on AFE and NFE were compatible. Although the posture effect was not significant (AFE: F 1,13 = 1.33, P = 0.27; NFE: F 1,13 = 2.18, P = 0.16), we found a supraposture effect on AFE (F 1,13 = 31.57, P < 0.01) and NFE (F 1,13 = 43.71, P < 0.01) with significant supraposture × posture interaction (AFE: F 1,13 = 42.58, P < 0.01; NFE: F 1,13 = 50.62, P < 0.01). Post‐hoc evaluation further revealed that the AFE and NFE of the static force‐matching were greater during unipedal stance than those during bipedal stance (US_static > BS_static) (P < 0.01). However, addition of the dynamic finger task led to an opposite stance effect on AFE and NFE (BS_dynamic > US_dynamic) (P < 0.01) (Fig. 3a). Hence, the stance effect on force‐matching errors varied with the version of added force‐matching.
Figure 2.
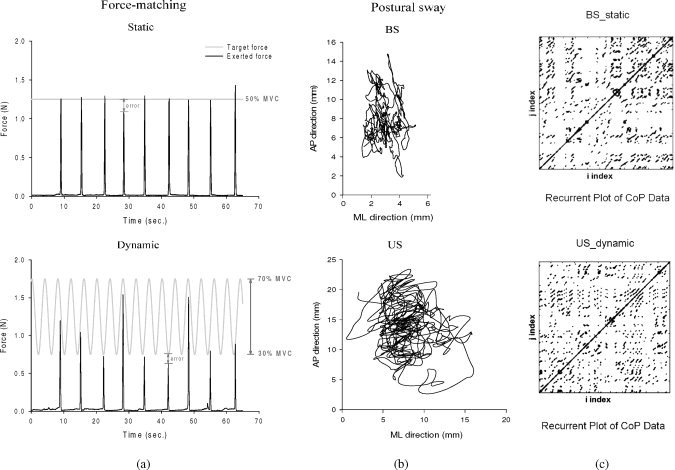
(a) A sample trial of static (top) and dynamic (bottom) force‐matching during bipedal stance. Each trial had a total of nine force‐matching events. The error of each force‐matching event was the mismatch between instantaneous target force (ITF) and peak precision grip force (PGF). (b) A sample trial of postural sway of bipedal (top) and unipedal (bottom) stances during static force matching. (c) A sample quantification of recurrence plots (RQA) of postural sway velocity during the CoP period in the BS_static (top) and US_dynamic (down) conditions. The recurrence plots (darkened points) in the phase space are constructed by plotting a pixel at coordinates (i, j), whenever pairs of data points (i and j) were separated by less than the preset threshold distance. The main diagonal line indicates a point compared with itself. (BS: bipedal stance, US: unipedal stance)
Figure 3.
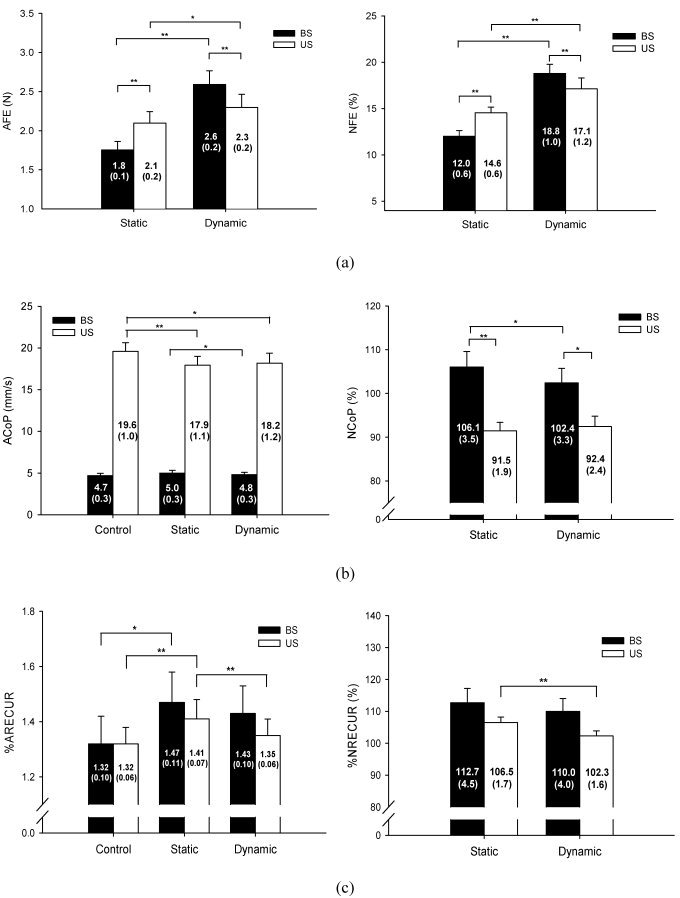
(a) The contrast of means and standard errors of absolute (left) and normalized (right) force error (AFE; NFE) among the four postural‐suprapostural tasks. (b) The contrast of means and standard errors of absolute (left) and normalized (right) center‐of‐pressure velocity (ACoP; NCoP) among the control conditions and four postural‐suprapostural tasks. (c) The contrast of means and standard errors of absolute (left) and normalized (right) percent recurrence (%RECUR) of the CoP velocity. (BS: bipedal stance, US: unipedal stance) (*P < 0.05, **P < 0.01)
Figure 3b displays the means and standard errors of the two postural sway parameters (ACoP and NCoP) in different experimental conditions. First, the ANOVA results suggested a significant posture effect (F2,12 = 248.31, P < 0.01) and supraposture effect (F2,12 = 4.26, P < 0.05) on ACoP with the interaction of both (F2,12 = 9.93, P < 0.01). In the BS condition, ACoP was greater for static force‐matching than for dynamic force‐matching (BS_static > BS _dynamic, P < 0.05)(Fig. 3b, left). In the US condition, ACoP was significantly suppressed with the addition of static and dynamic force‐matching as compared with that in the control condition (US_control > US_static, P < 0.01; US_control > US_dynamic, P < 0.05). Next, the ANOVA results suggested that NCoP was subject to stance pattern (F 1,13 = 12.72, P < 0.01), but not to force‐matching version (F 1,13 = 1.98, P = 0.18) with a significant interaction effect (F 1,13 = 4.99, P < 0.05). In the BS condition, the addition of dynamic force‐matching resulted in a greater reduction in NCoP than did the addition of static force‐matching (BS_static > BS _dynamic, P < 0.05)(Fig. 3b, right). However, NCoP in the US condition stance did not differ with the addition of force‐matching tasks (P > 0.05). We noted that whether static or dynamic force‐matching was superimposed, bipedal stance had a higher NCoP than unipedal stance (BS_static > US_static, P < 0.01; BS_dynamic > US_dynamic, P < 0.05). Figure 3c shows the means and standard errors of %ARECUR and %NRECUR in different experimental conditions. %ARECUR represented the degree of sway velocity repeating itself over time in a postural‐suprapostural condition, and %NRECUR was the regularity of sway velocity in reference to that of the control condition. The results of ANOVA showed a significant supraposture effect (F2,12 = 7.08, P < 0.01) rather than posture effect on %ARECUR (F2,12 = 0.17, P = 0.69) without an interaction effect (F2,12 = 1.99, P = 0.18). Irrespective of the bipedal and unipedal stances, static force‐matching always added values to the %ARECUR in reference to the control conditions (BS_static > BS_control, P < 0.05; US_static > US_control, P < 0.01). This finding suggests that sway data became less noisy for the supraposture effect. During unipedal stance, static force‐matching led to a greater %ARECUR (better sway regularity) than dynamic force‐matching (P < 0.01)(Fig. 3c, left). Correspondingly, %NRECUR was subject to force‐matching version only (F 1,13 = 5.85, P < 0.05), with %NRECUR in the US_static condition superior to that in the US_dynamic condition (P < 0.01). Overall, our postural data revealed that (1) postural sway and force‐matching error were differently affected by variations in stance pattern and force‐matching version (Fig. 3a, right vs. Figs. 3b,c, right); (2) the addition of a suprapostural motor task on unipedal stance led to a greater reduction in sway (<100% of control condition) than the addition of such a task on bipedal stance (>100% of control condition)(Fig. 3b, right); and (3) dynamic force‐matching produced a greater sway irregularity than did static force‐matching during unipedal stance (Fig. 3c).
ERP and MRP Data
Figures 4a,b show the population means of all the scalp‐recorded ERP and ERP components of interest (the N1, P2, and MRP) at the FC3 in the BS_static condition. The N1 and P2 waves presented after the presentation of the executive signals across postural‐suprapostural conditions. Following the P2 component, we observed a marked slow‐rising negative cortical potential (or MRP) that reached its maximal negativity before EMG onset of the FDI muscle. MRP was most likely to be relevant to preparation for the force‐matching movement. Figures 5a–d are typical ERP recordings showing the effects of posture and supraposture on N1 and P2. The ANOVA results suggested that the N1 amplitudes of most of the electrodes around the parietal cortex were subject to a significant posture effect (CP3: F 1,13 = 5.18, P < 0.05, CP4: F 1,13 = 5.14, P < 0.05, P3: F 1,13 = 6.84, P < 0.05; Pz: F 1,13 = 9.37, P < 0.01, P4: F 1,13 = 5.71, P < 0.05). However, the P2 amplitude was independent of the posture effect for all cortical areas (P > 0.05). Post‐hoc analysis further indicated that the N1 amplitude of bilateral parietal areas (CP3, CP4, P3, Pz, and P4) in the US condition was generally greater than that in the BS condition (P < 0.05; Fig. 6a), as the subjects concurrently conducted a static finger task. In the execution of dynamic force‐matching, the N1 amplitude was similarly greater in the US condition, but this augmentation tendency was limited to the electrodes in the left parietal cortex (CP3 and P3)(P < 0.05; Fig. 6b). On the other hand, a significant supraposture effect on P2 amplitude was noted in the frontal (FCz: F 1,13 = 6.30, P < 0.05), central (Cz: F 1,13 = 6.35, P < 0.05; C4: F 1,13 = 7.20, P < 0.05) and parietal cortices (CPz: F 1,13 = 13.93, P < 0.01; CP4: F 1,13 = 18.19, P < 0.01; P3: F 1,13 = 9.40, P < 0.01; Pz: F 1,13 = 14.85, P < 0.01; P4: F 1,13 = 8.06, P < 0.05). In the BS condition, the P2 component at the FCz, Cz, C4, CPz, CP4, Pz and P4 electrodes was greater during static force‐matching than during dynamic force‐matching (P < 0.05; Fig. 6c). In the US condition, a simultaneous static force‐matching task resulted in a larger P2 around the parietal cortex (CPz, CP4, P3, Pz, and P4 electrodes) than did the dynamic force‐matching task (P < 0.05; Fig. 6d). Overall, the P2 amplitude in the right parietal lobe waned with increasing task‐load of the supapostural motor task.
Figure 4.
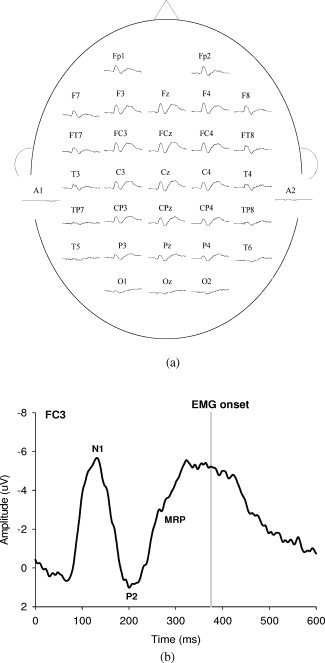
(a) Scalp‐recorded event‐related potentials related to the execution of static force‐matching in the bipedal stance condition (BS_static). (b) A grand average waveform of the FC3 electrode from all 14 subjects in the BS_static condition. In addition to N1 and P2 components, a slow negative MRP occurs immediately after the P2 component. The vertical line represents the onset of the FDI EMG.
Figure 5.
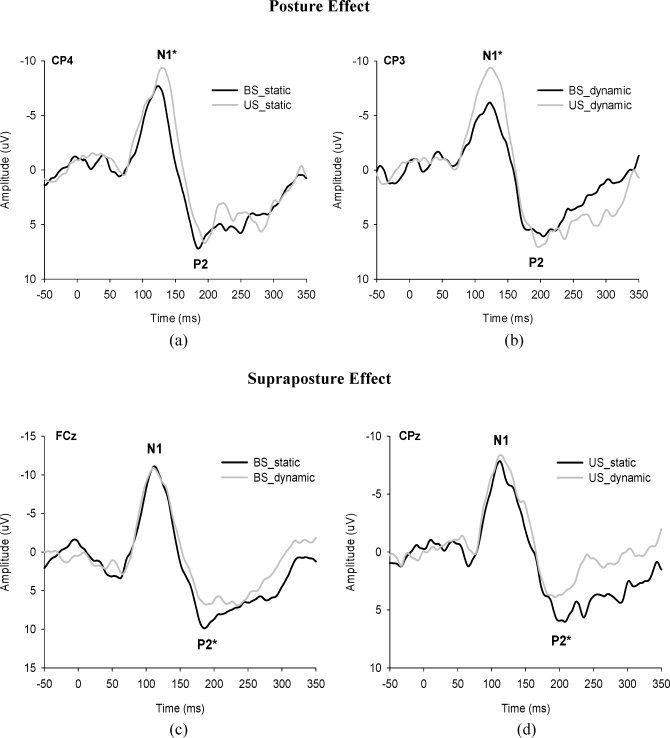
Average event‐related potentials evoked in the ongoing force‐matching for four postural‐suprapostural task patterns in the time window between 50 ms preceding and 350 ms following the executive tone from a typical subject. (a) The contrasts of N1 and P2 amplitudes between stances for static force‐matching, and (b) for dynamic force‐matching. (c‐d) The contrast of N1 and P2 amplitudes between versions of force‐matching during bipedal stance and during unipedal stance. (*P < 0.05)
Figure 6.
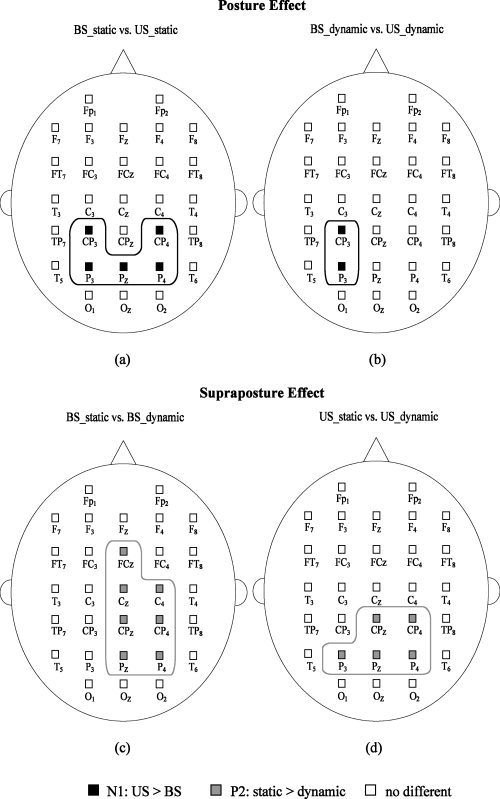
Topological plots showing significant posture effect and supraposture task effect on ERP amplitude. (a) Posture effect on N1 component in the static force‐matching condition; (b) posture effect on N1 component in the dynamic force‐matching condition; (c) supraposture effect on P2 component in the bipedal stance condition, and (d) supraposture effect on P2 component in the unipedal stance condition. (Black square: significant posture effect on N1 amplitude (US > BS); gray square: significant supraposture effect on P2 amplitude (static > dynamic); white square: no difference).
The earliest MRP onset was the first identifiable MRP among the F3, Fz, FC3, FCz, C3 and Cz sites (the contralateral frontal‐central electrodes). Figure 7 summarizes the means and standard errors of the earliest MRP onset for the four postural‐suprapostural setups. Despite insignificant main effects of posture and supraposture on the earliest MRP onset (posture: F 1,13 = 0.03, P = 0.86; supraposture: F 1,13 = 0.46, P = 0.50), there existed a significant posture × supraposture interaction (F 1,13 = 11.93, P < 0.05). Post‐hoc analysis revealed that the earliest MRP onset for execution of the static force‐matching was earlier in the BS condition than in the US condition (US_static > BS_static) (P < 0.01), but a converse stance effect on the earliest MRP onset was noted for dynamic force‐matching (BS_dynamic > US_dynamic) (P < 0.05). Linear interpolation with all electrodes across different time frames was computed, and the spatio‐temporal evolution of the MRP in the BS_static condition is shown in Fig. 8a. The MRP started with the left frontal‐central area and then spread gradually over the bilateral frontal‐central and parietal areas, with the largest negativity occurring at the left frontal‐central area (F3, Fz, FC3, FCz, C3, and Cz). Notably, the MRP evolved differentially among the four postural‐suprapostural conditions (Fig. 8b). It seemed that the task‐load of the suprapostural motor task affected the timing of bilateral activation of the MRP, which began visibly earlier in the case of dynamic force‐matching. The spreading of bilateral MRP for dynamic force‐matching tasks in the BS and US conditions presented at around 250‐260 ms (BS_dynamic and US_dynamic), roughly 10‐20 ms earlier than for the static force‐matching tasks in the BS/US conditions (BS_static and US_static).
Figure 7.
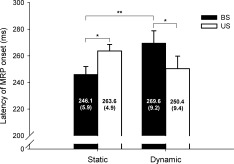
The contrast of means and standard errors of latency of MRP among the four postural‐suprapostural task conditions. (BS: bipedal stance, US: unipedal stance) (*P < 0.05, **P < 0.01).
Figure 8.
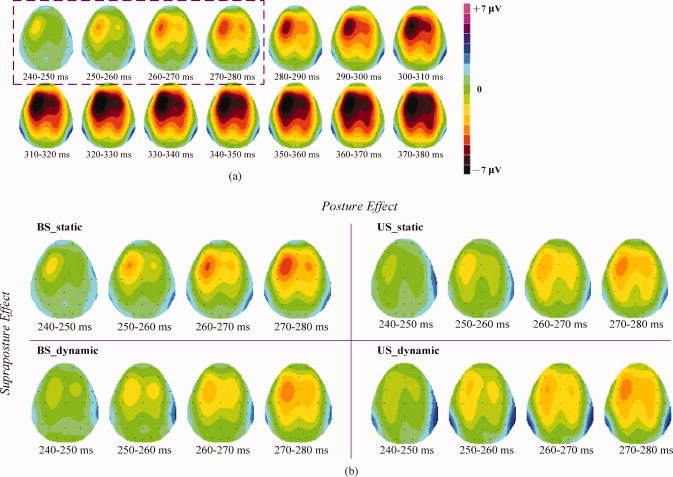
(a) Population means of topographic mapping of the MRP between 240 and 380 ms after the executive signal onset in the BS_static condition. (b) Population means of topographic mapping of all four postural‐suprapostural task conditions showing visible posture and supraposture effects on the MRP within the time frames of 240 and 280 ms after the executive signal onset. It is noticeable that MRP in the BS_static and US_dynamic conditions occurs earlier than MRP in the BS_dynamic and US_static conditions.
DISCUSSION
Force‐Matching Performance and Postural Sway
The present study showed a significant reciprocity effect of variations in task‐load between force‐matching and stance pattern on the performance of force‐matching. On the suprapostural side, task precision for static and dynamic force‐matching was differentially modulated by unipedal and bipedal stances (NFE: BS_static < US_static; BS_dynamic > US_dynamic)(Fig. 3a, right). In reference to the CoP sway in the control conditions, the addition of force‐matching on unipedal stance resulted in minimizing stance sway (US_static: 91.5%, US_dynamic: 92.4%), unlike the cases for bipedal stance (BS_static: 106.1%, BS_dynamic: 102.4%)(Fig. 3b, right). Also, the %RECUR results even revealed a lesser sway regularity for performing a more difficult force‐matching task in the unipedal conditions (US_dynamic < US_static)(Fig. 3c), contrary to the increase in sway regularity when somatosensory information is unavailable [Olivier et al.,2007]. The reciprocal results of parallel loading of postural and perceptual‐motor suprapostural tasks differed with classic posture‐cognition dual‐tasking (such as superimposition of Stroop, verbal, or numeric tasks on stance), under which postural threat mostly degrades performance of a cognitive task and/or an additive effect of the cognitive task constrains postural stability [Dault et al.,2001; Mitra and Fraizer,2004; Olivier et al.,2007; Yardley et al.,1999]. The conflicting relationship between cognitive processes and stance control suggests common central resource and general capacity limitations [Mitra and Fraizer,2004; Tombu and Jolicoeur,2004]. For resource competition, RT of the secondary cognitive task is also expected to multiply with increasing task‐load [Lajoie et al.,1993; Vuillerme and Nougier,2004]. However, common resource hypothesis and capacity limitations obtained empirically from posture‐cognition studies were not adequate for the present study, because (1) performance error for dynamic force‐matching was conversely smaller than that for static force‐matching during stance with a greater postural challenge (Fig. 3a); (2) RT of force‐matching was load‐invariant for all posture‐motor setups (P > 0.05); and (3) increasing suprapostural difficulty did not add to the amplitude and regularity of postural sway in the unipedal conditions (Figs. 3b,c). Under the stance conditions with a perceptual‐motor goal, postural and suprapostural tasks appeared not to interact in a competitive manner. Two motor subsystems for stance and perceptual‐motor supraposture could be functionally integrated [Stoffregen et al.,2007], as a task‐load increment might empower adaptive resource sharing or coercion of resource expansion. However, behavioral phenomena could hardly lend better insight into how the brain organizes the attentional function and task order in the postural‐suprapostural task with a perceptual‐motor goal.
Stance‐Related Modulation of N1 Negativity in a Postural‐Suprapostural Task
The present study appears to be the first to assess electrophysiological correlates (N1, P2, and MRP) for postural‐suprapostural tasks with postural and perpetual‐motor synergies. N1 is believed to represent the stimulus‐set mode of attention, and its magnitude is modifiable to a course of action that passively admits auditory input from a maintained set over the attended channel [Hillyard et al., 1971]. Previous studies reported a strongest auditory‐elicited N1 response at frontocentral sites for sudden postural perturbations under predictable conditions [Adkin et al.,2008]. Although we also noted the largest negativity of the N1 at fronto‐central sites (Fig. 4a), a posture effect on the N1 response was evident in parietal areas (Figs. 6a,b). Hence, enhanced N1 negativity associated with unipedal stance at early perceptual stage of postural‐suprapostural tasks was unlikely to be a sensorimotor set of pre‐selected responses for anticipated postural perturbations [Jacobs and Horak,2007]. The reason was that subjects stood during the whole experimental session in unperturbed stance for both the unipedal and bipedal conditions. They did not need to employ feedforward reactions to counter sudden postural threats [Kaluzny and Wiesendanger,1992; Pavol and Pai,2002], but just remained a relatively higher alert state for postural adjustments on a feedback basis during unipedal stance [Hauck et al.,2008]. When the postural control system is challenged by unipedal stance, maintenance of normal standing balance relies more on plantar sensation than bipedal stance [Meyer et al.,2004]. Hence, the postural effect on N1 response was evident principally around the somatosensory and sensorimotor cortices. In execution of the dynamic force‐matching task, postural effect on N1 was localized at CP3 and P3 with a larger N1 negativity during unipedal stance (Fig. 6b). This fact indicates that dynamic force‐matching coerced higher motor attention to stabilize postural sway during unipedal stance than during bipedal stance [Huang et al.,2009], pertaining to functioning of the left parietal cortex in adaptation to different posture conditions and updating spatial representation of the body to the environment [ Corradi‐Dell'Acqua et al.,2008], especially in the ankle joint [Johannsen et al.,2001]. Similar modulation of N1 negativity due to a perceived postural threat has also been reported in tasks with a high surface height [Adkin et al.,2008] and narrow stance [Dimitrov et al.,1996].
Perceptual‐Motor Suprapostural Task‐Related Modulation of P2 Positivity in a Postural‐Suprapostural Task
Contrary to the earlier N1, the P2 amplitude to an acoustical signal was just textured to the suprapostural task‐load with less positivity for dynamic force‐matching than that for static force‐matching (Figs. 5c,d and Figs. 6c,d). This neural effect clearly suggests a temporal order coordination of interfering processes for the particular postural‐suprapostural situations [Szameitat et al., 2002], by scheduling the stance response (N1) prior to the suprapostural response (P2). One attractive interpretation of the generation process of P2 positive deflection was that attention was directed to the behavioral distraction by novel stimuli (force‐matching), which led to deviation of auditory‐elicited neuronal responses that registers a background process of postural response [Luck and Hillyard,1994]. When attention for postural regulation was directed to a visual stimulus for force‐matching, a long‐lasting inhibitory process, typically peaking at 150‐240 ms after visual stimulus onset, exerted a negative deflection on P2 wave [Kotchoubey,2006]. Hence, P2 positive deflection could index brain processes for the force‐matching response due to a mismatched stimulus overlapping the memory representation of the preceding stimulus regarding stance arousal. Michie et al. (1993) reported a larger distracted‐related P2 potential for positivity during performance of a visual task with less difficult between‐source discriminations, in parallel to a smaller P2 positivity for more difficult dynamic force‐matching than simpler static force‐matching in this study (Figs. 6c,d). Intensive redirection of the focus of attention to dynamic force‐matching is assumed, for an aggravated visual‐load, to predict a periodically moving target as compared to a steady target during static force‐matching.
The suprapostural effect on the P2 amplitude was noted primarily in the posterior parietal areas (Figs. 6c,d), where discharge patterns of neurons in the areas are conditional to the display mode of a visual target [Lynch et al., 1997] and multi‐modal sensory information is integrated here for eye‐limb coordination [Hamzei et al.,2002; Ishihara and Imanaka,2007]. A decrease in P2 positivity in the parietal cortex was well congruent with the more taxing aspects for dynamic force‐matching that entailed a high attention level for on‐line comparison of intended and performed movement [Malhotra et al.,2009; Sarter et al.,2001]. Intriguingly, task‐dependent differences in P2 positivity between dynamic and static force‐matching caused increased activity in the central‐parietal and ipsilateral parietal regions, probably because the ipsilateral recruitment would aid in the more complex visuomotor processing for completion of dynamic force‐matching [Hamzei et al.,2002; Tomasi et al.,2007]. In contrast to load‐dependent activation in parietal areas for force‐matching, the task‐load increment of classic non‐perceptual cognitive operations mainly elicits increased activity of the prefrontal area in the neural network of multiple attentional subsystems [Hadland et al.,2001; Gruberet al.,2001]. Context‐dependent activations in response to superimposed events might lead to differing behavioral interferences. For instance, we did not observe a destabilization effect due to the addition of a perceptual‐motor task in the unipedal conditions, as predicted by capacity limited processing or conflicting postural‐suprapostural response.
Reciprocity Effect on MRP Onset
Subsequent to the P2 wave, a cortical MRP of widespread slow negativity was noted preceding the force‐matching tasks (Figs. 4a,b). As MRP was not present in the control conditions of the study, it appeared to be a pre‐motor potential due to neural processes in preparation for force‐matching execution. However, the MRP recorded in this study is not a simple readiness potential (or Bereitschaftspotential), which precedes a single self‐initiated movement by about 500 to 1000 ms [Jankelowitz and Colebatch,2002; Simonetta,1991]. Instead, for addition of a suprapostural task, MRP could be a cortical activity responsive to baseline postural reaction and planning of a force‐matching task, such that both MRP onset and force‐matching precision were similarly affected by the interference effect of the stance pattern and version of force‐matching (Fig. 3a and Fig. 7). The interaction effect on MRP implies existing stance modulation on preparation of a force‐matching maneuver, in support of the behavioral observation that stance control can be functionally integrated to suprapostural activity [Stoffregen et al.,2007].
Topological plots of MRP in Figs. 8a,b are helpful to visualize time‐dependent changes in attentional focus for the current postural‐suprapostural setting. After the 240‐250 ms from executive beep, the MRP spread globally to the distributed frontoparietal networks from the contralateral frontal and prefrontal areas to the bilateral premotor and parietal areas (Fig. 8a), a hierarchical cortical organization to resolve response conflicts between stance control and force‐matching. Early activations of the frontal and prefrontal cortices followed by the anterior cingulate during dual‐tasking was believed to provide a bias signal for appropriate response associations in the parietal cortex [Dreher and Grafman,2003], where a target's kinematic pattern was perceived and projected from the dorsal stream and primary visual cortex during a visuomotor task [Hamzei et al.,2002]. In addition, there was a visibly earlier MRP onset and additional volume recruitment in the ipsilateral hemisphere and/or a broader activated area across bilateral hemispheres, as task‐load increased under the conditions of dynamic force‐matching and unipedal stance (Fig. 8b). As resource capacity is a function of activated area and activated duration in the brain [Kok,1997], adaptive resource sharing or resource expansion might explain the load‐invariant RT of force‐matching under various posture‐motor combinations.
CONCLUSION
This paper first presented three ERP components (N1, P2, and MRP) in a postural‐suprapostural task with a perceptual‐motor goal. Perceptual‐motor and postural synergy conformed to task‐order coordination in performance and the “posture first principle,” in light of dissociable posture‐dependent N1 and supraposture‐dependent P2. MRP topological mapping revealed that the common frontoparietal network was responsible for implicit response conflicts between upright stance and force‐matching. Our behavioral and neurophysiological data suggested that limited central capacity and resource competition are not adequate for stance control with a perceptual‐motor suprapostural task, which could be flexibly integrated into posture synergy depending on relative task load.
Acknowledgements
We thank Dr. Rong‐Ju Cherng for her equipment support and comments on an earlier version of the manuscript.
REFERENCES
- Adkin AL, Quant S, Maki BE, McIlroy WE ( 2006): Cortical responses associated with predictable and unpredictable compensatory balance reactions. Exp Brain Res 172: 85–93. [DOI] [PubMed] [Google Scholar]
- Adkin AL, Campbell AD, Chua R, Carpenter MG ( 2008): The influence of postural threat on the cortical response to unpredictable and predictable postural perturbations. Neurosci Lett 435: 120–125. [DOI] [PubMed] [Google Scholar]
- Andersson G, Hagman J, Talianzadeh R, Svedberg A, Larsen HC ( 2002): Effect of cognitive load on postural control. Brain Res Bull 58: 135–139. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam R, Riley MA, Turvey MT ( 2000): Specificity of postural sway to the demands of a precision task. Gait Posture 11: 12–24. [DOI] [PubMed] [Google Scholar]
- Başar‐Eroglu C, Demiralp T, Schürmann M, Başar E ( 2001): Topological distribution of oddball 'P300′ responses. Int J Psychophysiol 39: 213–220. [DOI] [PubMed] [Google Scholar]
- Corradi‐Dell'Acqua C, Hesse MD, Rumiati RI, Fink GR ( 2008): Where is a nose with respect to a foot? The left posterior parietal cortex processes spatial relationships among body parts. Cereb Cortex 18: 2879–2890. [DOI] [PubMed] [Google Scholar]
- Dault MC, Geurts AC, Mulder TW, Duysens J ( 2001): Postural control and cognitive task performance in healthy participants while balancing on different support‐surface configurations. Gait Posture 14: 248–255. [DOI] [PubMed] [Google Scholar]
- Dimitrov B, Gavrilenko T, Gatev P ( 1996): Mechanically evoked cerebral potentials to sudden ankle dorsiflexion in human subjects during standing. Neurosci Lett 208: 199–202. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J ( 2003): Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex 13: 329–339. [DOI] [PubMed] [Google Scholar]
- Fraizer EV, Mitra S ( 2008): Methodological and interpretive issues in posture‐cognition dual‐tasking in upright stance. Gait Posture 27: 271–279. [DOI] [PubMed] [Google Scholar]
- Gruber O, Indefrey P, Steinmetz H, Kleinschmidt A ( 2001): Dissociating neural correlates of cognitive components in mental calculation. Cereb Cortex 11: 350–359. [DOI] [PubMed] [Google Scholar]
- Gontier E, Le Dantec C, Leleu A, Paul I, Charvin H, Bernard C, Lalonde R, Rebaï M ( 2007): Frontal and parietal ERPs associated with duration discriminations with or without task interference. Brain Res 1170: 79–89. [DOI] [PubMed] [Google Scholar]
- Hamzei F, Dettmers C, Rijntjes M, Glauche V, Kiebel S, Weber B, Weiller C ( 2002): Visuomotor control within a distributed parieto‐frontal network. Exp Brain Res 146: 273–281. [DOI] [PubMed] [Google Scholar]
- Hadland KA, Rushworth MF, Passingham RE, Jahanshahi M, Rothwell JC ( 2001): Interference with performance of a response selection task that has no working memory component: an rTMS comparison of the dorsolateral prefrontal and medial frontal cortex. J Cogn Neurosci 13: 1097–1108. [DOI] [PubMed] [Google Scholar]
- Hauck LJ, Carpenter MG, Frank JS ( 2008): Task‐specific measures of balance efficacy, anxiety, and stability and their relationship to clinical balance performance. Gait Posture 27: 676–682. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW ( 1973): Electrical signs of selective attention in the human brain. Science 182: 177–180. [DOI] [PubMed] [Google Scholar]
- Huang CY, Cherng RJ, Yang ZR, Chen YT, Hwang IS ( 2009): Modulation of soleus H reflex due to stance pattern and haptic stabilization of posture. J Electromyogr Kinesiol 19: 492–499. [DOI] [PubMed] [Google Scholar]
- Huang CY, Cherng RJ, Hwang IS ( 2010): Reciprocal influences on performances of a postural‐suprapostural task by manipulating the level of task‐load. J Electromyogr Kinesiol 20: 413–419. [DOI] [PubMed] [Google Scholar]
- Ishihara M, Imanaka K ( 2007): Motor preparation of manual aiming at a visual target manipulated in size, luminance contrast, and location. Perception 36: 1375–1390. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Horak FB ( 2007): Cortical control of postural responses. J Neural Transm 114: 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankelowitz SK, Colebatch JG ( 2002): Movement‐related potentials associated with self‐paced, cued and imagined arm movements. Exp Brain Res 147: 98–107. [DOI] [PubMed] [Google Scholar]
- Johannsen P, Christensen LO, Sinkjaer T, Nielsen JB ( 2001): Cerebral functional anatomy of voluntary contractions of ankle muscles in man. J Physiol 535: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaluzny P, Wiesendanger M ( 1992): Feedforward postural stabilization in a distal bimanual unloading task. Exp Brain Res 92: 173–182. [DOI] [PubMed] [Google Scholar]
- Klingberg T ( 2000): Limitations in information processing in the human brain: neuroimaging of dual task performance and working memory tasks. Prog Brain Res 126: 95–102. [DOI] [PubMed] [Google Scholar]
- Kok A ( 1997): Event‐related‐potential (ERP) reflections of mental resources: A review and synthesis. Biol Psychol 45: 19–56. [DOI] [PubMed] [Google Scholar]
- Kotchoubey B ( 2006): Event‐related potentials, cognition, and behavior: A biological approach. Neurosci Biobehav Rev 30: 42–65. [DOI] [PubMed] [Google Scholar]
- Lafond D, Corriveau H, Hébert R, Prince F ( 2004): Intrasession reliability of center of pressure measures of postural steadiness in healthy elderly people. Arch Phys Med Rehabil 85: 896–901. [DOI] [PubMed] [Google Scholar]
- Lang W, Zilch O, Koska C, Lindinger G, Deecke L ( 1989): Negative cortical DC shifts preceding and accompanying simple and complex sequential movements. Exp Brain Res 74: 99–104. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Bard C, Fleury M ( 1993): Attentional demands for static and dynamic equilibrium. Exp Brain Res 97: 139–144. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA ( 1994): Electrophysiological correlates of feature analysis during visual search. Psychophysiology 31: 291–308. [DOI] [PubMed] [Google Scholar]
- Lynch JC, Mountcastle VB, Talbot WH, Yin TC ( 1977): Parietal lobe mechanisms for directed visual attention. J Neurophysiol 40: 362–389. [DOI] [PubMed] [Google Scholar]
- Maki BE, McIlroy WE ( 1996): Influence of arousal and attention on the control of postural sway. J Vestib Res 6: 53–59. [PubMed] [Google Scholar]
- Malhotra P, Coulthard EJ, Husain M ( 2009): Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain 132: 645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNevin NH, Wulf G ( 2002): Attentional focus on supra‐postural tasks affects postural control. Hum Mov Sci 21: 187–202. [DOI] [PubMed] [Google Scholar]
- Meyer PF, Oddsson LI, De Luca CJ ( 2004): The role of plantar cutaneous sensation in unperturbed stance. Exp Brain Res 156: 505–512. [DOI] [PubMed] [Google Scholar]
- Michie PT, Solowij N, Crawford JM, Glue LC ( 1993): The effects of between‐source discriminability on attended and unattended auditory ERPs. Psychophysiology 30: 205–220. [DOI] [PubMed] [Google Scholar]
- Mitra S, Fraizer EV ( 2004): Effects of explicit sway‐minimization on postural‐suprapostural dual‐task performance. Hum Mov Sci 23: 1–20. [DOI] [PubMed] [Google Scholar]
- Näätänen R ( 1992): Attention and brain function. Hillsdale: Lawrence Erlbaum Associates, Inc. 120–130 p. [Google Scholar]
- Olivier I, Cuisinier R, Vaugoyeau M, Nougier V, Assaiante C ( 2007): Dual‐task study of cognitive and postural interference in 7‐year‐olds and adults. Neuroreport 18: 817–821. [DOI] [PubMed] [Google Scholar]
- Pellecchia GL ( 2003): Postural sway increases with attentional demands of concurrent cognitive task. Gait Posture 18: 29–34. [DOI] [PubMed] [Google Scholar]
- Pavol MJ, Pai YC ( 2002): Feedforward adaptations are used to compensate for a potential loss of balance. Exp Brain Res 145: 528–538. [DOI] [PubMed] [Google Scholar]
- Quant S, Adkin AL, Staines WR, Maki BE, McIlroy WE ( 2004): The effect of a concurrent cognitive task on cortical potentials evoked by unpredictable balance perturbations. BMC Neurosci 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymakers JA, Samson MM, Verhaar HJ ( 2005): The assessment of body sway and the choice of the stability parameter(s). Gait Posture 21: 48–58. [DOI] [PubMed] [Google Scholar]
- Reilly DS, van Donkelaar P, Saavedra S, Woollacott MH ( 2008): Interaction between the development of postural control and the executive function of attention. J Mot Behav 40: 90–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley MA, Balasubramaniam R, Turvey MT ( 1999): Recurrence quantification analysis of postural fluctuations. Gait Posture 9: 65–78. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP ( 2001): The cognitive neuroscience of sustained attention: where top‐down meets bottom‐up. Brain Res Brain Res Rev 35: 146–160. [DOI] [PubMed] [Google Scholar]
- Sigman M, Dehaene S ( 2008): Brain mechanisms of serial and parallel processing during dual‐task performance. J Neurosci 28: 7585–7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetta M, Clanet M, Rascol O ( 1991): Bereitschaftspotential in a simple movement or in a motor sequence starting with the same simple movement. Electroenceph Clin Neurophysiol 81: 129–134. [DOI] [PubMed] [Google Scholar]
- Stoffregen TA, Hove P, Bardy BG, Riley M, Bonnet CT ( 2007): Postural stabilization of perceptual but not cognitive performance. J Mot Behav 39: 126–138. [DOI] [PubMed] [Google Scholar]
- Swan L, Otani H, Loubert PV ( 2007): Reducing postural sway by manipulating the difficulty levels of a cognitive task and a balance task. Gait Posture 26: 470–474. [DOI] [PubMed] [Google Scholar]
- Szameitat AJ, Lepsien J, von Cramon DY, Sterr A, Schubert T ( 2006): Task‐order coordination in dual‐task performance and the lateral prefrontal cortex: an event‐related fMRI study. Psychol Res 70: 541–552. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli EC, Ernst T ( 2007): Different activation patterns for working memory load and visual attention load. Brain Res 1132: 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombu M, Jolicoeur P ( 2004): Virtually no evidence for virtually perfect time‐sharing. J Exp Psychol Hum Percept Perform 30: 795–810. [DOI] [PubMed] [Google Scholar]
- Vuillerme N, Nougier V ( 2004): Attentional demand for regulating postural sway: the effect of expertise in gymnastics. Brain Res Bull 63: 161–165. [DOI] [PubMed] [Google Scholar]
- Webber CL Jr., Zbilut JP ( 1994): Dynamical assessment of physiological systems and states using recurrence plot strategies. J Appl Physiol 76: 965–973. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Hillyard SA ( 1991): Modulation of early auditory processing during selective listening to rapidly presented tones. Electroencephalogr Clin Neurophysiol 79: 170–191. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Shumway‐Cook A ( 2002): Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 16, 1–14. [DOI] [PubMed] [Google Scholar]
- Woollacott M, Vander Velde T ( 2008): Non‐visual spatial tasks reveal increased interactions with stance postural control. Brain Res 1208: 95–102. [DOI] [PubMed] [Google Scholar]
- Wulf G, Mercer J, McNevin N, Guadagnoli MA ( 2004): Reciprocal influences of attentional focus on postural and suprapostural task performance. J Mot Behav 36: 189–199. [DOI] [PubMed] [Google Scholar]
- Yardley L, Gardner M, Leadbetter A, Lavie N ( 1999): Effect of articulatory and mental tasks on postural control. Neuroreport 10: 215–219. [DOI] [PubMed] [Google Scholar]


