Abstract
Diffusion tensor MRI‐based tractography was used to investigate white matter (WM) changes in the major limbic (i.e., fornix and cingulum) and cortico‐cortical association pathways [i.e., the uncinate fasciculus, the inferior fronto‐occipital fasciculus, the inferior longitudinal fasciculus (ILF), the superior longitudinal fasciculus, and the corpus callosum] in 25 Alzheimer's disease (AD) patients, 19 amnestic mild cognitive impairment (aMCI) patients, and 15 healthy controls (HC). Mean diffusivity (MD), fractional anisotropy (FA), as well as axial (DA) and radial (DR) diffusivities were measured for each tract, using an atlas‐based tractography approach. The association of WM tract integrity with hippocampal volume was also assessed. MD values were significantly different among groups in all WM tracts (P values ranging from 0.002 to 0.03), except in the fornix (P = 0.06) and the inferior fronto‐occipital fasciculus (P = 0.09). Conversely, FA was significantly different among groups in the fornix only (P = 0.02). DA values were significantly different among groups in all WM tracts (P values ranging from 0.001 to 0.01), except in the fornix (P = 0.13) and the cingulum (P = 0.29). Significantly different DR values among groups were found in the fornix (P = 0.02) and the ILF (P = 0.01). In the fornix and cingulum, DR was significantly more increased than DA in both patient groups compared to HC. No difference in DA versus DR was found in cortico‐cortical WM tracts. DA values in the fornix were significantly correlated with the hippocampal volume. This study demonstrates a different pattern of WM involvement in the limbic and cortico‐cortical association pathways in aMCI and AD patients. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: Alzheimer's disease, mild cognitive impairment, magnetic resonance imaging, diffusion tensor, white matter tracts, limbic network, cortico‐cortical association tracts
INTRODUCTION
Alzheimer's disease (AD) is a clinically heterogeneous condition, characterized by the accumulation of extracellular, insoluble beta‐amyloid (Aβ) plaques and intracytoplasmic tau‐associated neurofibrillary tangles [Arnold et al., 1991; Braak and Braak, 1995; Gomez‐Isla et al., 1996]. There is a growing body of evidence that suggests that the AD pathological process involves a “programmed” sequence of biochemical and structural changes which begins at the cellular and synaptic level and ultimately culminates in neuronal death and degeneration of white matter (WM) tracts [Brun and Englund, 1986; Selkoe, 2002]. At present, the regional patterns of WM changes in AD are largely unknown and the potential mechanisms leading to them are poorly understood [Bartzokis, 2004; Delbeuck et al., 2003; Englund, 1998]. Postmortem studies [Bronge et al., 2002; Englund, 1998; Englund and Brun, 1990; Englund et al., 1988] have suggested that in AD the degeneration of WM tracts can be due to multiple processes. Microstructural WM degradation can be secondary to gray matter (GM) pathology. Cellular processes, such as the deposition of Aβ around neuronal cell bodies and the accumulation of tau protein in neuronal cells, can lead to neurodegeneration including loss of axons and myelin (i.e., secondary degeneration) [Englund, 1998]. Oligodendrocytes death and reactive gliosis that are more characteristic of a direct WM insult rather than of a primary GM damage have also been reported [Englund and Brun, 1990; Englund et al., 1988; Sjobeck and Englund, 2003; Sjobeck et al., 2005]. WM degeneration tends to be more severe in WM regions that myelinate late in the course of brain development, such as neocortical association and allocortical fibers, possibly because of an increased vulnerability of oligodendrocytes in these regions. According to this model (retrogenesis), myelin breakdown would be a primary event in AD, which develops in a pattern that is reverse to myelogenesis [Bartzokis, 2004; Reisberg et al., 1999]. Finally, comorbid factors, such as infarctions and small vessel disease with a cerebrovascular/hypoperfusional origin, have the potential to cause WM changes in AD [Englund et al., 1988].
By measuring directional changes in water diffusivity, diffusion tensor (DT) MRI allows to investigate in vivo the microstructure of the brain WM [Basser et al., 1994; Pierpaoli et al., 1996]. Two diffusivity metrics are commonly employed as indices of tissue pathology: mean diffusivity (MD), which is the average of the three eigenvalues of the diffusion tensor and measures the magnitude of water molecules diffusion, and fractional anisotropy (FA), which is defined as a coefficient of variation of the eigenvalues and is an index of the degree of directionality of water diffusivity that provides information on tissue geometry [Basser et al., 1994; Pierpaoli et al., 1996].
DT MRI metrics are sensitive to the pathology of a number of neurological conditions that results in neuroaxonal loss and/or disruption of myelin sheath, including AD [Hess, 2009]. Using a regions‐of‐interest (ROIs) analysis, the majority of the DT MRI studies in AD patients reported increased MD and reduced FA values in WM regions, which include the temporal and parietal lobes, and, at a lesser extent, the frontal lobes [Bozzali et al., 2002; Fellgiebel et al., 2004, 2005; Head et al., 2004; Naggara et al., 2006; Rose et al., 2000; Stahl et al., 2007; Takahashi et al., 2002; Yoshiura et al., 2002]. More recently, regionally unbiased, voxel‐based DT MRI studies showed FA reductions in the parietal and temporal lobes of AD patients, including the splenium of the corpus callosum [Medina et al., 2006; Rose et al., 2008; Teipel et al., 2007; Xie et al., 2006]. Although DT MRI results are considerably more variable in patients with mild cognitive impairment (MCI), in part reflecting the known clinical heterogeneity of this population, regions located in the posterior hemispheric WM have been found to be affected early in the course of the disease [Fellgiebel et al., 2005; Huang et al., 2007; Medina et al., 2006; Rose et al., 2006; Stahl et al., 2007; Stenset et al., in press; Xie et al., 2006; Zhang et al., 2007].
Because of the intrinsic limitations of ROI‐ and voxel‐based analyses, previous studies could only infer which specific tracts were involved. On the contrary, DT MRI‐based tractography allows to gain quantitative information on the localization of damage to specific neuronal pathways [Basser et al., 2000]. However, only a few studies have assessed the integrity of WM tracts in AD patients and showed the presence of diffusivity abnormalities in the splenium of the corpus callosum, posterior cingulum, and uncinate fasciculi [Taoka et al., 2006; Xie et al., 2005; Yasmin et al., 2008; Zhang et al., 2009]. To our knowledge, the only previous DT MRI‐based tractography study that included MCI patients in a group analysis identified DT MRI changes in the posterior cingulum relative to controls, while no difference was found between MCI and early AD subjects in the uncinate fasciculi, the posterior cingulum, and the corticospinal tracts [Kiuchi et al., 2009]. Moreover, previous studies in AD and MCI investigated only a few selected tracts and did not carry out a comprehensive assessment of the major WM pathways.
In the majority of DT MRI studies of AD, the investigation of WM abnormalities has been limited to the assessment of FA changes or to a combined analysis of FA and MD changes. However, contributions to FA and MD alterations could be due to changes of diffusion either parallel or perpendicular to the principal direction of the tensor, and it is known that the substrates of one or the other of these two indices may differ. Axonal damage, as occurs in secondary degeneration, is likely to result in a decreased parallel diffusivity [i.e., axial diffusivity (DA)] [Pierpaoli et al., 2001], while myelin breakdown has been found to be associated with an increased perpendicular diffusivity [i.e., radial diffusivity (DR)] and a normal DA [Pierpaoli et al., 2001; Song et al., 2002, 2003]. Increases of both DR and DA are thought to be related to chronic ischemia [Pierpaoli et al., 2001; Sotak, 2002]. DA and DR have been used to characterize WM changes associated with normal aging and several neurological diseases, including epilepsy and multiple sclerosis [Concha et al., 2006; Hasan et al., 2009; Henry et al., 2003; Roosendaal et al., 2009; Zhang et al., in press]. To date, only a few studies interrogated DA and DR in patients with AD [Choi et al., 2005; Huang et al., 2007; Salat et al., in press; Stenset et al., in press; Stricker et al., 2009; Zhang et al., 2009], and only two studies in those with MCI [Huang et al., 2007; Stenset et al., in press]. However, the results of these reports were discordant since some authors found decreased [Huang et al., 2007] and others increased DA values [Choi et al., 2005; Salat et al., in press; Stenset et al., in press; Stricker et al., 2009] in the temporal lobe of AD and MCI patients.
In this study, we used DT MRI‐based tractography to comprehensively investigate WM changes in AD and amnestic MCI (aMCI) patients. We assessed WM tracts of the limbic system, which are known to be affected in AD, and the major cortico‐cortical association tracts, which play an important role in subserving cognitive functions. To ascertain the structural integrity of WM tracts, MD, FA, DA, and DR values were obtained. We also measured hippocampal atrophy, which is an early pathological feature of AD and aMCI and contributes to the presence of cognitive deficits [Petersen et al., 2000].
MATERIALS AND METHODS
Subjects
Patients were recruited from the Outpatient Memory Clinic of the IRCCS Centro San Giovanni di Dio Fatebenefratelli (National Center for Alzheimer's Disease), Brescia, Italy, between February 2006 and November 2008. From this group, we selected patients with a clinical diagnosis of AD [McKhann et al., 1984] or aMCI [Petersen et al., 2001], based on the results of a standardized protocol including physical, neurological, and neuropsychological evaluations. History was taken with a structured interview from patients' relatives (usually spouses or relatives). Neuropsychological assessment was performed by an expert psychologist blinded to the MRI results and evaluated: (i) global cognitive functioning with the Mini Mental State Examination (MMSE) [Folstein et al., 1975]; (ii) memory function with the Babcock Story Recall (Immediate and Delayed Recall) [Barigazzi, 1987], Rey's Word List Immediate and Delayed Recall Tests [Carlesimo et al., 1996], and Rey's Figure Delayed Recall Test [Caffarra et al., 2002]; (iii) frontal‐executive functions with the Trail Making Test A and B [Reitan, 1958]; (iv) language functions with the Phonological and Semantic Fluency [Novelli, 1986] and Token [De Renzi and Vignolo, 1962; Spinnler, 1987] Tests; and (v) visuospatial abilities with the Rey's Figure Copy Test [Caffarra et al., 2002]. Patients were excluded if they had (a) evidence of depression or disthymia; (b) abnormal laboratory tests, including vitamin B12, folate, and thyroid hormone levels; (c) significant medical illnesses or substance abuse that could interfere with cognitive functioning; and (d) any other major systemic, psychiatric, or neurological illnesses.
We included 25 patients with AD (women/men: 11/14, mean age: 75 ± 9 years) and 19 patients with aMCI (women/men: 9/10, mean age: 68 ± 8 years). To characterize better the aMCI population, the presence of the supportive features indicated by the revised research criteria for the diagnosis of AD [Dubois et al., 2007] was assessed. For the 19 aMCI patients, routine structural MRI (i.e., medial temporal lobe volume), 18F‐FDG PET (i.e., glucose metabolism pattern in the parieto‐temporal regions), and cerebrospinal fluid (CSF) biomarkers (i.e., Aβ42, total tau, and phospho‐tau concentrations) were available. Fifteen aMCI patients had at least one abnormal biomarker suggestive of AD pathology; of these, five patients had converted to AD (mean time to conversion = 15 months). AD biomarkers were negative in the other four aMCI patients.
Fifteen healthy controls (HC, women/men: 9/6, mean age: 70 ± 6 years) were recruited among relatives of patients and by word of mouth. HC underwent multidimensional assessment, including clinical, neurological, and neuropsychological evaluation, and were included into the study only when all of them were normal. During the selection process, neuroimaging findings were used only to exclude other causes of focal or diffuse brain damage, including lacunae, and extensive cerebrovascular disorders in both patients and HC. An experienced radiologist reviewed the severity of the cerebrovascular disease according to the Wahlund rating scale [Wahlund et al., 2001]. Subjects were excluded when the Wahlund rating scale was above the 90th percentile of the distribution from a previously published healthy elderly population [Galluzzi et al., 2009]. The local ethics committee approved the study. Written informed consent was obtained from all the subjects prior to enrolment into the study.
MRI Acquisition
Conventional MRI and DT MRI scans were acquired using a 1.5 Tesla system (Avanto, Siemens, Erlangen, Germany). The following pulse sequences were obtained from all patients and HC: (i) dual‐echo (DE) turbo spin echo [TR = 2,650 ms, first echo TE = 28 ms, second echo TE = 113 ms, echo train length = 5, number of slices = 50, slice thickness = 2.5 mm with no gap, matrix size = 512 × 512, field of view (FOV) = 250 mm × 250 mm] and (ii) pulsed‐gradient spin‐echo echo‐planar (TR = 6,500 ms, TE = 95 ms, number of slices = 40, slice thickness = 2.5 mm with no gap, matrix size = 128 × 128, FOV = 240 mm × 240 mm), with diffusion‐encoding gradients applied in 12 noncollinear directions (b factor = 1,000 s/mm2, number of averages = 8).
High‐resolution T1‐weighted MRI scans for hippocampal volumetry analysis were acquired from AD and aMCI patients using a 1.0 Tesla system (Philips, Gyroscan, Best, The Netherlands) with a fast field echo sequence (TR = 20 ms, TE = 5 ms, flip angle = 30°, slice thickness = 1.3 mm, matrix size = 256 × 256, FOV = 220 mm × 220 mm), within 15 days from the conventional and DT MRI scans.
MRI Analysis
All MRI analysis was performed by a single experienced observer, blinded to clinical and neuropsychological findings. WM hyperintensities (WMHs), if any, were identified on DE scans. An experienced radiologist reviewed the severity of WMHs according to the Wahlund rating scale [Wahlund et al., 2001]. WMH load was also measured using the JIM software package (Version 4.0, Xinapse Systems, Northants, UK, http://www.xinapse.com).
DT MRI analysis
DT analysis was carried out using an in‐house software. Diffusion‐weighted images were first corrected for distortion induced by eddy currents [Studholme et al., 1997], then the DT was estimated by linear regression [Basser et al., 1994], and MD and FA maps were computed [Basser and Pierpaoli, 1996]. In addition, DA (which is equivalent to the magnitude of the largest eigenvalue of the tensor) and DR (which is the average of the two smallest eigenvalues of the tensor) maps were calculated. To investigate the normal appearing WM tissue and limit CSF contamination secondary to brain atrophy, WMHs and CSF were masked out from the diffusivities and anisotropy maps. CSF segmentation was conducted on DE images using the FMRIB's Automated Segmentation Tool (FAST) implemented within FSL4 library (http://www.fmrib.ox.ac.uk/fsl).
An atlas‐based automated approach was used to obtain DT MRI‐derived metrics of WM tracts. This procedure involves (1) the creation of a reference FA image in the standard space (the FA atlas) using a group of healthy subjects (reference group), (2) the definition of WM tract probability maps on the FA atlas, (3) the nonlinear alignment of individual study subjects' MD, FA, DA, and DR maps to the FA atlas, and (4) the application of the WM tract probability maps to the individual study subject's images to measure mean tract MD, FA, DR, and DA. In detail, the analysis was performed as follows:
FA atlas creation
Since the direct application of tractography in elderly subjects is hampered by the presence of regions with decreased FA (e.g., WMHs) and brain atrophy, the FA atlas was obtained using DT MRI images from 24 healthy volunteers (reference group) aged between 20 and 45 years (women/men: 15/9; mean age = 31 ± 8 years) with no history of neurological or psychiatric disorders, as previously described [Pagani et al., 2008]. Briefly, their DE scans were registered to the standard Montreal Neurological Institute (MNI) space [Mazziotta et al., 2001] with affine transformation using the VTK CISG Registration Toolkit [Hartkens et al., 2002]. This transformation was then applied to FA images to correct for differences in head size between controls. FA maps were then nonlinearly transformed with an iterative procedure to produce an average shape and intensity image atlas (i.e., FA atlas) [Pagani et al., 2008].
WM tract maps
On FA maps of reference healthy subjects, fiber tracking [Pagani et al., 2005] was performed to segment the major brain WM tracts bilaterally [Catani et al., 2002]. These included WM tracts of the limbic system (i.e., fornix and cingulum) and the major cortico‐cortical tracts [i.e., uncinate fasciculus, inferior fronto‐occipital fasciculus, inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), and corpus callosum]. The reconstruction of the selected WM tracts in a representative subject from the reference group is illustrated in Figure 1. WM tracts of the reference subjects were then registered to the FA atlas, using the transformation matrices previously computed (see “FA atlas creation” section), and summed up to produce WM tract probability maps. In each probability map, values range between 0 and 100% and represent the frequency at which a given voxel belongs to that tract in the 24 reference subjects. These maps were then thresholded at 40% (i.e., only voxels with a frequency greater than 40 have been considered) and binarized to produce binary WM maps. Finally, a skeleton (http://www.fmrib.ox.ac.uk/fsl/data/FMRIB58_FA.html) was applied to the tract probability maps to consider only the center of each tract and thus avoid any further partial volume effect. Right and left WM tracts were sampled together to produce a single measure for each tract. WM tract probability maps obtained in the reference group are shown in Figure 2.
Figure 1.
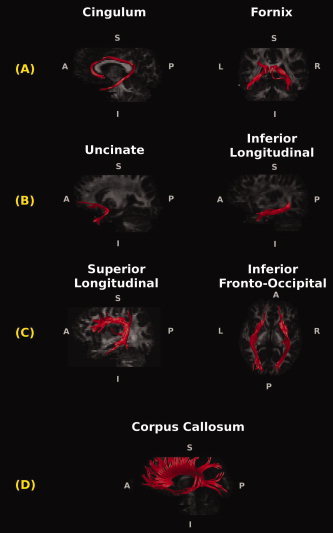
Reconstructions of the white matter (WM) tracts in a reference healthy control superimposed on the single subject's fractional anisotropy images. The cingulum and the fornix (A) are the major WM connections passing through the medial temporal lobe. The uncinate and the inferior longitudinal fasciculi (B) connect the anterior temporal lobe with frontal and occipital cortices. The superior longitudinal and the inferior fronto‐occipital fasciculi (C) connect the frontal cortex with the occipital and parieto‐temporal lobes. The corpus callosum (D) is the major interhemispheric WM tract.
Figure 2.
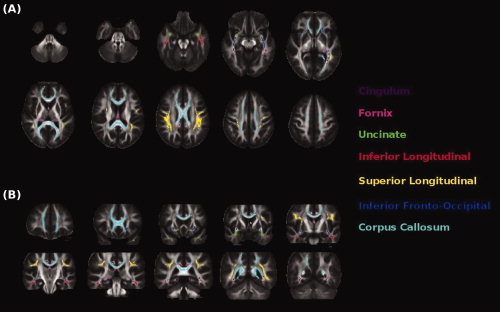
Illustration of the white matter (WM) tract probability maps obtained from reference healthy subjects. Probability maps are superimposed on (A) axial and (B) coronal sections of the fractional anisotropy atlas.
DT MRI‐derived metrics of WM tracts
The nonlinear transformations between the FA atlas and the FA maps of individual study subjects were estimated [Rohde et al., 2003] and then applied to each subject's MD, FA, DA, and DR maps. WM tract probability maps were then used as masks to extract mean tract measures for MD, FA, DR, and DA in each study subject.
Hippocampal volumetry
The hippocampal volume was measured with a manual tracing technique, as described elsewhere [Frisoni et al., 2007]. The 3D images were corrected for magnetic field nonuniformities, normalized for intensity, and linearly registered to ICBM152 standard template in the stereotaxic space [Collins et al., 1994], using a software developed at the McConnel Brain Imaging Centre (Montreal Neurological Institute, McGill University, Montreal, Canada). Using the DISPLAY software (McGill University, Montreal, Canada), the hippocampi were manually traced on the reoriented, normalized, 1.5‐mm‐thick coronal images by a single tracer, blinded to clinical, neuropsychological, and DT MRI findings, who simultaneously checked tracing accuracy on the sagittal and axial planes. The hippocampi were outlined following a standardized and validated protocol [Pruessner et al., 2000]. The test–retest reliability coefficients of this method have been reported previously [Frisoni et al., 2007]. To obtain the native hippocampal volume, the ROIs were back‐transformed from stereotaxic to native space. Left and right native hippocampal volumes were combined and then normalized to the total intracranial volume, obtained by manually tracing the entire intracranial cavity (the lower boundary being the foramen magnum) on 7‐mm‐thick coronal slices using the DISPLAY software (McGill University, Montreal, Canada).
Statistical Analysis
Statistical analysis was performed using the SPSS package, release 13.0 (SPSS, Chicago, IL). Socio‐demographic, clinical, and neuropsychological variables were compared between groups using an analysis of variance (ANOVA) model, with Bonferroni correction for multiple comparisons. Subject's age entered all the statistical analyses as a covariate. Scores on neuropsychological tests were age‐, sex‐, and education‐corrected by using normative values taken from an Italian standard population [Amodio et al., 2002; Caffarra et al., 2002; Carlesimo et al., 1996; De Renzi and Vignolo, 1962; Novelli, 1986; Spinnler, 1987]. To reduce the number of cognitive variables included in the analysis, cognitive composite scores were computed for each subject by transforming age‐, sex‐, and education‐corrected scores into standardized Z scores (i.e., computing the difference between each subject score and the average score, divided by the standard deviation of the score). Composite scores were obtained for memory function by averaging the Z scores of Babcock Story Recall, Rey's Word List Immediate and Delayed Recall, and Rey's Figure Delayed Recall tests, for language by averaging Z scores of Token, and Phonological and Semantic Fluency tests, and for frontal‐executive function by averaging Z scores of Trail Making Test A and B. Standardized scores for visuospatial abilities were obtained by converting the Rey's Figure Copy test into Z scores.
DT MRI indices (i.e., average MD, FA, DA, and DR values of WM tracts) and volumetric measures (hippocampal volumes and WMHs) were compared among groups using the General Linear Model by entering subject's age and WMH load as covariates and the group as the independent variable. Post‐hoc comparisons were tested with Bonferroni correction for multiple comparisons. To investigate further the contribution of eigenvalues (i.e., DA and DR) to MD and FA changes, DA and DR percentage values in AD and aMCI patients compared to those of controls were also assessed. DA and DR percentage changes were compared using a repeated measure ANOVA analysis by entering group (aMCI and AD) as a between‐subject factor, eigenvalues percentage changes compared to HC as a within‐subject factor, and by assessing the main effect of eigenvalues. Percentage values were used rather than raw values, since, by definition, DR values are smaller than DA values and, as a consequence, normalization is required before comparisons are performed. In AD and aMCI patients, correlations between DT MRI changes and hippocampal volumes were assessed using the Pearson's correlation coefficient. The significance threshold was set at P < 0.05.
RESULTS
Socio‐Demographic, Clinical, and Neuropsychological Features
Table I shows the main socio‐demographic, clinical, and neuropsychological findings of the subjects included. AD patients were older than aMCI patients (P = 0.02), had a lower educational level than HC (P = 0.01), and a lower MMSE score than HC and aMCI patients (P < 0.001). Both AD and aMCI patients showed significant memory (P < 0.001 for both AD and aMCI), language (P < 0.001 in AD and P = 0.003 in aMCI), and frontal‐executive (P < 0.001 in AD and P = 0.03 in aMCI) deficits. Compared with HC, visuospatial functions were significantly impaired in AD (P < 0.001), but not in aMCI patients (P = 0.07).
Table I.
Socio‐demographic, clinical, and neuropsychological features of healthy controls, patients with amnestic mild cognitive impairment, and Alzheimer's disease
| HC | aMCI | AD | P a | |
|---|---|---|---|---|
| Number of individuals | 15 | 19 | 25 | — |
| Socio‐demographic and clinical features | ||||
| Age (years) | 69.8 (6.0) | 68.5 (7.9) | 75.3 (8.7)* | 0.01 |
| Sex, women | 9 (60%) | 9 (47%) | 11 (44%) | 0.61 |
| Education (years) | 12.3 (3.6) | 9.3 (4.2) | 7.4 (3.8)** | 0.01 |
| Disease duration (months) | — | 26 (15) | 35 (22) | 0.17 |
| Cognitive functionsb | ||||
| Mini Mental State Exam | 28.8 (1.5) | 26.0 (1.2) | 19.1 (5.8)**,* | <0.001 |
| Memory (Z score) | 1.86 (0.83) | 0.30 (0.59)*** | −0.50 (0.42)**,* | <0.001 |
| Visuospatial (Z score) | 1.16 (0.12) | 0.38 (0.63) | −0.66 (0.92)**,* | <0.001 |
| Language (Z score) | 1.32 (0.56) | 0.29 (0.48)*** | −0.53 (0.78)**,* | <0.001 |
| Frontal‐executive (Z score) | 1.16 (0.12) | 0.41 (0.71)*** | −0.66 (0.66)**,* | <0.001 |
Abbreviations: HC, healthy controls; aMCI, amnestic mild cognitive impairment; AD, Alzheimer's disease.
Values are mean (SD) or number (%).
Post‐hoc analysis with Bonferroni correction: *P < 0.05 in AD versus aMCI; **P < 0.05 in AD versus HC; ***P < 0.05 in aMCI versus HC. See text for further details.
P values refer to ANOVA or chi‐square tests.
Standardised (Z) scores were computed according to the formula Z = mean score/SD, and by averaging the Z scores of four memory, three language, and two frontal‐executive tests. See text for further details.
MRI Findings
WMHs
Wahlund rating scale scores were similar between groups (P = 0.59). The mean WMH load was 4.99 ml [standard deviation (SD) = 8.06] in HC, 4.55 ml (SD = 4.61) in aMCI patients, and 8.96 ml (SD = 7.03) in AD patients (P = 0.62).
MD and FA values of WM tracts
Group differences of WM tracts' MD and FA values are shown in Table II. MD values were significantly different among groups in all WM tracts (P values ranging from 0.002 to 0.03; Fig. 3), except in the fornix (P = 0.06) and the inferior fronto‐occipital fasciculus (P = 0.09). Conversely, FA was significantly different among groups in the fornix only (P = 0.02; Fig. 3). Post‐hoc comparisons showed that, compared with HC, AD patients had increased MD in the cingulum, the uncinate fasciculus, the ILF, the SLF, and the corpus callosum (P < 0.05). FA of the fornix was reduced in AD patients versus HC (P < 0.05). Compared with HC and AD patients, aMCI patients did not show any significant MD and FA difference.
Table II.
Mean diffusivity and fractional anisotropy values of the assessed white matter tracts in healthy controls, patients with amnestic mild cognitive impairment, and patients with Alzheimer's disease
| HC | aMCI | AD | P | |
|---|---|---|---|---|
| Fornix | ||||
| MD | 0.87 (0.07) | 0.93 (0.06) | 0.93 (0.10) | 0.06 |
| FA | 0.42 (0.03) | 0.41 (0.03) | 0.38 (0.03)* | 0.05 |
| Cingulum | ||||
| MD | 0.74 (0.03) | 0.76 (0.03) | 0.78 (0.03)* | 0.02 |
| FA | 0.56 (0.04) | 0.55 (0.04) | 0.53 (0.05) | 0.55 |
| Uncinate | ||||
| MD | 0.81 (0.04) | 0.84 (0.05) | 0.86 (0.04)* | 0.03 |
| FA | 0.40 (0.04) | 0.41 (0.03) | 0.39 (0.02) | 0.45 |
| Inferior fronto‐occipital | ||||
| MD | 0.81 (0.04) | 0.82 (0.04) | 0.84 (0.04) | 0.09 |
| FA | 0.52 (0.04) | 0.54 (0.04) | 0.51 (0.03) | 0.12 |
| ILF | ||||
| MD | 0.80 (0.06) | 0.84 (0.05) | 0.88 (0.05)* | 0.002 |
| FA | 0.46 (0.03) | 0.46 (0.02) | 0.45 (0.03) | 0.70 |
| SLF | ||||
| MD | 0.74 (0.03) | 0.76 (0.03) | 0.78 (0.04)* | 0.02 |
| FA | 0.48 (0.04) | 0.48 (0.03) | 0.48 (0.03) | 0.73 |
| Corpus callosum | ||||
| MD | 0.76 (0.03) | 0.78 (0.04) | 0.81 (0.05)* | 0.02 |
| FA | 0.60 (0.04) | 0.61 (0.03) | 0.59 (0.04) | 0.82 |
Abbreviations: HC, healthy controls; aMCI, amnestic mild cognitive impairment; AD, Alzheimer's disease; GLM, general linear model; MD, mean diffusivity; FA, fractional anisotropy; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus.
MD values are ×10−3 mm2 s−1.
Values are mean (standard deviation).
Significantly different (P < 0.05) between AD and HC in Bonferroni‐corrected post‐hoc analysis.
Figure 3.
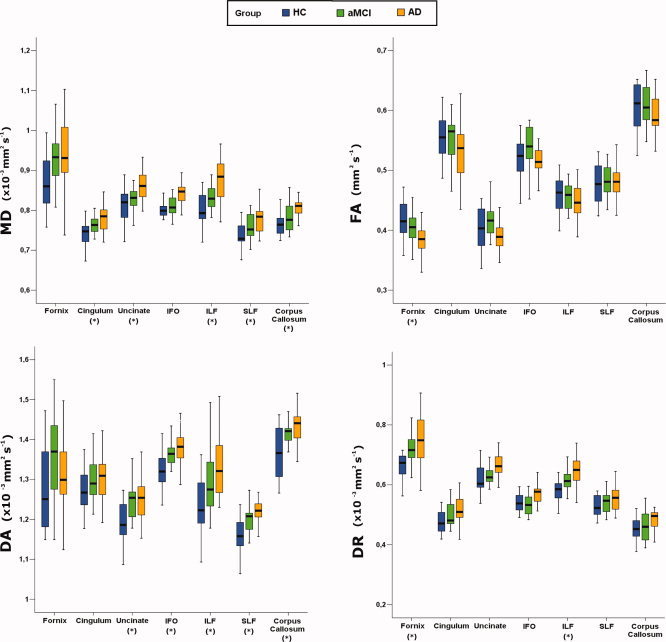
Boxplots showing the distribution of diffusion tensor MRI indices in patients with amnestic mild cognitive impairment, patients with Alzheimer's disease, and healthy controls. The boxes represent the interquartile ranges, which contain 50% of individual subjects' values. The whiskers are lines that extend from the box to the highest and lowest values, excluding outliers. A line across the box indicates the median value. Abbreviations: HC, healthy controls; aMCI, amnestic mild cognitive impairment; AD, Alzheimer's disease; FA, fractional anisotropy; MD, mean diffusivity; DA, axial diffusivity; DR, radial diffusivity; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; IFO, inferior frontooccipital fasciculus. *P < 0.05 on General Linear Model test.
DA and DR values of WM tracts
Table III shows DA and DR values of WM tracts in HC and patients. DA values were significantly different among groups in all WM tracts (P values ranging from 0.001 to 0.01), except in the fornix (P = 0.13) and the cingulum (P = 0.29). Significantly different DR values among groups were found in the fornix (P = 0.02) and the ILF (P = 0.01). Post‐hoc comparisons showed that DA values were significantly increased in both aMCI and AD patients compared with HC in all cortico‐cortical association tracts, except in the ILF where the differences were significant in AD patients only (Table III). DR values were significantly increased in the fornix (both patient groups vs HC) and in the ILF (AD vs HC) (Table III). The repeated measure ANOVA analysis (Table III) showed that the fornix and the cingulum had greater increase in DR than in DA (P < 0.001 in the fornix, P = 0.01 in the cingulum). In the fornix, a significant “group x eigenvalue” interaction was also detected (P = 0.02), indicating a different pattern of changes between AD and aMCI (unchanged vs increased DA, respectively). No DA versus DR increase difference was found in the cortico‐cortical association tracts (Table III).
Table III.
Axial and radial diffusivities values of the assessed white matter tracts in healthy controls, patients with amnestic mild cognitive impairment, and patients with Alzheimer's disease
| HC [mean (SD)] | aMCI [mean (SD)] | AD [mean (SD)] | P (GLM) | aMCI (% change vs HC) | AD (% change vs HC) | ANOVA repeated measures | ||
|---|---|---|---|---|---|---|---|---|
| Main effect eigenvalue | Interaction, Group × eigenvalue | |||||||
| Fornix | ||||||||
| DA | 1.28 (0.11) | 1.35 (0.11) | 1.30 (0.12) | 0.13 | 5.3 (8.7) | 1.6 (9.1) | <0.001 | 0.02 |
| DR | 0.67 (0.06) | 0.72 (0.05)** | 0.75 (0.09)* | 0.02 | 8.2 (8.0) | 12.5 (13.4) | ||
| Cingulum | ||||||||
| DA | 1.27 (0.05) | 1.30 (0.05) | 1.30 (0.05) | 0.29 | 2.1 (4.3) | 2.2 (4.3) | 0.01 | 0.61 |
| DR | 0.48 (0.04) | 0.50 (0.04) | 0.52 (0.05) | 0.12 | 4.2 (8.5) | 8.8 (10.6) | ||
| Uncinate | ||||||||
| DA | 1.19 (0.06) | 1.24 (0.05)** | 1.25 (0.05)* | 0.003 | 4.4 (4.0) | 5.0 (4.2) | 0.48 | 0.08 |
| DR | 0.62 (0.05) | 0.63 (0.05) | 0.67 (0.04) | 0.14 | 1.8 (8.2) | 7.1 (6.8) | ||
| Inferior fronto‐occipital | ||||||||
| DA | 1.33 (0.05) | 1.37 (0.04)** | 1.37 (0.05)* | 0.01 | 3.3 (2.8) | 3.7 (3.6) | 0.20 | 0.054 |
| DR | 0.55 (0.05) | 0.54 (0.05) | 0.58 (0.04) | 0.17 | −1.5 (8.9) | 5.6 (7.5) | ||
| ILF | ||||||||
| DA | 1.24 (0.08) | 1.29 (0.08) | 1.34 (0.07)* | 0.004 | 4.5 (6.8) | 8.1 (6.0) | 0.16 | 0.86 |
| DR | 0.59 (0.05) | 0.62 (0.04) | 0.65 (0.05)* | 0.01 | 5.1 (7.4) | 10.1 (8.3) | ||
| SLF | ||||||||
| DA | 1.16 (0.05) | 1.20 (0.03)** | 1.22 (0.04)* | 0.001 | 3.5 (3.0) | 5.2 (3.4) | 0.50 | 0.86 |
| DR | 0.53 (0.04) | 0.54 (0.04) | 0.56 (0.04) | 0.31 | 2.0 (7.0) | 5.3 (7.3) | ||
| Corpus callosum | ||||||||
| DA | 1.37 (0.06) | 1.42 (0.03)** | 1.43 (0.05)* | 0.001 | 3.5 (2.0) | 4.7 (3.3) | 0.42 | 0.40 |
| DR | 0.45 (0.04) | 0.46 (0.05) | 0.49 (0.05) | 0.27 | 1.9 (10.0) | 8.4 (11.7) | ||
DA and DR values are ×10−3 mm2 s−1.
Abbreviations: SD, standard deviation; HC, healthy controls; aMCI, amnestic mild cognitive impairment; AD, Alzheimer's disease; GLM, general linear model; DA, axial diffusivity; DR, radial diffusivity; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus.
Significantly different (P < 0.05) in *AD versus HC and **aMCI versus HC in Bonferroni‐corrected post‐hoc analysis.
Associations with hippocampal volume
Mean normalized hippocampal volumes were 3,702 ± 654 mm3 in AD patients and 4,589 ± 488 mm3 in aMCI patients (P < 0.001). In both AD (P < 0.001) and aMCI patients (P < 0.001), hippocampal volumes were decreased compared with that of a reference healthy elderly population [Galluzzi et al., 2009]. The individual mean hippocampal volumes were 1.5 SDs below the mean hippocampal volume of the reference group in 19/25 AD and 8/18 aMCI patients. In AD and aMCI patients, hippocampal volumes were significantly correlated with fornix DA values (r = 0.50, P = 0.001; Fig. 4). No correlation was found between hippocampal volumes and WM tracts MD, FA, and DR values.
Figure 4.
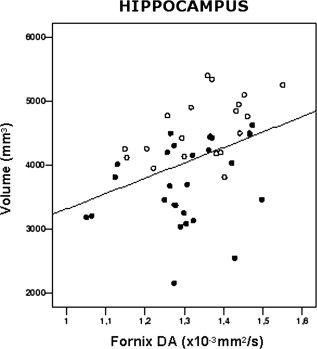
Scatterplot showing the significant relationships between axial diffusivity (DA) of the fornix and hippocampal volume in patients with amnestic mild cognitive impairment (empty circles) and Alzheimer's disease (filled circles).
DISCUSSION
To date, controversy exists regarding the extent of WM damage in AD, its pathological substrates, and the association of WM injury with disease‐related cognitive impairment. In this study, we used DT MRI‐based tractography to investigate the integrity of the major WM tracts in AD and aMCI patients. Significant WM damage in aMCI and AD was found in both the limbic pathways (i.e., fornix and cingulum) and the major cortico‐cortical WM tracts (i.e., the uncinate fasciculus, the inferior fronto‐occipital fasciculus, the ILF, the superior longitudinal fasciculus, and the corpus callosum) subserving association cortices. The analysis of the DT MRI eigenvalues also allowed us to gain insight into the possible underlying substrates of such a WM damage. On the basis of our results and current interpretation of the DT indices [Pierpaoli et al., 2001; Song et al., 2002, 2003; Sotak, 2002], we speculate that secondary degeneration and myelin damage are likely to contribute to the observed DT MRI changes of the limbic network (this is suggested by the greater increase in DR than DA in these WM tracts, and the correlation found between the DA values and the hippocampal volume), whereas microvascular pathology associated with a mild secondary degeneration may be the underlying pathological changes of WM damage in the cortico‐cortical association tracts; this is suggested by the increased DA values detected in these WM tracts and the known greater vulnerability of these regions to vascular pathology [Englund, 1998; Gootjes et al., 2004].
Previous postmortem MRI pathology correlation studies in elderly focused on the relationship between the pathological heterogeneity of WMHs and conventional MRI findings [Fazekas et al., 1991, 1993; Scheltens et al., 1995]. Pathological correlates of WMHs included myelin and axonal loss, reduction of olygodentrocytes, microglial activation, astrogliosis, and dilated perivascular spaces [Fazekas et al., 1991, 1993; Scheltens et al., 1995]. Conversely, postmortem DT MRI studies in demented patients are only a few. Englund et al. [ 2004] showed a correlation between myelin loss and FA decrease in two demented patients. In a single, pathology‐proven, frontotemporal dementia case, DT MRI showed decreased FA in frontal regions, where histopathology revealed typical frontal lobe degeneration [Larsson et al., 2004]. A recent postmortem DT MRI study in 11 AD patients [Gouw et al., 2008] showed that the strongest predictor of the FA decrease in both WMHs and normal appearing WM is axonal loss, while the relationship with myelin loss and astrogliosis are largely determined by their correlation with axonal density. Taken together, these findings suggest that DT MRI is sensitive to WM pathological changes in AD brain. However, to the best of our knowledge, the pathological correlates of MD, DA, and DR changes in AD or the topographical distribution of the DT MRI‐postmortem correlations have never been investigated.
WM Changes in the Limbic System
In the limbic network, decreased FA and unchanged MD was found in the fornix, while the cingulum showed increased MD without significant FA changes. The analysis of the eigenvalues showed that both tracts had DR values relatively greater than DA values. In the cingulum, however, the increased DR was lower than that of fornix and did not result in FA changes. This pattern of DT MRI abnormalities (i.e., DR greater than DA) has been shown to occur with normal aging [Zhang et al., in press]. Similar results have also been reported in the WM of the MTL in AD patients by two recent voxel‐based DT MRI studies [Salat et al., in press; Stricker et al., 2009]. These authors claimed that their findings argued against the “classic” secondary degeneration model of the disease [Pierpaoli et al., 2001; Song et al., 2003] and suggested that primary myelin damage rather than axonal injury is the pathological signature in these WM tracts. However, such a hypothesis is challenged by the severe atrophy of the hippocampus and entorhinal cortex in AD patients [Braak and Braak, 1995; Price et al., 2001; Schonheit et al., 2004], which makes the presence of secondary degeneration highly plausible. In our study, we found a positive significant correlation between hippocampal volumes and DA values of the fornix, which is in agreement with a role of secondary degenerative phenomena. As a consequence, it seems more likely that the observed WM changes in the limbic pathways reflect the presence of both demyelination and axonal loss rather than isolated myelin pathology. The fornix provides the main cholinergic input to the hippocampus and it also projects from the hippocampus to other regions [Copenhaver, 2006]. The disruption of the connections between the hippocampus and the anterior thalamic nuclei, mammillary bodies, striatum, and prefrontal cortex via the fornix has been postulated as a possible cause of deficit in episodic memory [Copenhaver, 2006]. Moreover, although subjects with significant vascular pathology on conventional MRI scans were not included in the present study and the effect of WMHs was excluded from the DT MRI analysis (both by controlling the analysis for global WMH load and masking out WMHs from subjects' DT MRI maps), we cannot rule out that microvascular ischemic disease may have had an impact on the observed normal appearing WM DT MRI changes. Indeed, in the fornix and the cingulum, myelin degeneration and vascular mechanisms would have an additive effect on DR, whereas a combination of vascular pathology and secondary degeneration would have an opposite effect on DA (increasing and reducing DA values, respectively), thus possibly leading to a DA “pseudo‐normalization,” rather than to a net DA decrease as it is the case for the “classic” secondary degeneration model.
WM Changes in the Cortico‐Cortical Association Tracts
Another intriguing finding of this study is that the major WM cortico‐cortical association tracts are also affected in AD patients. This is consistent with previous ROI‐based and voxel‐based DT MRI studies which showed damage to several WM regions outside the MTL network, including various portions of the occipital, temporal, and parietal WM and, albeit less consistently, frontal WM [Bozzali et al., 2002; Fellgiebel et al., 2004, 2005; Head et al., 2004; Rose et al., 2000; Stahl et al., 2007; Takahashi et al., 2002; Yoshiura et al., 2002]. In our study, cortico‐cortical tracts showed increased MD and DA, without significant changes in FA and DR. Again, this pattern of DT MRI changes may reflect the concomitant presence of axonal and myelin pathology, but at a different relative extent compared with that observed in the limbic network. Since the pathology burden (i.e., neuronal loss) in brain regions subserved by cortico‐cortical WM tracts is not as severe as in the MTL, it is tempting to speculate that secondary degeneration (associated with decreased DA values) may not be a major pathological feature in these tracts. On the other hand, vascular pathology (associated with increased DA and DR values) commonly affects the frontal and parietal WM regions to a greater extent than it involves temporal WM [Englund, 1998; Gootjes et al., 2004]. Another possible explanation of these findings is that decreased tissue density occurs in AD which increases water diffusivity while the underlying directional structure is maintained [Bronge et al., 2002; Englund, 1998]. In addition, our findings do not seem to support the retrogenesis hypothesis, since we did not find a more pronounced damage to late‐myelinated WM tracts.
The majority of AD patients (76%) had a prominent pattern of hippocampal atrophy. In the present study, we did not assess quantitatively the atrophy in other cortical regions. However, greater parietal than hippocampal atrophy is commonly found in early‐onset AD [Frisoni et al., 2007]. In our sample, there were four patients with a disease onset earlier than 65 years. The sample size of this subgroup is too small to infer on the possible effect of cortical atrophy on DA values of cortico‐cortical association tracts. No previous study, to our knowledge, investigated the differences in DT MRI pattern between early and late‐onset AD. On a speculative ground, in the hypothesis that axonal loss is secondary to atrophy, one might expect to see greater damage in cortico‐cortical association tracts than in the limbic system in early‐onset AD patients.
WM Changes in Preclinical AD (MCI Stage)
Between‐group comparisons showed a significant increase in DR and DA values in aMCI patients compared with elderly HC both in the limbic network and in the cortico‐cortical pathways, whereas MD and FA values were not different. Previous ROI‐based DT MRI studies in MCI subjects gave inconsistent results. Some authors found decreased FA in limbic regions [Fellgiebel et al., 2005; Huang et al., 2007; Medina et al., 2006; Rose et al., 2006; Zhang et al., 2007], but others did not [Kantarci et al., 2001; Stahl et al., 2007]. Our findings are in agreement with a recent voxel‐based DT MRI study [Damoiseaux et al., 2009] which found no MD and FA difference in MCI patients relative to AD and HC. To our knowledge, the only available tractography study in patients with MCI showed significantly decreased FA only in the posterior cingulum [Kiuchi et al., 2009], a result which cannot be directly compared to ours, since we have assessed the entire cingulum. Two possible explanations may account for these results. In agreement with the notion of aMCI as a transitional stage between normal aging and AD [Petersen et al., 2001], changes in MD and FA may be subtle in aMCI patients. This might suggest DA and DR as potential early markers of AD pathology in WM. Secondly, the heterogeneity of the aMCI group may have, at least partially, masked DT MRI changes. However, the majority of aMCI subjects included in our study can be classified as incipient AD following the novel “research” AD criteria [Dubois et al., 2007], thus suggesting that their biological—and not just clinical/neuropsychological—profiles resemble those of AD patients. It is worth noting that insufficient statistical power, possibly related to the relatively small sample size studied, could also be responsible for the absence of a between‐group effect.
Limitations and Conclusions
Some limitations of our study ought to be mentioned. First, we do not have histopathological data on our patients, and therefore, we can only speculate on the possible pathological substrates underlying the observed DT MRI changes. However, this is not easy to be obtained, especially in aMCI patients, unless many years have been passed between MRI and histopathology, which would inevitably make challenging the interpretation of DT MRI abnormalities. Second, the aMCI patient sample was relatively small and this may have biased some of the results, when this group was involved. Third, since we have information on conversion from aMCI to AD in only five patients, it remains to be seen whether the DT MRI findings in aMCI were related to AD or other types of dementia in the remaining subjects. Finally, some methodological problems related to DT MRI should be addressed. Regions of crossing fibers are characterized by low anisotropy, and thus, the determination of fiber orientation may be difficult in these voxels [Henry et al., 2003], leading to unreliable tensor estimates. However, the fasciculi that we investigated are major WM tracts associated with high anisotropy, and therefore, the effect of crossing fibers should not have affected our results at a great deal [Henry et al., 2003]. We are also aware that cortical and WM atrophy may cause partial volume effects from the CSF and, as a consequence, lead to false positive findings. However, in our DT MRI analysis, particular attention was paid to minimize this. Indeed, the effect of WM atrophy in elderly has been reduced by using an FA atlas based on young healthy subjects. The atlas‐based approach permits to obtain a reference image (i.e., the FA atlas), which is highly representative of the mean morphology (shape) and intensity of the group, on which individuals maps were spatially aligned. Moreover, compared to ROI‐based and standard tractography procedures, such an approach allows to minimize the manual drawing of ROIs, which may vary from subject to subject especially when applied to a pathological brain. Then, DT MRI indices from WM tracts were derived after CSF masking, WM thresholding, and skeletonizing the WM tract probability maps (i.e., considering only the core of the tracts). These procedures are important especially for small WM tracts such as the fornix, which runs close to the ventricles and is known to be atrophic in AD patients [Copenhaver et al., 2006]. WM tract skeletonizing can also be applied as a general rule in DT MRI, which ensures that voxels included in the analysis are well inside the tract. Clearly, this goes at the price of excluding part of the tracts from the comparison. Given the population of this study, we preferred this conservative approach. The use of thin slices (2.5 mm) should also have reduced the contribution of partial volume effects in an unprecedented way, since previous studies used thicker slices (3–6 mm) [Huang et al., 2007; Kiuchi et al., 2009; Medina et al., 2006; Rose et al., 2008; Stahl et al., 2007; Stenset et al., in press; Stricker et al., 2009; Taoka et al., 2006; Xie et al., 2006; Yasmin et al., 2008; Zhang et al., 2007, 2009].
Despite such limitations, this study demonstrates that different patterns of WM damage occur in limbic network and cortico‐cortical association tracts in AD and aMCI patients and sheds light onto the complex anatomical basis of DT MRI changes in AD.
REFERENCES
- Amodio P, Wenin H, Del Piccolo F, Mapelli D, Montagnese S, Pellegrini A, Musto C, Gatta A, Umilta C ( 2002): Variability of trail making test, symbol digit test and line trait test in normal people. A normative study taking into account age‐dependent decline and sociobiological variables. Aging Clin Exp Res 14: 117–131. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW ( 1991): The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex 1: 103–116. [DOI] [PubMed] [Google Scholar]
- Barigazzi ( 1987): Esplorazione testistica della memoria di prosa. Ric Psicol 1: 50–80. [Google Scholar]
- Bartzokis G ( 2004): Age‐related myelin breakdown: A developmental model of cognitive decline and Alzheimer's disease. Neurobiol Aging 25: 5–18; author reply 49–62. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C ( 1996): Microstructural and physiological features of tissues elucidated by quantitative‐diffusion‐tensor MRI. J Magn Reson B 111: 209–219. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D ( 1994): Estimation of the effective self‐diffusion tensor from the NMR spin echo. J Magn Reson B 103: 247–254. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A ( 2000): In vivo fiber tractography using DT‐MRI data. Magn Reson Med 44: 625–632. [DOI] [PubMed] [Google Scholar]
- Bozzali M, Falini A, Franceschi M, Cercignani M, Zuffi M, Scotti G, Comi G, Filippi M ( 2002): White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry 72: 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1995): Staging of Alzheimer's disease‐related neurofibrillary changes. Neurobiol Aging 16: 271–278; discussion 278–284. [DOI] [PubMed] [Google Scholar]
- Bronge L, Bogdanovic N, Wahlund LO ( 2002): Postmortem MRI and histopathology of white matter changes in Alzheimer brains. A quantitative, comparative study. Dement Geriatr Cogn Disord 13: 205–212. [DOI] [PubMed] [Google Scholar]
- Brun A, Englund E ( 1986): A white matter disorder in dementia of the Alzheimer type: A pathoanatomical study. Ann Neurol 19: 253–262. [DOI] [PubMed] [Google Scholar]
- Caffarra P, Vezzadini G, Dieci F, Zonato F, Venneri A ( 2002): Rey–Osterrieth complex figure: Normative values in an Italian population sample. Neurol Sci 22: 443–447. [DOI] [PubMed] [Google Scholar]
- Carlesimo GA, Caltagirone C, Gainotti G ( 1996): The Mental Deterioration Battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36: 378–384. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK ( 2002): Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 17: 77–94. [DOI] [PubMed] [Google Scholar]
- Choi SJ, Lim KO, Monteiro I, Reisberg B ( 2005): Diffusion tensor imaging of frontal white matter microstructure in early Alzheimer's disease: A preliminary study. J Geriatr Psychiatry Neurol 18: 12–19. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Concha L, Gross DW, Wheatley BM, Beaulieu C ( 2006): Diffusion tensor imaging of time‐dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage 32: 1090–1099. [DOI] [PubMed] [Google Scholar]
- Copenhaver BR, Rabin LA, Saykin AJ, Roth RM, Wishart HA, Flashman LA, Santulli RB, McHugh TL, Mamourian AC ( 2006): The fornix and mammillary bodies in older adults with Alzheimer's disease, mild cognitive impairment, and cognitive complaints: A volumetric MRI study. Psychiatry Res 147: 93–103. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Smith SM, Witter MP, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Zarei M, Rombouts SA ( 2009): White matter tract integrity in aging and Alzheimer's disease. Hum Brain Mapp 30: 1051–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbeuck X, Van der Linden M, Collette F ( 2003): Alzheimer's disease as a disconnection syndrome? Neuropsychol Rev 13: 79–92. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Vignolo LA ( 1962): The token test: A sensitive test to detect receptive disturbances in aphasics. Brain 85: 665–678. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger‐Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O'brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P ( 2007): Research criteria for the diagnosis of Alzheimer's disease: Revising the NINCDS‐ADRDA criteria. Lancet Neurol 6: 734–746. [DOI] [PubMed] [Google Scholar]
- Englund E ( 1998): Neuropathology of white matter changes in Alzheimer's disease and vascular dementia. Dement Geriatr Cogn Disord 9 Suppl 1: 6–12. [DOI] [PubMed] [Google Scholar]
- Englund E, Brun A ( 1990): White matter changes in dementia of Alzheimer's type: The difference in vulnerability between cell compartments. Histopathology 16: 433–439. [DOI] [PubMed] [Google Scholar]
- Englund E, Brun A, Alling C ( 1988): White matter changes in dementia of Alzheimer's type. Biochemical and neuropathological correlates. Brain 111 ( Pt 6): 1425–1439. [DOI] [PubMed] [Google Scholar]
- Englund E, Sjobeck M, Brockstedt S, Latt J, Larsson EM ( 2004): Diffusion tensor MRI post mortem demonstrated cerebral white matter pathology. J Neurol 251: 350–352. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Payer F, Schmidt R, Kleinert G, Radner H, Lechner H ( 1991): The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am J Neuroradiol 12: 915–921. [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H ( 1993): Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology 43: 1683–1689. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P ( 2004): Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: A diffusion tensor imaging study. Dement Geriatr Cogn Disord 18: 101–108. [DOI] [PubMed] [Google Scholar]
- Fellgiebel A, Muller MJ, Wille P, Dellani PR, Scheurich A, Schmidt LG, Stoeter P ( 2005): Color‐coded diffusion‐tensor‐imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol Aging 26: 1193–1198. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR ( 1975): “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Frisoni GB, Pievani M, Testa C, Sabattoli F, Bresciani L, Bonetti M, Beltramello A, Hayashi KM, Toga AW, Thompson PM ( 2007): The topography of grey matter involvement in early and late onset Alzheimer's disease. Brain 130: 720–730. [DOI] [PubMed] [Google Scholar]
- Galluzzi S, Testa C, Boccardi M, Bresciani L, Benussi LRG, Beltramello A, Bonetti M, Bono G, Falini A, Magnani G, Minonzio G, Piovan E, Binetti G, Frisoni GB ( 2009): The Italian Brain Normative Archive of structural MR scans: Norms for medial temporal atrophy and white matter lesions. Aging Clin Exp Res 21( 4/5), 266–276. [DOI] [PubMed] [Google Scholar]
- Gomez‐Isla T, Price JL, McKeel DW,Jr , Morris JC, Growdon JH, Hyman BT ( 1996): Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci 16: 4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootjes L, Teipel SJ, Zebuhr Y, Schwarz R, Leinsinger G, Scheltens P, Moller HJ, Hampel H ( 2004): Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer's disease and healthy aging. Dement Geriatr Cogn Disord 18: 180–188. [DOI] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, Vrenken H, van der Flier WM, Rozemuller JM, Barkhof F, Scheltens P, Geurts JJ ( 2008): Heterogeneity of white matter hyperintensities in Alzheimer's disease: Post‐mortem quantitative MRI and neuropathology. Brain 131: 3286–3298. [DOI] [PubMed] [Google Scholar]
- Hartkens T, Rueckert D, Schnabel JA, Hawkes DJ, Hill DLG ( 2002): VTK CISG Registration Toolkit: An open source software package for affine and non‐rigid registration of single‐ and multimodal 3D images In: von Monika Meiler H, Saupe D, Kruggel F, Handels H, Lehmann T, editors. Bildverarbeitung für die Medizin 2002. Algorithmen, Systeme, Anwendungen. Leipzig: Springer. [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, Kramer LA, Ashtari M, Cirino PT, Papanicolaou AC, Fletcher JM, Ewing‐Cobbs L ( 2009): Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res 1276: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ ( 2004): Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex 14: 410–423. [DOI] [PubMed] [Google Scholar]
- Henry RG, Oh J, Nelson SJ, Pelletier D ( 2003): Directional diffusion in relapsing‐remitting multiple sclerosis: A possible in vivo signature of Wallerian degeneration. J Magn Reson Imaging 18: 420–426. [DOI] [PubMed] [Google Scholar]
- Hess CP ( 2009): Update on diffusion tensor imaging in Alzheimer's disease. Magn Reson Imaging Clin N Am 17: 215–224. [DOI] [PubMed] [Google Scholar]
- Huang J, Friedland RP, Auchus AP ( 2007): Diffusion tensor imaging of normal‐appearing white matter in mild cognitive impairment and early Alzheimer disease: Preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am J Neuroradiol 28: 1943–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Jack CR,Jr , Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC ( 2001): Mild cognitive impairment and Alzheimer disease: Regional diffusivity of water. Radiology 219: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi K, Morikawa M, Taoka T, Nagashima T, Yamauchi T, Makinodan M, Norimoto K, Hashimoto K, Kosaka J, Inoue Y, Kichikawa K, Kishimoto T ( 2009): Abnormalities of the uncinate fasciculus and posterior cingulate fasciculus in mild cognitive impairment and early Alzheimer's disease: A diffusion tensor tractography study. Brain Res 1287: 184–191. [DOI] [PubMed] [Google Scholar]
- Larsson EM, Englund E, Sjobeck M, Latt J, Brockstedt S ( 2004): MRI with diffusion tensor imaging post‐mortem at 3.0 T in a patient with frontotemporal dementia. Dement Geriatr Cogn Disord 17: 316–319. [DOI] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero‐Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Le Goualher G, Boomsma D, Cannon T, Kawashima R, Mazoyer B ( 2001): A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos Trans R Soc Lond B Biol Sci 356: 1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM ( 1984): Clinical diagnosis of Alzheimer's disease: Report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34: 939–944. [DOI] [PubMed] [Google Scholar]
- Medina D, DeToledo‐Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT ( 2006): White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging 27: 663–672. [DOI] [PubMed] [Google Scholar]
- Naggara O, Oppenheim C, Rieu D, Raoux N, Rodrigo S, Dalla Barba G, Meder JF ( 2006): Diffusion tensor imaging in early Alzheimer's disease. Psychiatry Res 146: 243–249. [DOI] [PubMed] [Google Scholar]
- Novelli ( 1986): Tre test clinici di ricerca e produzione lessicale. Taratura su soggetti normali. Arch Psicol Neurol Psichiatr 47: 477–506. [Google Scholar]
- Pagani E, Filippi M, Rocca MA, Horsfield MA ( 2005): A method for obtaining tract‐specific diffusion tensor MRI measurements in the presence of disease: Application to patients with clinically isolated syndromes suggestive of multiple sclerosis. Neuroimage 26: 258–265. [DOI] [PubMed] [Google Scholar]
- Pagani E, Agosta F, Rocca MA, Caputo D, Filippi M ( 2008): Voxel‐based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage 41: 657–667. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Jack CR,Jr , Xu YC, Waring SC, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Boeve BF, Kokmen E ( 2000): Memory and MRI‐based hippocampal volumes in aging and AD. Neurology 54: 581–587. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B ( 2001): Current concepts in mild cognitive impairment. Arch Neurol 58: 1985–1992. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G ( 1996): Diffusion tensor MR imaging of the human brain. Radiology 201: 637–648. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P ( 2001): Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13: 1174–1185. [DOI] [PubMed] [Google Scholar]
- Price CJ, Warburton EA, Moore CJ, Frackowiak RS, Friston KJ ( 2001): Dynamic diaschisis: Anatomically remote and context‐sensitive human brain lesions. J Cogn Neurosci 13: 419–429. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC ( 2000): Volumetry of hippocampus and amygdala with high‐resolution MRI and three‐dimensional analysis software: Minimizing the discrepancies between laboratories. Cereb Cortex 10: 433–442. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Franssen EH, Hasan SM, Monteiro I, Boksay I, Souren LE, Kenowsky S, Auer SR, Elahi S, Kluger A ( 1999): Retrogenesis: Clinical, physiologic, and pathologic mechanisms in brain aging, Alzheimer's and other dementing processes. Eur Arch Psychiatry Clin Neurosci 249 Suppl 3: 28–36. [DOI] [PubMed] [Google Scholar]
- Reitan ( 1958): Validity of the trail making test as an indication of organic brain damage. Percept Mot Skills 8: 271–276. [Google Scholar]
- Rohde GK, Aldroubi A, Dawant BM ( 2003): The adaptive bases algorithm for intensity‐based nonrigid image registration. IEEE Trans Med Imaging 22: 1470–1479. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Geurts JJ, Vrenken H, Hulst HE, Cover KS, Castelijns JA, Pouwels PJ, Barkhof F ( 2009): Regional DTI differences in multiple sclerosis patients. Neuroimage 44: 1397–1403. [DOI] [PubMed] [Google Scholar]
- Rose SE, Chen F, Chalk JB, Zelaya FO, Strugnell WE, Benson M, Semple J, Doddrell DM ( 2000): Loss of connectivity in Alzheimer's disease: An evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. J Neurol Neurosurg Psychiatry 69: 528–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SE, McMahon KL, Janke AL, O'Dowd B, de Zubicaray G, Strudwick MW, Chalk JB ( 2006): Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry 77: 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SE, Janke AL, Chalk JB ( 2008): Gray and white matter changes in Alzheimer's disease: A diffusion tensor imaging study. J Magn Reson Imaging 27: 20–26. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, van der Kouwe AJ, Greve DN, Pappu V, Lee SY, Hevelone ND, Zaleta AK, Growdon JH, Corkin S, Fischl B, Rosas HD ( 2010): White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiol Aging 31: 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W ( 1995): Histopathologic correlates of white matter changes on MRI in Alzheimer's disease and normal aging. Neurology 45: 883–888. [DOI] [PubMed] [Google Scholar]
- Schonheit B, Zarski R, Ohm TG ( 2004): Spatial and temporal relationships between plaques and tangles in Alzheimer‐pathology. Neurobiol Aging 25: 697–711. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ ( 2002): Alzheimer's disease is a synaptic failure. Science 298: 789–791. [DOI] [PubMed] [Google Scholar]
- Sjobeck M, Englund E ( 2003): Glial levels determine severity of white matter disease in Alzheimer's disease: A neuropathological study of glial changes. Neuropathol Appl Neurobiol 29: 159–169. [DOI] [PubMed] [Google Scholar]
- Sjobeck M, Haglund M, Englund E ( 2005): Decreasing myelin density reflected increasing white matter pathology in Alzheimer's disease—A neuropathological study. Int J Geriatr Psychiatry 20: 919–926. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH ( 2002): Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–1436. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH ( 2003): Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20: 1714–1722. [DOI] [PubMed] [Google Scholar]
- Sotak CH ( 2002): The role of diffusion tensor imaging in the evaluation of ischemic brain injury—A review. NMR Biomed 15: 561–569. [DOI] [PubMed] [Google Scholar]
- Spinnler ( 1987): Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci 6: 1–20. [PubMed] [Google Scholar]
- Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO ( 2007): White matter damage in Alzheimer disease and mild cognitive impairment: Assessment with diffusion‐tensor MR imaging and parallel imaging techniques. Radiology 243: 483–492. [DOI] [PubMed] [Google Scholar]
- Stenset V, Bjornerud A, Fjell AM, Walhovd KB, Hofoss D, Due‐Tonnessen P, Gjerstad L, Fladby T: Cingulum fiber diffusivity and CSF T‐tau in patients with subjective and mild cognitive impairment. Neurobiol Aging (in press). [DOI] [PubMed] [Google Scholar]
- Stricker NH, Schweinsburg BC, Delano‐Wood L, Wierenga CE, Bangen KJ, Haaland KY, Frank LR, Salmon DP, Bondi MW ( 2009): Decreased white matter integrity in late‐myelinating fiber pathways in Alzheimer's disease supports retrogenesis. Neuroimage 45: 10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studholme C, Hill DL, Hawkes DJ ( 1997): Automated three‐dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys 24: 25–35. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yonezawa H, Takahashi J, Kudo M, Inoue T, Tohgi H ( 2002): Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurosci Lett 332: 45–48. [DOI] [PubMed] [Google Scholar]
- Taoka T, Iwasaki S, Sakamoto M, Nakagawa H, Fukusumi A, Myochin K, Hirohashi S, Hoshida T, Kichikawa K ( 2006): Diffusion anisotropy and diffusivity of white matter tracts within the temporal stem in Alzheimer disease: Evaluation of the “tract of interest” by diffusion tensor tractography. AJNR Am J Neuroradiol 27: 1040–1045. [PMC free article] [PubMed] [Google Scholar]
- Teipel SJ, Stahl R, Dietrich O, Schoenberg SO, Perneczky R, Bokde AL, Reiser MF, Moller HJ, Hampel H ( 2007): Multivariate network analysis of fiber tract integrity in Alzheimer's disease. Neuroimage 34: 985–995. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, Wallin A, Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P; for European Task Force on Age‐Related White Matter Changes ( 2001): A new rating scale for age‐related white matter changes applicable to MRI and CT. Stroke 32: 1318–1322. [DOI] [PubMed] [Google Scholar]
- Xie S, Xiao JX, Wang YH, Wu HK, Gong GL, Jiang XX ( 2005): Evaluation of bilateral cingulum with tractography in patients with Alzheimer's disease. Neuroreport 16: 1275–1278. [DOI] [PubMed] [Google Scholar]
- Xie S, Xiao JX, Gong GL, Zang YF, Wang YH, Wu HK, Jiang XX ( 2006): Voxel‐based detection of white matter abnormalities in mild Alzheimer disease. Neurology 66: 1845–1849. [DOI] [PubMed] [Google Scholar]
- Yasmin H, Nakata Y, Aoki S, Abe O, Sato N, Nemoto K, Arima K, Furuta N, Uno M, Hirai S, Masutani Y, Ohtomo K ( 2008): Diffusion abnormalities of the uncinate fasciculus in Alzheimer's disease: Diffusion tensor tract‐specific analysis using a new method to measure the core of the tract. Neuroradiology 50: 293–299. [DOI] [PubMed] [Google Scholar]
- Yoshiura T, Mihara F, Ogomori K, Tanaka A, Kaneko K, Masuda K ( 2002): Diffusion tensor in posterior cingulate gyrus: Correlation with cognitive decline in Alzheimer's disease. Neuroreport 13: 2299–2302. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW ( 2007): Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology 68: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Du AT, Rosen HJ, Kramer JH, Gorno‐Tempini ML, Miller BL, Weiner MW ( 2009): White matter damage in frontotemporal dementia and Alzheimer's disease measured by diffusion MRI. Brain 132: 2579–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Du AT, Hayasaka S, Jahng GH, Hlavin J, Zhan W, Weiner MW, Schuff N: Patterns of age‐related water diffusion changes in human brain by concordance and discordance analysis. Neurobiol Aging (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]


