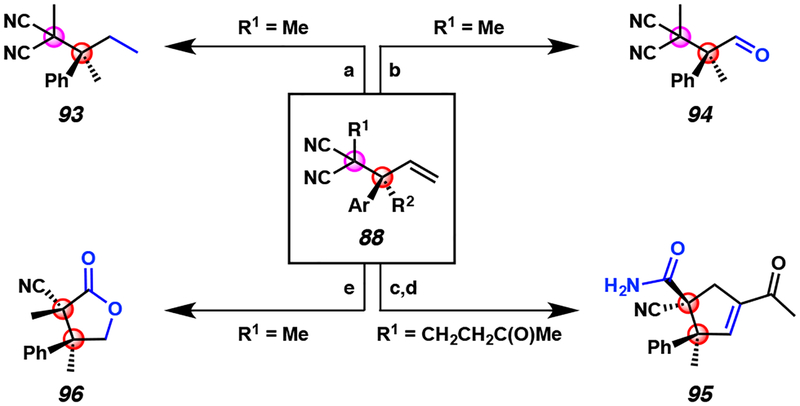
Figure 8.
Product derivatizations of iridium-catalyzed allylic alkylation products 88 bearing vicinal all-carbon quaternary centers
aConditions: (a) RhCl(PPh3)3, H2 (balloon), benzene, 23 °C, 18 h, 92% yield. (b) O3, pyridine, CH2Cl2, −78 °C, 4 min, 93% yield. (c) i. O3, pyridine, CH2Cl2, −78 °C, 4 min, ii. p-TsOH, benzene, reflux, 18 h, 47% yield. (d) NaOH, EtOH/H2O (1:1), 60 °C, 18 h, 38% yield. 1:11 dr. (e) i. O3, MeOH,−78 °C, 0.5 h, ii. NaBH4, 0 °C, 3 h, 65% yield, 1:2.5 dr.