Table 2.
Optimization of iridium-catalyzed allylic alkylation reaction of alkyl-substituted electrophile 43a
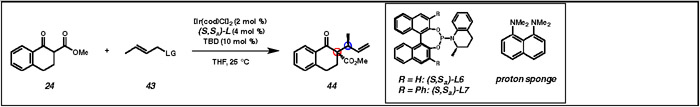 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | L | Base (200 mol %) |
Additive (mol %) |
LG | yield (%)b | b:lc | drc | ee (%)d |
| 1 | L6 | - | LiBr (100) | OCO2Me | 100 | 55:45 | 6.4:1 | - |
| 2 | L6 | LiOt-Bu | - | OCO2Me | 85 | 34:66 | 5.3:1 | - |
| 3 | L6 | LiOt-Bu | LiCI (100) | OCO2Me | 69 | 50:50 | 7.2:1 | - |
| 4 | L6 | LiOt-Bu | LiCI (100) | CI | 94 | 86:14 | 4.8:1 | - |
| 5 | L6 | Proton sponge | LiCI (100) | CI | 100 | 93:7 | 7.9:1 | 66 |
| 6 | L7 | Proton sponge | LiCI (100) | CI | 46 | 95:5 | 6.0:1 | 96 |
| 7 | L7 | Proton sponge | LiCI (400) | Cl | 78 | 94:6 | 6.7:1 | 97 |
Reactions performed on 0.1 mmol of 43, 0.2 mmol of 24 in THF (0.1 M) for 18 h.
1H NMR yield of the mixture of diastereomers based on internal standard.
Determined by 1H NMR analysis of the crude reaction mixture.
Determined by chiral SFC analysis.
Proton sponge = 1,8-bis(dimethylamino)naphthalene.
