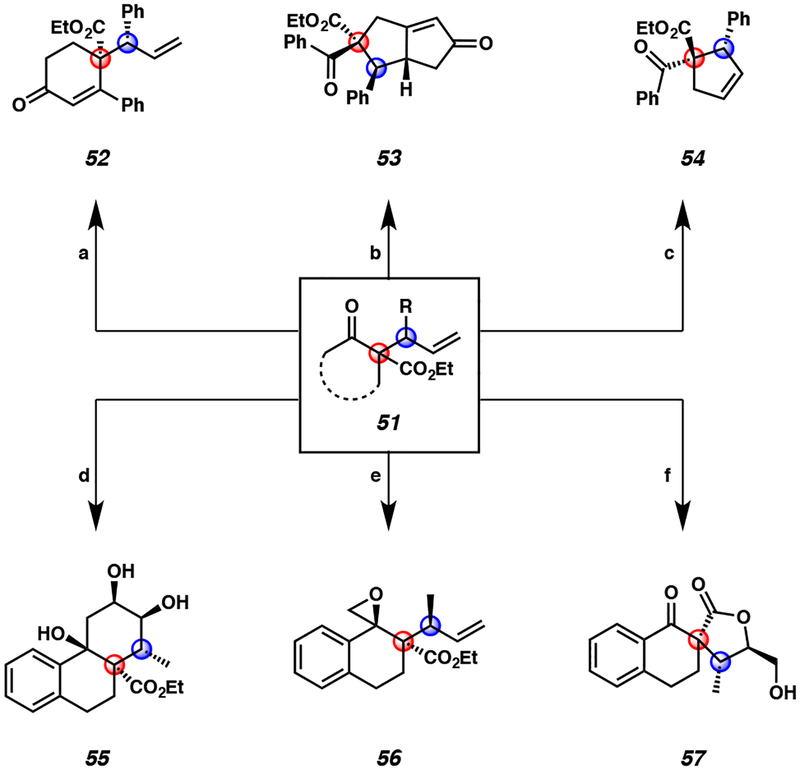
Figure 4.
Select examples of diverse product transformations of enantio- and diastereoselective iridium-catalyzed allylic alkylation products 51a
aConditions: (a) pyrrolidine, AcOH, t-BuOMe, reflux, 95% yield. (b) Co2(CO)8, CH2Cl2, then Me3NO×2H2O, >20:1 dr, 99% yield. (c) HG-II (10 mol %), CH2Cl2, 40 °C, 96% yield. (d) i) allylmagnesium chloride, THF, −78 °C, 71% yield, ii) HG-II, CH2Cl2, 81% yield, iii) K2OsO4, NMO, THF/H2O, 59% yield. (e) Me3S(O)I, NaH, DMSO, 82% yield. (f) K2OsO4, NMO, THF/H2O, 65% yield.