Table 1.
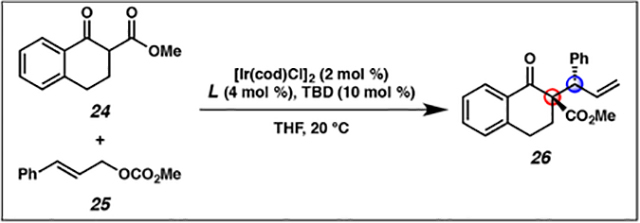
Development of conditions for the iridium-catalyzed allylic alkylation reaction of cyclic nucleophiles forming vicinal tertiary and all-carbon quaternary stereocentersa
 | |||||
|---|---|---|---|---|---|
| Entry | L | Additive (X mol %) |
b:lb | drb | ee (%)c |
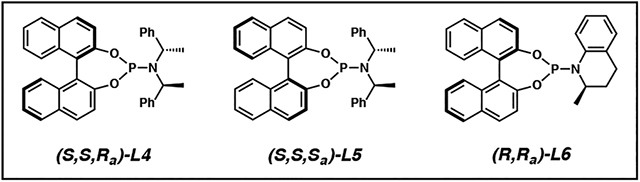
| 1 | L4 | NaH (200) | >95:5 | 1:1 | 99 |
| 2 | L5 | NaH (200) | >95:5 | 1:2 | 32 |
| 3 | L6 | NaH (200) | 95:5 | >20:1 | 98 |
| 4 | L6 | - | 80:20 | 11:1 | 96 |
| 5 | L6 | LiCl (100) | 88:12 | 14:1 | 98 |
| 6 | L6 | LiBr (100) | 95:5 | >20:1 | >99 |
 | |||||
Reactions performed with 0.1 mmol of 25, 0.2 mmol of 24 in THF (0.1M) and allowed to proceed to complete consumption of 25.
Determined by 1H NMR and UHPLC-MS analysis of the crude mixture.
Determined by chiral HPLC analysis of the major diastereomer.
TBD = 1,3,5-triazabicyclo[4.4.0]dec-5-ene.
