Abstract
Two of the most striking features in alcoholism are the irresistible craving for alcohol and the proceeding neglect of other activities and pleasures that were formerly relevant. Craving has been investigated extensively and is commonly due to a dysfunctional reward system. The neural basis of the neglect of self‐relevant interests, which can be described as altered personal reference, and its association to the reward system, however, remains unclear. Using fMRI, we investigated neural activity during a paradigm that tested for both reward and personal reference with regard to the same stimuli, i.e., alcoholic and nonalcoholic pictures, in healthy subjects and abstinent alcoholic patients. Alcoholic patients showed slightly reduced signal changes in the brain stem adjacent to ventral tegmental area (VTA) and in the ventromedial prefrontal cortex (VMPFC) during the reward task, while we found no alterations in the right and left ventral striatum (VS). The same regions (VS, VTA, and VMPFC), however, showed reduced signal changes during personal reference with lack of neural differentiation between high and low referenced stimuli in alcoholic patients. In summary, we demonstrate for the first time neurophysiological alterations in reward circuitry during personal reference in alcoholic patients. Our results underline the important role of the reward circuitry during personal reference in the pathophysiology of alcohol addiction. Hum Brain Mapp 2009. © 2008 Wiley‐Liss, Inc.
Keywords: fMRI, reward, alcoholism, personal reference, brain imaging, addiction
INTRODUCTION
Patients who suffer from addiction are afflicted with a variety of severe symptoms, which include among others a strong yearning and craving for the substance and the inability to control its intake [American Psychiatric Press, 1994; Baler and Volkow, 2006; Goldstein et al. 2007]. Recent imaging studies showed dysfunctions in reward circuitry, e.g., ventral striatum (VS) and ventromedial prefrontal cortex (VMPFC), as being involved in substance craving. While addicted patients show less activation in these regions (and other regions as, e.g., nucleus caudatus, thalamus, dorsolateral prefrontal cortex, anterior cingulate cortex, orbitofrontal cortex) during anticipation of monetary reward [Wrase et al., 2007], the same regions as well as another key region of the reward system, the ventral tegmental area (VTA), showed, in contrast, increased neural activity during mere presentation of alcoholic stimuli independent of reward [Braus et al., 2001; George et al., 2001; Grusser et al., 2004; Kareken et al., 2004; Modell and Mountz, 1995; Myrick et al., 2004; Wrase et al., 2007]. Taken together, these findings indicate reduced neural activity in reward circuitry in response to nonalcoholic reward‐indicating cues and increased activity during the presentation of alcoholic stimuli in alcoholism. Similar findings of reduced neural activity in reward circuitry during a reward task have been made for other addictions such as cocaine [Kalivas and Volkow, 2005; Volkow et al., 2003; Volkow et al., 1997, 2004a, b] or pathological gambling [Reuter et al., 2005].
In addition to craving and yearning, alcoholic patients develop a proceeding neglect of formerly important interests and habits with an increasing personal reference to alcoholic stimuli. Personal reference can empirically be tested for by letting subjects decide about the level of personal association with alcoholic and nonalcoholic stimuli. The here applied concept of personal reference bears strong resemblance with the one of self‐relatedness [de Greck et al., 2008; Kelley et al., 2002; Northoff and Bermpohl, 2004; Northoff et al., 2007]. Previous imaging studies in healthy subjects have demonstrated the at least partial involvement of reward circuitry in self‐relatedness including the VS, VTA, and the VMPFC [de Greck et al., 2008; Northoff et al., 2006, 2007; Phan et al., 2004]. This led us to our suggestion that alcoholic patients might not only show abnormalities in these regions during reward but also during personal reference.
The aim of our preliminary study was to assess neural activity in reward circuitry during both reward, i.e., the perception of monetary wins and losses, and personal reference, i.e., the evaluation of high and low referenced stimuli. Based on the above‐described findings, we first hypothesized that alcoholic patients show reduced neural activity in VS, VTA, and VMPFC during the perception of reward. Secondly, we hypothesized altered neural activity in these regions during evaluation of personal reference in alcoholic patients. Moreover, we expected to find decreased activation during the evaluation of stimuli with high personal reference. Using event‐related fMRI, we applied a previously established paradigm that tested for reward, i.e., win and loss of money as well as for personal reference [de Greck et al., 2008; Reuter et al., 2005]. The latter task included the evaluation of visual stimuli as either high or low referenced. We used the functional localizer approach [Saxe et al., 2006], which allowed us to investigate neural activity during personal reference in exactly those regions recruited by reward.
METHODS AND MATERIALS
Subjects
We investigated 10 healthy right‐handed subjects (2 women, 8 men; mean age = 34.3 ± 7.9 SD; range: 26–50 years) without any psychiatric, neurological, or medical illness and 10 abstinent right‐handed alcoholics matched according to age and sex (2 women, 8 men; mean age = 38.7 ± 9.1 SD; range: 24–55 years). After a detailed explanation of the study design and any potential risks, all subjects gave their written informed consent. The study was approved by the institutional review board of the University of Magdeburg, Germany.
All patients were diagnosed as alcohol dependent according to ICD‐10 and DSM‐IV criteria and had no other psychiatric axis I disorders and no past history of dependency or current abuse of other drugs. The alcoholics had been dependent for 9.7 ± 5.70 years (range: 3–23 years) and abstinent from alcohol for 5.35 ± 1.49 months (range: 3–8 months). The maximum amount of alcohol consumed during dependency had been 497 ± 224.76 g/day (range: 225–840 g/day). Both healthy subjects and alcoholics had been free of benzodiazepine and clomethiazole use for at least one week. One patient was treated with 25 mg doxepin per night, but no other psychotropic drugs were taken. No subject was in a nicotine withdrawal state during the fMRI session.
Paradigm
We implemented a well‐established paradigm, which was already successfully used in healthy subjects by our group [de Greck et al., 2008]. The experiment contained three different types of tasks. During reward trials, subjects entered a gambling situation, in which they could either win or lose money. During reference‐evaluation trials, subjects were supposed to gauge whether a stimulus was high or low in relation to them. The third task was a control task, in which subjects had to assess the orientation of a presented stimulus. The sequence of all trial types was designed to be as similar as possible to allow comparisons (see Fig. 1).
Figure 1.
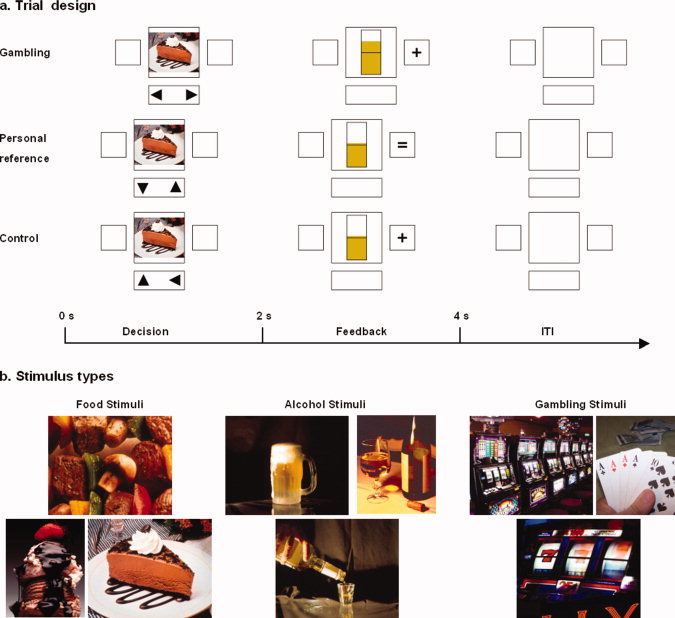
Study design. (a) Trial design: the experiment contained three types of trials with a similar structure but different tasks. Each trial began with a decision phase (2‐s duration) followed by a feedback phase (2‐s duration). Prior to the beginning of the next trial a short intertrial interval (ITI, duration 1 or 2 s) was presented. Gambling trials: subjects had to bet for either the left or right side in the decision phase. Whether they had won or lost was symbolized by the presentation of plus or minus sign on the chosen side in the feedback phase; win or lose were indicated by the bar and +/‐ signs. Personal reference trials: subjects were asked to assess the presented picture during the decision phase. In the feedback phase, the answer was represented by an equal sign to avoid confusion with the other two feedback tasks. For consistency purposes, the state bar was also presented showing two alternating values. Subjects were instructed that the presentation of the state bar was irrelevant during personal reference trials. Control trials: subjects had to evaluate the alignment of the stimulus whether it had a horizontal or a vertical orientation. The correct and incorrect answers were symbolized by the presentation of a plus or minus sign in the feedback period. The state bar was also presented in the feedback phase with an alternating irrelevant value. (b) Stimulus types: Three different types of stimuli were used in each of the three tasks. Food stimuli were taken from the International Affective Picture System (IAPS). These stimuli contained different types of food. The gambling stimuli contained slot machines, card playing situations, or roulette scenes. Theses stimuli were designed especially for this study. Alcohol stimuli were taken from the Normative Appetitive Picture System (NAPS), and they showed different kinds of alcohol liquids. In total, we used 40 different stimuli of each stimulus type in the experiment. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
All trials began with the presentation of a decision phase (2‐s duration), in which subjects had to perform a button press with either their the left or right hand. During this phase, a picture was displayed in the center of the screen and two little triangles at the bottom symbolized the task that had to be performed. The decision phase was directly followed by a feedback phase (2‐s duration), in which subjects got a short symbolized feedback. The display contained a symbol on the site of their response and a state bar in the center. Every location on the screen in which pictures or symbols could appear was surrounded by a thin frame. Before each next trial, a short intertrial interval (ITI, duration 1 or 2 s) was presented in which only the four empty location frames were presented. While the ITI was identical in all three tasks, the specific content of the two phases was different for each task.
In the decision phase of gambling trials, subjects were asked to bet by deciding for the left or right site of the display. In the following feedback phase, they were informed whether they had won or lost. This was symbolized by the presentation of a plus or minus sign on the chosen site. Additionally, the state bar that reflected the actual amount of the subject's payment increased or decreased. In orientation on the study performed by Reuter et al. [Reuter et al., 2005], our subjects were made to believe that they were gambling against the computer and that their luck during the gambling trials had a direct influence on the amount of their payment. They were not aware that, in fact, the proportion of wins and losses was predefined and almost identical for all subjects.
During the decision phase of personal reference trials, subjects had to evaluate whether the presented picture was of high or low personal reference. In the feedback phase of these trials, an equality sign was presented when the button press was delivered in time. In contrast to both of the other tasks, the minus sign was only presented when no response occurred. We decided to present an equality sign instead of the plus sign to make sure that this task had no rewarding component. The state bar was presented in these trials as well for the purpose of consistency. Although subjects were instructed that it had no meaning, the actual value fluctuated around the midline.
In control trials, it was the subject's task to identify the alignment of the presented picture during the decision phase. All stimuli had the shape of a rectangle, half of the stimuli were horizontally aligned and half of them vertically. When subjects gave the right answer, a plus sign was presented in the feedback phase and a minus sign in false trials, respectively. As described earlier, the feedback display contained the fluctuating state bar that was irrelevant in these trials.
A total of 120 stimuli were presented four times during the experiment; once during evaluation of personal reference and control trials and twice during gambling trials. The twofold presentation of stimuli during the gambling trials resulted from our orientation on the reward paradigm introduced by Reuter et al. [Reuter et al., 2005], which contained a total number of 250 trials. To achieve the same effect, we also included a total number of 240 trials, which made the twofold presentation of our stimuli unavoidable. The 120 stimuli included 40 food pictures, 40 alcohol pictures, and 40 gambling pictures. The food pictures were taken from the International Affective Picture System [Lang et al., 1999] and slightly modified. As alcohol stimuli served pictures from the Normative Appetitive Picture System [Breiner et al., 1995; Stritzke et al., 2004] that were modified as well. The gambling stimuli comprised typical gambling scenes and were developed especially for this study. Likewise the state bar presented in the feedback phase had three different colors reflecting the actual stimulus category. The selection of stimuli was driven by our question which kind of stimulus might be best suited to investigate the relationship between reward and personal reference. Based on previous imaging experiments, we decided to take stimuli that showed a strong reward value like natural reinforcers, e.g., food stimuli [Killgore et al., 2003; Wang et al., 2004]. Clinical experience with alcoholic patients led us to implement pictures containing alcohol stimuli, as alcoholics report a strong reference toward these stimuli on the one hand and show a pronounced craving for these stimuli on the other [Modell and Mountz, 1995; Park et al., 2007]. We were also curious as to why these patients developed alcoholism, which is a substance‐related addictive disorder and not a nonsubstance‐related disorder such as pathological gambling. For this reason, we integrated pictures containing gambling stimuli.
Trials were presented in rows of 10 stimuli of the same category. Hereafter, a baseline event occurred that lasted for 4, 5, or 6 s. The experiment was divided in eight sessions with the same task, i.e., four gambling sessions, two sessions with evaluation of personal reference, and two control sessions. Sessions and trials were presented in a pseudorandomized order. At the end of each session, a short evaluation period was presented in which subjects were asked to state their actual situation. They had to describe their general contentment, hungriness, craving for alcohol, and craving for gambling by virtually moving a bar on a visual analog scale.
The experiment was executed on a ordinary desktop personal computer using the software package Presentation (Neurobehavioral Systems, http://www.neurobs.com). Subjects were lying inside the scanner and were watching the projected experiment on a matt screen through a mirror attached on the head coil.
Subsequent to the fMRI session, all subjects performed a postscanning experiment in which they evaluated all presented stimuli in respect to their personal reference and the craving that was induced by the stimulus. After the 2‐s presentation of the stimulus, subjects had to assess whether they could consent to two displayed statements (personal reference: “The content has a lot to do with me”; craving: “I have a strong craving for the content”) by virtually moving a bar on the screen. The postscanning paradigm was likewise displayed on an ordinary desktop personal computer using the experimentation software package Presentation (Neurobehavioral Systems, http://www.neurobs.com).
Behavioral Data Analysis
Behavioral data have been examined using repeated measurements analysis of variance (ANOVA) and paired‐samples t‐tests as well as independent‐samples t‐tests for group comparisons. Different factors have been included in the ANOVAs: Within‐subjects factor condition (win, lose, reward trials with no response, high personal reference, low personal reference, reference trials with no response, correct control trials, incorrect control trials, control trials with no response), within‐subjects factor personal reference (high and low), within‐subjects factor stimulus category (natural, gambling and alcohol), and the between‐subjects factor group (healthy and alcoholics).
fMRI Data Acquisition and Analysis
Functional measurements were performed on a 3‐T whole body MRI system (Siemens Trio, Erlangen, Germany) with echo planar imaging (EPI) using an eight‐channel head coil. The slices were acquired parallel to the AC–PC plane in an odd–even interleaved acquisition order. Thirty‐two T2*‐weighted echo planar images per volume with blood oxygenation level‐dependent (BOLD) contrast were obtained (matrix: 64 × 64; 32 slices per volume; FoV: 224 × 224 mm; spatial resolution: 3.5 × 3.5 × 4 mm; TE = 30 ms; TR = 2000 ms; flip angle = 80°). Functional data were recorded in eight scanning sessions containing 210 volumes per session for each subject. The first four volumes were discarded. The fMRI data were preprocessed and statistically analyzed by the general linear model approach [Friston et al., 1995] using the SPM2 software package (spm2, http://www.fil.ion.ucl.ac.uk) and MATLAB 6.5 (The Mathworks, Natick, MA). All functional images were slice time corrected with reference to the first slice acquired, corrected for motion artifacts by realignment to the last volume, and spatially normalized to a standard T2‐weighted SPM template [Ashburner and Friston, 1999]. The normalization was realized by warping the subject's last functional image to the SPM template and applying these parameters to the other functional images. The images were resampled to 2 × 2 × 2 mm and smoothed with an isotropic 6‐mm full‐width half‐maximum Gaussian kernel.
The time‐series fMRI data were filtered using a high pass filter and cutoff of 128 s. A statistical model for each subject was computed by applying a canonical response function [Friston et al., 1998]. All relevant periods, i.e., the decision phase, the feedback phase, and the baseline phase were included in the SPM model. Regionally specific condition effects were tested by employing linear contrasts for each subject and different conditions. The resulting contrast images were submitted to a second‐level random‐effects analysis. Here, one‐sample t‐tests were used on images obtained for each subjects' volume set and different conditions. To control for the multiple‐testing problem, we performed a false discovery rate correction [Nichols and Hayasaka, 2003]. The anatomical localization of significant activations was assessed with reference to the standard stereotactic atlas by superimposition of the SPM maps on a standard brain template provided by SPM2.
In a second step, we analyzed the fMRI raw data using the Marseille Region of Interest Toolbox software package [Brett et al., 2002; MarsBaR 1.86, http://www.sourceforge.net/projects/marsbar]. Using a sphere‐shaped region‐of‐interest (ROI, radius 5 mm) we extracted the raw data from activations found in the second level analysis. To control for baseline drifts, we applied a linear baseline shift correction. Mean normalized fMRI signal values from two following time steps (6 and 8 s after feedback onset) of the BOLD were included in the statistical analysis using repeated measurements analysis of variance (ANOVA) and paired‐samples t‐tests as well as independent‐samples t‐tests for group comparisons [Dreher et al., 2006; Yarkoni et al., 2005]. Different factors have been included in the ANOVAs. Within‐subject's factor reward (win and lose), within‐subject's factor personal reference (high and low), within‐subject's factor stimulus category (natural, gambling, and alcohol), and the between‐subject's factor group (healthy and alcoholics). As the ratio of high and low personal reference trials could not be predefined as the ratio of win and lose trials, we performed Levene's tests to check for possible inhomogeneity of variances.
It should be mentioned at this point that our paradigm did not allow for comparisons of activations during the reward task (i.e., monetary wins and losses) with activations during the personal reference task (i.e., evaluation of pictures with high and low personal reference), as the time point of the main event of both tasks was different.
RESULTS
Behavioral Data
Reaction times
Reaction times were analyzed with regard to possible group differences and the effects of different tasks and conditions. Repeated measurements ANOVAs and independent‐samples t‐tests revealed no significant group differences. The statistical analysis for the different conditions showed slowest reaction times during high personal reference events compared with all other conditions in healthy subjects. Alcoholics did not show any significant difference in the reaction times for different conditions (see Supplementary Material 1a and 1b).
Personal reference
Behavioral testing of personal reference, using independent samples t‐tests, revealed that alcoholic patients showed a significantly higher number of high referenced stimuli (t(18) = 2.735; p = 0.014) and a lower number of low referenced stimuli (t(18) = −2.703; p = 0.015) when compared with healthy subjects.
The effects of stimulus categories on ratings of personal reference were investigated using a 2 × 3 × 2 factorial repeated measurements ANOVA (personal reference, stimulus category, group). We obtained significant effects for the interaction between personal reference and group as well as for the interaction between personal reference and stimulus category. The interaction between the factors personal reference, stimulus category, and group revealed a statistical trend as did the personal reference factor (see Supplementary Material 1c). Post‐hoc analysis with paired‐samples t‐tests showed that healthy subjects rated both alcohol stimuli and gambling stimuli significantly more often as low referenced than as high referenced (t(9) = 3.930; p = 0.003 and t(9) = 3.228; p = 0.010, respectively). While alcoholic patients did not show any significant difference between the numbers of high and low referenced alcoholic and gambling stimuli, respectively, we found a statistical trend for more high personal reference ratings of natural stimuli (t(9) = 2.085; p = 0.067; see also Supplementary Material 1d and Fig. 2). This was further confirmed by post‐hoc group comparison using independent‐samples t‐tests where alcoholic patients showed significantly higher numbers of high and lower numbers of low referenced alcoholic stimuli when compared with healthy subjects (t(18) = 3.224; p = 0.005 and t(18) = −2.993; p = 0.008, respectively; see also Supplementary Material 1d and Fig. 2).
Figure 2.
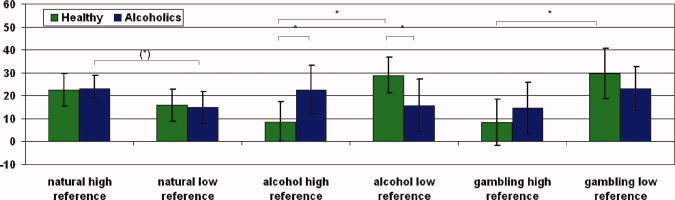
Ratings of personal reference in different stimulus categories. The number of trials (y‐axis) in different conditions and stimulus categories (error bar: standard deviation) have been examined within each group using paired t‐tests. Furthermore, between‐group comparisons have been performed using independent‐samples t‐tests. In healthy subjects, no significant difference in the number of high and low referenced pictures of natural stimuli has been observed. Gambling pictures have been significantly rated less frequently high referenced than low referenced as it has been for alcohol stimuli. In alcoholics, the comparison of the number of high and low referenced pictures of natural stimuli revealed a statistical trend. No statistical difference between the number of high and low referenced gambling or alcohol pictures has been observed. In the group comparison, we only found significant differences for the performed personal reference ratings on alcohol stimuli. Abbreviations, *p < 0.05. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Personal Ratings of General Contentment, Hungriness, Craving for Alcohol, and Craving for Gambling
The analysis of these ratings over all subjects and sessions revealed no significant results (see Supplementary Material 1e).
FMRI Data
Changes in the reward system during win and lose in alcoholic subjects
As a first step, we aimed to ascertain the reward regions of our whole sample group, i.e., healthy subjects and alcoholic patients collected as one group. Following the approach of Goldstein et al. [Goldstein and collegues, 2007], we performed a one sample t‐test on the contrast win > lose. We applied this method for detecting our ROI, as we wanted neither the group of healthy subjects nor the group of our alcoholic patients to have a dominant influence on the determination of the ROI. Corresponding to the well‐established data about the reward system (see introduction and discussion for details), we observed neural activity in right and left VS, in the brain‐stem adjacent to VTA (in the following simply described as VTA), and left VMPFC (coordinates with reference to the MNI stereotactic space: right VS (18, 20, −6); left VS (−18, 16, −2); right VTA (6, −28, −32); left VMPFC (−4, 48, −6); see images on the left in Figure 3) and other regions including the left inferior frontal gyrus, the right lateral globus pallidus and the right putamen (see Table I). Further analysis focused exclusively on the right and left VS, the VTA and the left VMPFC, as these regions have been demonstrated to be crucially involved in the reward system in both healthy and alcoholic subjects (see Introduction and Discussion for details).
Figure 3.
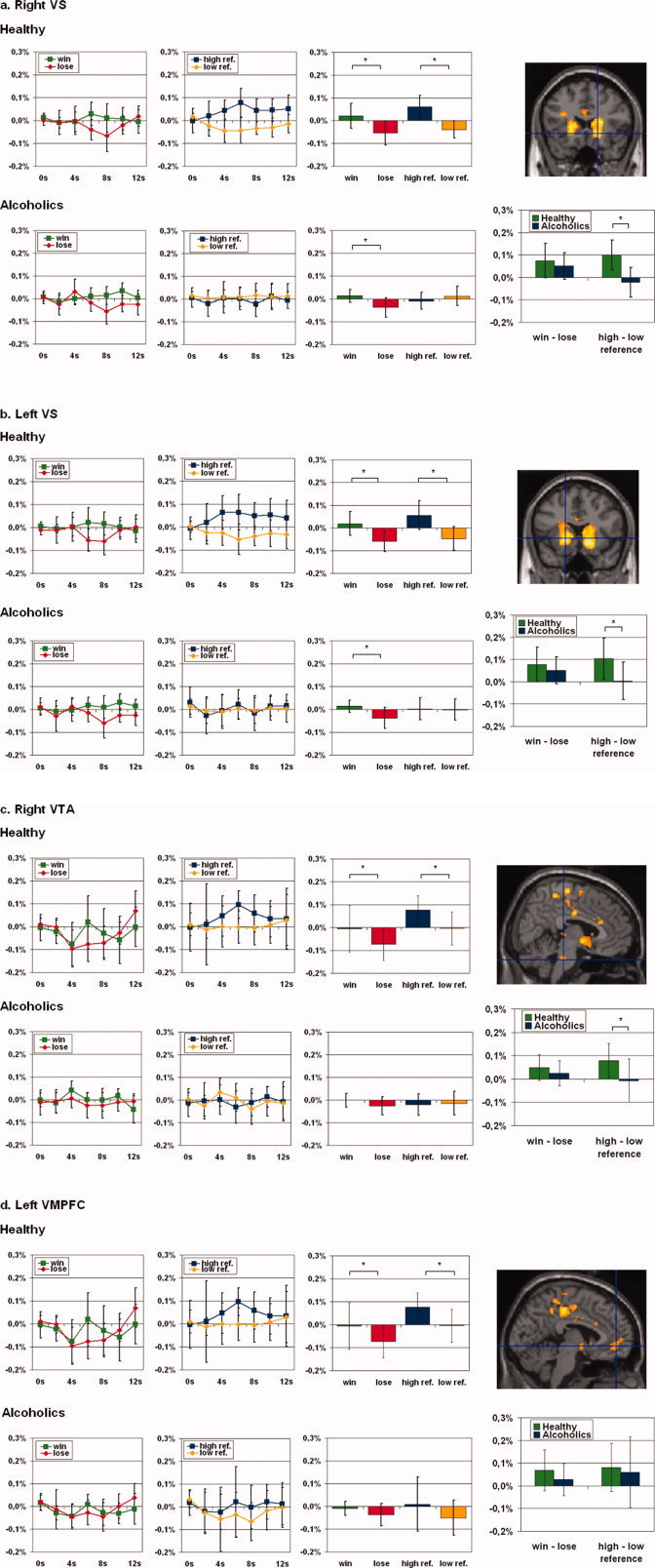
Activations and fMRI signal changes in reward regions during reward and personal reference. The second level group statistic for the contrast win > lose revealed activations in the right and left ventral striatum (VS), the left ventral tegmental area (VTA), and the left ventromedial prefrontal cortex (VMPFC). The top picture on the right side of each paragraph shows the t‐contrast calculated with SPM2. The two diagrams at the left of each line show the mean normalized fMRI signal changes (y‐axis) for the conditions win, lose, high personal reference, and low personal reference (error bar: standard deviation) with t = 0 for the start of the feedback phase in healthy and in alcoholics respectively. The box diagrams on the right display the mean normalized fMRI values (y‐axis) for the time points 6–8 s. The box diagrams below each picture on the far right side illustrate the mean differences of the mean normalized fMRI signal (y‐axis) for the time points 6–8 s (error bars: standard deviation). The mean normalized fMRI values of each group have been analyzed by paired‐samples t‐tests, the differences of the mean normalized fMRI signal have been examined with independent‐samples t‐tests between the two groups. (a) Right VS (18, 20, −6; Z = 5.32; pFDR = 0.001). We found a higher mean fMRI signal for win events compared to lose events in healthy and in alcoholics and a higher mean fMRI signal for high personal reference events compared with low personal reference events only in healthy but not in alcoholics. While the difference of win and lose events showed no significant difference in the group comparison of healthy and alcoholics, this comparison revealed significant higher difference of high referenced and low referenced events in healthy individuals. (b) Left VS (−18, 16, −2; Z = 5.22; pFDR = 0.001). We found a higher mean fMRI signal for win events compared with lose events in healthy and in alcoholics and a higher mean fMRI signal for high personal reference events compared with low personal reference events in healthy, but not in alcoholics. While the difference of win and lose events showed no significant difference in the group comparison of healthy and alcoholics, this comparison revealed a significantly higher difference of high personal reference and low personal reference events in healthy individuals. c. Right VTA (6, −28, −32; Z = 4.29; pFDR = 0.002). We found a higher mean fMRI signal for win events compared with lose events only in healthy individuals, but not in alcoholics. The contrast of high personal reference events compared with low personal reference events revealed a significantly higher fMRI signal during high personal reference events only in healthy individuals. While the difference of win and lose events showed no significant difference in the group comparison of healthy individuals and alcoholics, this comparison revealed a significantly higher difference of high personal reference and low personal reference events in healthy individuals. (d) Left VMPFC (−4, 48, −6; Z = 3.85; pFDR = 0.006). We found a higher mean fMRI signal for win events compared to lose events only in healthy, not in alcoholics and a higher mean fMRI signal for high personal reference events compared to low–personal reference events only in healthy, not in alcoholics. Both the difference of win–lose events and of high personal reference low personal reference events showed no significant difference in the group comparison of healthy and alcoholics. Abbreviations: VS = ventral striatum; VTA = ventral tegmental area; VMPFC = ventromedial prefrontal cortex; * = p < 0.05. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table I.
Activations for the contrast [reward win] > [reward lose]
| Region | x, y, z [mm*] | Z | p (FDR) | |||
|---|---|---|---|---|---|---|
| Right ventral striatum | 18 | 20 | −6 | 5.32 | 0.001 | |
| Left ventral striatum | −18 | 16 | −2 | 5.22 | 0.001 | |
| Right Pons | 6 | −28 | −32 | 4.29 | 0.002 | |
| Left ventromedial prefrontal cortex | −4 | 48 | −6 | 3.85 | 0.006 | |
| Left inferior frontal gyrus | −38 | 34 | −18 | 5.11 | 0.001 | |
| Right lateral globus pallidus | 12 | 6 | −6 | 5.22 | 0.001 | |
| Right putamen | 22 | −2 | 16 | 5.47 | 0.001 | |
| Left inferior temporal gyrus | −58 | −54 | −8 | 4.57 | 0.001 | |
| Left precentral gyrus | −42 | −16 | 46 | 4.39 | 0.002 | |
| Left nucleus caudatus | −18 | −38 | 18 | 4.39 | 0.002 | |
| Right anterior cingulum | 6 | 26 | 22 | 4.13 | 0.003 | |
| Left postcentral gyrus | −40 | −28 | 36 | 4.22 | 0.003 | |
| Left insula | −28 | −38 | 18 | 4.14 | 0.003 | |
| Left fusiform gyrus | −42 | −26 | −20 | 3.99 | 0.004 | |
| Right temporal gyrus | 38 | −28 | 10 | 4.05 | 0.004 | |
| Left superior temporal gyrus | −64 | −40 | 18 | 4.01 | 0.004 | |
| Left cingulate gyrus | −6 | −44 | 44 | 4.02 | 0.004 | |
| Right middle temporal gyrus | 68 | −30 | 0 | 3.97 | 0.005 | |
| Right fusiform gyrus | 50 | −42 | −22 | 3.96 | 0.005 | |
| Left middle frontal gyrus | −46 | 34 | 18 | 3.85 | 0.006 | |
| Anterior cingulum | 0 | 18 | 26 | 3.84 | 0.006 | |
| Right middle temporal gyrus | −58 | −6 | −8 | 3.85 | 0.006 | |
| Right inferior temporal gyrus | 50 | −30 | −20 | 3.82 | 0.006 | |
| Right middle temporal gyrus | 52 | −38 | −14 | 3.86 | 0.006 | |
| Left paracentral lobule | −18 | −42 | 48 | 3.81 | 0.006 | |
| Left precuneus | −38 | −66 | 36 | 3.85 | 0.006 | |
| Left middle frontal gyrus | −40 | 52 | −4 | 3.70 | 0.008 | |
| Right middle temporal gyrus | 56 | 6 | −30 | 3.70 | 0.008 | |
| Right caudate nucleus | 26 | −40 | 20 | 3.60 | 0.009 | |
| Left precuneus | −24 | −78 | 14 | 3.63 | 0.009 | |
Coordinates refer to the MNI stereotactic space.
Based on the regions as elucidated in the first step (see above and Methods for details), we compared signal intensities for win and lose between both groups. A 2 × 2 factorial ANOVA (reward, group) revealed a significant main effect for the factor reward in each of the four regions but not for the reward × group interaction (see Supplementary Material 2a). Paired‐samples t‐tests in healthy subjects revealed significant differences in signal intensities between win and lose in all of the four regions (right VS: t(9) = 3.041, p = 0.014; left VS: t(9) = 3.122, p = 0.012; right VTA: t(9) = 2.800; p = 0.021; left VMPFC: t(9) = 2.428; p = 0.038; see also Supplementary Material 2b and Fig. 3). Alcoholic patients also showed significant differences between win and lose in the right and left VS, but they failed to show this in the VTA and the VMPFC (right VS: t(9) = 2.673; p = 0.025; left VS: t(9) = 2.658; p = 0.026; right VTA: t(9) = 1.465; p = 0.177; left VMPFC: t(9) = 1.227; p = 0.251; see also Supplementary Material 2b and bar diagrams on the right in Figure 3 for win and lose separately in both groups). Despite these differences, this did not amount to statistically significant differences between groups in the difference between win and lose (right VS: t(18) = 0.778; p = 0.447; left VS: t(18) = 0.842; p = 0.411; right VTA: t(18) = 0.990; p = 0.335; left VMPFC: t(18) = 1.148; p = 0.266; see also Supplementary Material 2b and Fig. 3). Levene's tests for equality of variances did not yield significant values for any of the comparisons.
Changes in the reward system during high and low personal reference in alcoholic patients
We then investigated signal intensities during high and low personal reference in those regions that were recruited during win and lose in both groups. A 2 × 2 factorial ANOVA (evaluation of personal reference, group) revealed a significant main effect for the factor evaluation of personal reference in three of the four regions (i.e. right and left VS, left VMPFC) and for the personal reference × group interaction in three of the four regions (i.e., right and left VS, right VTA, see Supplementary Material 2c). In contrast to healthy subjects who revealed significant higher fMRI signals during trials with the evaluation of high personal reference when compared with low personal reference trials (right VS: t(9) = 4.762; p = 0.001; left VS: t(9) = 3.534; p = 0.006; right VTA: t(9) = 3.429; p = 0.008; left VMPFC: t(9) = 2.409; p = 0.039; see also Supplementary Material 2d and Fig. 3), alcoholic patients did not show significant differences in signal intensities between high and low referenced stimuli in both right and left VS, VTA, and VMPFC (right VS: t(9) = −1.055; p = 0.319; left VS: t(9) = 0.142; p = 0.890; right VTA: t(9) = 0.222; p = 0.830; left VMPFC: t(9) = 1.214; p = 0.256; see also Supplementary Material 2d and Fig. 3). Comparisons of the difference between high and low personal reference yielded significant differences between groups in both right and left VS and VTA but not in VMPFC (right VS: t(18) = 4.110; p = 0.001; left VS: t(18) = 2.527; p = 0.021; right VTA: t(18) = 2.288; p = 0.034; left VMPFC: t(18) = 0.346; p = 0.734; see also Supplementary Material 2d and Fig. 3). Levene's tests for equality of variances did not yield significant values for any of the comparisons.
To demonstrate that our fMRI signal changes during the evaluation of personal reference are not due to nonspecific cognitive‐evaluative confounds, we directly compared fMRI signal changes from the ascertained reward regions during the evaluation of personal reference with those during the control condition. The results showing that the activations in reward circuitry during high personal reference are caused by the degree of personal reference (and not by the cognitive‐evaluative confounds) are presented in Supplementary Material 3.
Effects of stimulus category on signal intensities during win and lose and high and low personal reference
To control for specific effects of stimulus categories (natural, gambling, alcohol) during win and lose, we conducted a 3 × 2 × 2 factorial repeated measures ANOVA (stimulus category, personal reference, group). This did not reveal any significant interaction between the stimulus category and group in any of the four regions during win and lose (see Supplementary Material 2e). Similarly, we did not observe any effects neither of personal reference, e.g., whether the stimuli were high or low referenced, nor of the factor stimulus category or of the personal reference × group interaction on signal intensities during win and lose in any of the four regions (see also Supplementary Material 2e). Finally, we did not observe any significant interaction between stimulus category, personal reference, and group during win and lose in both right and left VS and in the VTA (see Supplementary Material 2e). Taken together, these results suggest that there was only some influence of different stimulus categories on signal intensities during win and lose in the VMPFC but not in the other three regions in both groups.
We then conducted similar analyses for investigating the effects of stimulus category on signal intensities during the evaluation of high and low personal reference. A 3 × 2 factorial repeated measurements ANOVA (stimulus category, group) revealed a significant effect of the factor stimulus category only in the VTA (see Supplementary Material 2f and the figure of Supplementary Material 4). In healthy subjects, post‐hoc paired t‐tests for this region revealed a higher difference between high referenced and low referenced stimuli in alcohol trials compared with gambling trials (T(7) = 3.835; p = 0.006). However, in alcoholic patients, post‐hoc paired samples t‐tests for this region did not reveal any significant difference. We, however, did not observe any significant interaction between stimulus category and group in the VTA or any of the other regions (see also Supplementary Material 2f). Taken together, these results indicate some effects of stimulus category in healthy subjects in the VTA, concerning the difference between gambling and alcoholic stimuli.
DISCUSSION
We investigated reward function and personal reference in reward circuitry in abstinent alcoholic patients. Contrary to our first hypothesis, we were not able to replicate previous findings that clearly showed reduced activity in reward regions during the perception of reward [Wrase et al., 2007]. We could not find any statistically significant reduction in signal changes in all four regions observed (VS, VTA, and VMPFC) during the perception of monetary reward in alcoholic patients when compared with healthy subjects. However, we observed some slight (though statistically nonsignificant) differences of signal intensity in the VTA and the VMPFC, which is in accordance with previous findings [Wrase et al., 2007]. The most striking outcome of our study was that alcoholic patients, although showing no major alterations in reward function, revealed reduced signal changes during personal reference, i.e., the evaluation of high referenced stimuli, in the very same reward regions, which confirms our second hypothesis. We thus demonstrate for the first time altered neural activity in reward circuitry during personal reference in alcoholic patients.
Several studies found altered neural activity during reward tasks in reward circuitry in alcoholic patients [Wrase et al., 2007] and patients suffering from other forms of addictive diseases such as cocaine [Volkow et al., 1997, 2004a] and pathological gambling [Reuter et al., 2005]. Although we tested for changes in reward activity using a well‐established paradigm that had shown altered reward function in pathological gamblers before [Reuter et al., 2005], we were not able to detect statistically significant evidence for disturbed reward function in our alcoholic subjects. Although we observed less differentiation in signal changes between win and lose in the VTA and the VMPFC during the reward task in alcoholic patients, these did not yield statistically significant differences when compared with healthy subjects. The possible reasons for the lack of reduced reward function might include the following methodological and clinical explanations. While Wrase et al. [Wrase et al., 2007], relying on the Monetary Incentive Delay Task (MID‐Task) established by Knutson et al. [Knutson et al., 2001a], focused on the anticipatory period of monetary gains and losses, we here investigated the gain and loss periods themselves. One may consequently speculate that the VS may specifically be associated with deficits in anticipation of reward rather than with deficits in the perception of reward. This interpretation is supported by the findings from Knutson et al. [Knutson et al., 2001a, b, 2003], who associated neural activity in the VS with the anticipation of reward, whereas the VMPFC was active during the gain period itself. This neural differentiation however needs to be investigated in future studies that directly compare anticipation and perception of reward in alcoholic patients. Another difference between the two studies concerns the stage during which alcoholic patients were investigated. While the study by Wrase et al. [ 2007] and others [George et al., 2001; Grusser et al., 2004; Kareken et al., 2004; Myrick et al., 2004] investigated acutely detoxified alcoholic patients, our patients were already abstinent for at least three months. Braus [Braus et al., 2001] as well as others [Heinz et al., 1996; Laine et al., 1999] presented some evidence that altered neural activity in reward circuitry may disappear and thus normalize after at least three weeks of abstinence. Deficiency in reward circuitry during the acute state may be due to a decrease in VS dopamine transporters that recovers after a few days of abstinence [Laine et al., 1999]. The long duration of abstinence in our alcoholic patients (at least three months of abstinence) may thus possibly account for the observed absence of abnormalities in VS (and the absence of between‐group effects in the other regions) during reward. The hypothesized neural differentiation between the acute and abstinent state suggests that deficits in reward regions may potentially be regarded as a state marker of acute alcohol abuse.
The most striking finding is that alcoholic patients showed considerably reduced signal changes in reward circuitry during the evaluation high personal reference when compared to healthy subjects; this has not been investigated before. Recent studies in healthy subjects demonstrated involvement of the VS and the VMPFC in personal reference [Northoff, 2007; Northoff and Bermpohl, 2004; Northoff et al., 2006; Phan et al., 2004]. These findings are supported by a recent study of our group, which explicitly investigated the association of reward and evaluation of personal reference [de Greck et al., 2008]. We were able to demonstrate that reward circuitry (VS, VMPFC, and VTA) is recruited during the evaluation of high referenced stimuli. How can we explain such apparent overlap between reward and personal reference? We suggest that both reward and personal reference might present distinct aspects of the so‐called valuation system [Montague and Berns, 2002; Montague et al., 2006]. The valuation system does not only assign immediate value to a stimulus, indicating the reward value, but also its long‐term value for the organism reflecting what can be called personal reference. The shared valuation system might neuronally be represented in basic reward circuitry including VTA, VS, and VMPFC. The present findings indicate that the neural basis of this valuation system may be altered in alcoholic patients. While our patients exhibited only slight abnormalities (e.g., no between group differences) in reward circuitry during reward function, i.e., win and loss, they showed major abnormalities in the same regions during the evaluation of personal reference. Such functional dissociation between reward and personal reference in reward circuitry suggests a possible dissociation between the assignment of immediate and long‐term value to stimuli in alcoholic patients. While the immediate value assignment and thus reward function may recover with the duration of abstinence, the deficits in long‐term assignment of personal significance, i.e., personal reference may persist beyond the state of acute detoxification. One may consequently speculate that clinically neural deficits in reward function may be considered a state marker of acute addiction, whereas neural deficits in personal reference may rather be a trait marker of alcoholism describing the predisposition of a person to addiction. This rather speculative hypothesis, however, awaits further confirmation from longitudinal studies investigating both reward and personal reference.
Clinically, deficits in personal reference may manifest in the patient's attribution of abnormally high personal significance to alcohol. This corresponds well to our behavioral data showing that alcoholic patients evaluated alcoholic stimuli more high personal referenced when compared with healthy subjects. Behavioral attribution of higher personal reference to alcoholic stimuli was however not accompanied by higher signal changes in either region of reward circuitry, e.g., VS, VMPFC, and VTA, during alcohol‐related stimuli. In other terms, we could not observe any specific effects of alcoholic stimuli in reward circuitry during either reward or personal reference in alcoholic patients. This indicates a basic deficit in reward circuitry remaining independent of the kind of stimulus in alcoholic patients. Investigating pathological gamblers, Reuter [Reuter et al., 2005] argue that organisms try to maintain a homeostatic baseline level of dopamine in the ventral striatum. While healthy subjects are able to reach and maintain a sufficient baseline level of dopamine activity in the VS by weak reinforcers found in everyday life, addicted patients like pathological gamblers lack this ability and seek for stronger reinforcers, as e.g. gambling or drugs. This hypothesis is supported by our data since we showed that the simple evaluation of stimuli with high personal reference leads to activity in VS (and other reward regions like VTA and VMPFC) only in healthy subjects but not in abstinent alcoholic patients. In other words, while healthy subjects show activation in reward circuitry during the evaluation of high referenced stimuli and hence can stabilize their homeostatic baseline activity in the VS, abstinent alcoholic patients lack this activation and are dependant on stronger reinforcers.
Some methodological limitations of our study should be mentioned. First, the number of patients investigated here is rather low so that our results should be considered preliminary, awaiting further support from larger samples. Second, one might criticize that our concept of reward needs to be parsed into distinct aspects of reward as proposed by Berridge and Robinson [ 2003]. Unfortunately, our design does not allow us to clearly distinguish between the appetitive preconsummatory aspects (wanting) on the one hand and hedonic consummatory aspects (liking) on the other. The paradigm used in our study relied on a similar design that was established by Reuter et al. [Reuter et al., 2005] and showed also robust effects in a previous study conducted by our group [de Greck et al., 2008]. The focus lay on the hedonic and consummatory phase. Third, one may argue that the concept of personal reference is rather ill‐defined. We here referred to previous studies on personal reference [Northoff and Bermpohl, 2004; Northoff et al. 2006, 2007] that let subjects explicitly decide whether the presented stimulus is high or low referenced. Finally one might miss that in our paradigm we did not test for any interaction between reward and personal reference. On the one hand, we intended to clearly identify reward‐associated regions without any active reference component to use them as a functional localizer. On the other hand, we aimed to introduce a personal reference task without any traces of a reward component to elucidate whether personal reference recruits reward circuitry using the functional localizer approach [Saxe et al., 2006]. To exclude any potential pictorial differences in both tasks, we presented the same stimuli. This design allowed us to test the hypothesis whether personal reference recruits neural activity in those regions that are involved in reward. This design, however did not allow us to test for any interaction effects between personal reference and reward at all; for that a different design that includes a high and low reward component within the reference evaluation task would be needed. One nevertheless has to consider that we cannot completely exclude some impact of reward even during the personal reference task because we used primary reinforcers, i.e., food, which subjects had to evaluate with regard to their personal reference. Although we cannot exclude such interaction completely, our observation that neural effects of personal reference occurred in all three stimulus categories argues against this interpretation. However, to completely exclude such interaction, a design that totally relinquishes primary reinforcers would be needed.
In conclusion, we demonstrate reduced neural activity in reward circuitry during personal reference in abstinent alcoholic patients. Our data show that alcoholic patients remain unable to appropriately increase neural activity in reward circuitry during the evaluation of high referenced stimuli. This may not only contribute to better understand the neural basis of alterations in personal reference in these patients but also to establish deficient reward circuitry as diagnostic and therapeutic marker of addiction.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supplementary Material.
Acknowledgements
The authors thank Sascha Moerth and Michael Rotte for their comments on conception and design and Rabea Paus, Diana Moritz, Ulrike Proesch, and Ulrike Bruer for assistance in data collection and analysis as well as Eva Stockum and Bjoern Enzi for their helpful comments on the manuscript. The authors also thank the staff members in Neurology II for their support and collaboration. The study was supported by a Heisenberg grant from the German Research Foundation (DFG, 304/4‐1 to G.N.), the Salus Foundation (to G.N), the Swiss National Research Foundation (3100A0‐100830) to G.N.
REFERENCES
- American Psychiatric Press . 1994. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC. [Google Scholar]
- Ashburner J,Friston KJ( 1999): Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler RD,Volkow ND( 2006): Drug addiction: The neurobiology of disrupted self‐control. Trends Mol Med 12: 559–566. [DOI] [PubMed] [Google Scholar]
- Berridge KC,Robinson TE( 2003): Parsing reward trends Neurosci 26: 507–513. [DOI] [PubMed] [Google Scholar]
- Braus DF,Wrase J,Grusser S,Hermann D,Ruf M,Flor H,Mann K,Heinz A( 2001): Alcohol‐associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm 108: 887–894. [DOI] [PubMed] [Google Scholar]
- Breiner MJ,Stritzke WGK,Lang AR( 1995): The Normative Appetitive Picture System (Photographic Slides). Florida State University; Tallahassee, FL. [Google Scholar]
- Brett M,Anton JL,Valabregue R,Poline JB ( 2002): Region of interest analysis using an SPM toolbox [abstract]. Presented at the 8th International Conferance on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. Available on CD‐ROM in NeuroImage, Vol 16, No 2, abstract 497.
- de Greck M,Rotte M,Paus R,Moritz D,Thiemann R,Proesch U,Bruer U,Moerth S,Tempelmann C,Bogerts B,Northoff G ( 2008): Is our self based on reward? Self‐relatedness recruits neural activity in the reward system. Neuroimage 39: 2066–2075. [DOI] [PubMed] [Google Scholar]
- Dreher JC,Kohn P,Berman KF( 2006): Neural coding of distinct statistical properties of reward information in humans. Cereb Cortex 16: 561–573. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Fletcher P,Josephs O,Holmes A,Rugg MD,Turner R( 1998): Event‐related fMRI: Characterizing differential responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Friston KJ,Holmes AP,Worsley KJ,Poline JB,Frith C,Frackowiak RSJ( 1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- George MS,Anton RF,Bloomer C,Teneback C,Drobes DJ,Lorberbaum JP,Nahas Z,Vincent DJ ( 2001): Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol‐specific cues. Arch Gen Psychiatr 58: 345–352. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ,Alia‐Klein N,Tomasi D,Zhang L,Cottone LA,Maloney T,Telang F,Caparelli EC,Chang L,Ernst T et al. ( 2007): Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self‐control in cocaine addiction? Am J Psychiatr 164: 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusser SM,Wrase J,Klein S,Hermann D,Smolka MN,Ruf M,Weber‐Fahr W,Flor H,Mann K,Braus DF,Heinz A ( 2004): Cue‐induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 175: 296–302. [DOI] [PubMed] [Google Scholar]
- Heinz A,Dufeu P,Kuhn S,Dettling M,Graf K,Kurten I,Rommelspacher H,Schmidt LG ( 1996): Psychopathological and behavioral correlates of dopaminergic sensitivity in alcohol‐dependent patients. Arch Gen Psychiatr 53: 1123–1128. [DOI] [PubMed] [Google Scholar]
- Kalivas PW,Volkow ND ( 2005): The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatr 162: 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kareken DA,Claus ED,Sabri M,Dzemidzic M,Kosobud AE,Radnovich AJ,Hector D,Ramchandani VA,O'Connor SJ,Lowe M et al. ( 2004): Alcohol‐related olfactory cues activate the nucleus accumbens and ventral tegmental area in high‐risk drinkers: preliminary findings. Alcohol Clin Exp Res 28: 550–557. [DOI] [PubMed] [Google Scholar]
- Kelley WM,Macrae CN,Wyland CL,Caglar S,Inati S,Heatherton TF ( 2002): Finding the self? An event‐related fMRI study. J Cogn Neurosci 14: 785–794. [DOI] [PubMed] [Google Scholar]
- Killgore WD,Young AD,Femia LA,Bogorodzki P,Rogowska J,Yurgelun‐Todd DA ( 2003): Cortical and limbic activation during viewing of high‐ versus low‐calorie foods. Neuroimage 19: 1381–1394. [DOI] [PubMed] [Google Scholar]
- Knutson B,Adams CM,Fong GW,Hommer D ( 2001a): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B,Fong GW,Adams CM,Varner JL,Hommer D ( 2001b) Dissociation of reward anticipation and outcome with event‐related fMRI. Neuroreport 12: 3683–3687. [DOI] [PubMed] [Google Scholar]
- Knutson B,Fong GW,Bennett SM,Adams CM,Hommer D ( 2003): A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event‐related fMRI. Neuroimage 18: 263–272. [DOI] [PubMed] [Google Scholar]
- Laine TP,Ahonen A,Torniainen P,Heikkila J,Pyhtinen J,Rasanen P,Niemela O,Hillbom M ( 1999): Dopamine transporters increase in human brain after alcohol withdrawal. Mol Psychiatr 4: 189–191;104–105. [DOI] [PubMed] [Google Scholar]
- Lang PJ,Bradley MM,Cuthbert BN. 1999. International Affective Picture System. The Center for Research in Psychophysiology, University of Florida.
- MarsBaR 1.86 . Available at: http://www.sourceforge.net/projects/marsbar.
- Modell JG,Mountz JM ( 1995): Focal cerebral blood flow change during craving for alcohol measured by SPECT. J Neuropsychiatr Clin Neurosci 7: 15–22. [DOI] [PubMed] [Google Scholar]
- Montague PR,Berns GS ( 2002): Neural economics and the biological substrates of valuation. Neuron 36: 265–284. [DOI] [PubMed] [Google Scholar]
- Montague PR,King‐Casas B,Cohen JD ( 2006): Imaging valuation models in human choice. Annu Rev Neurosci 29: 417–448. [DOI] [PubMed] [Google Scholar]
- Myrick H,Anton RF,Li X,Henderson S,Drobes D,Voronin K,George MS ( 2004): Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology 29: 393–402. [DOI] [PubMed] [Google Scholar]
- Nichols T,Hayasaka S ( 2003): Controlling the familywise error rate in functional neuroimaging: A comparative review. Stat Methods Med Res 12: 419–446. [DOI] [PubMed] [Google Scholar]
- Northoff G ( 2007): Subcortical regions and the self. Behav Brain Sci 30: 100–101.19696906 [Google Scholar]
- Northoff G,Bermpohl F ( 2004): Cortical midline structures and the self. Trends Cogn Sci 8: 102–107. [DOI] [PubMed] [Google Scholar]
- Northoff G,Heinzel A,de Greck M,Bermpohl F,Dobrowolny H,Panksepp J ( 2006): Self‐referential processing in our brain—A meta‐analysis of imaging studies on the self. Neuroimage 31: 440–457. [DOI] [PubMed] [Google Scholar]
- Northoff G,Schneider F,Rotte M,Matthiae C,Tempelmann C,Wiebking C,Bermpohl F,Heinzel A,Danos P,Heinze HJ,Bogerts B,Walter M,Panksepp J ( 2007): Differential parametric modulation of self‐relatedness and emotions in different brain regions. Hum Brain Mapp, Dec 6. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MS,Sohn JH,Suk JA,Kim SH,Sohn S,Sparacio R ( 2007): Brain substrates of craving to alcohol cues in subjects with alcohol use disorder. Alcohol Alcohol 42: 417–422. [DOI] [PubMed] [Google Scholar]
- Phan KL,Taylor SF,Welsh RC,Ho SH,Britton JC,Liberzon I ( 2004): Neural correlates of individual ratings of emotional salience: A trial‐related fMRI study. Neuroimage 21: 768–780. [DOI] [PubMed] [Google Scholar]
- Reuter J,Raedler T,Rose M,Hand I,Glascher J,Buchel C ( 2005): Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci 8: 147–148. [DOI] [PubMed] [Google Scholar]
- Saxe R,Brett M,Kanwisher N ( 2006): Divide and conquer: A defense of functional localizers. Neuroimage 30: 1088–1096; discussion 1097‐1099. [DOI] [PubMed] [Google Scholar]
- Stritzke WG,Breiner MJ,Curtin JJ,Lang AR ( 2004): Assessment of substance cue reactivity: Advances in reliability, specificity, and validity. Psychol Addict Behav 18: 148–159. [DOI] [PubMed] [Google Scholar]
- Volkow ND,Fowler JS,Wang GJ ( 2003): The addicted human brain: insights from imaging studies. J Clin Invest 111: 1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND,Fowler JS,Wang GJ ( 2004a): The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology 47( Suppl 1): 3–13. [DOI] [PubMed] [Google Scholar]
- Volkow ND,Fowler JS,Wang GJ,Swanson JM ( 2004b) Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatr 9: 557–569. [DOI] [PubMed] [Google Scholar]
- Volkow ND,Wang GJ,Fowler JS,Logan J,Gatley SJ,Hitzemann R,Chen AD,Dewey SL,Pappas N ( 1997): Decreased striatal dopaminergic responsiveness in detoxified cocaine‐dependent subjects. Nature 386: 830–833. [DOI] [PubMed] [Google Scholar]
- Wang GJ,Volkow ND,Telang F,Jayne M,Ma J,Rao M,Zhu W,Wong CT,Pappas NR,Geliebter A,Fowler JS ( 2004): Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage 21: 1790–1797. [DOI] [PubMed] [Google Scholar]
- Wrase J,Schlagenhauf F,Kienast T,Wustenberg T,Bermpohl F,Kahnt T,Beck A,Strohle A,Juckel G,Knutson B,Heinz A ( 2007): Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 35: 787–794. [DOI] [PubMed] [Google Scholar]
- Yarkoni T,Gray JR,Chrastil ER,Barch DM,Green L,Braver TS ( 2005): Sustained neural activity associated with cognitive control during temporally extended decision making. Brain Res Cogn Brain Res 23: 71–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supplementary Material.


