Abstract
Recent neuroimaging studies make contradictory predictions about the involvement of left Brodmann's area (BA) 44 in processing local syntactic violations in determiner phrases (DPs). Some studies suggest a role for BA 44 in detecting local syntactic violations, whereas others attribute this function to the left premotor cortex. Therefore, the present event‐related functional magnetic resonance imaging (fMRI) study investigated whether left‐cytoarchitectonic BA 44 was activated when German DPs involving syntactic gender violations were compared with correct DPs (correct: ‘der Baum’—the[masculine] tree[masculine]; violated: ‘das Baum’—the[neuter] tree[masculine]). Grammaticality judgements were made for both visual and auditory DPs to be able to generalize the results across modalities. Grammaticality judgements involved, among others, left BA 44 and left BA 6 in the premotor cortex for visual and auditory stimuli. Most importantly, activation in left BA 44 was consistently higher for violated than for correct DPs. This finding was behaviourally corroborated by longer reaction times for violated versus correct DPs. Additional brain regions, showing the same effect, included left premotor cortex, supplementary motor area, right middle and superior frontal cortex, and left cerebellum. Based on earlier findings from the literature, the results indicate the involvement of left BA 44 in processing local syntactic violations when these include morphological features, whereas left premotor cortex seems crucial for the detection of local word category violations. Hum Brain Mapp, 2010. © 2010 Wiley‐Liss, Inc.
Keywords: fMRI, auditory, visual, phrase, Broca's area, Broca's region
INTRODUCTION
The processing of syntactic structures has since long been associated with Broca's region in the left inferior frontal lobe, in particular, with Brodmann's area (BA) 44 (for reviews of the literature see, for example, Friederici [2002] and Grodzinsky [2000]). Such processing involves syntactic information at the word level, for example, word‐category information or syntactic gender [Friederici et al.,2000; Heim et al.,2003; Hernandez et al.,2007; Longoni et al.,2005]. Furthermore, Broca's region is involved in integrating these types of information into syntactically complex structures [e.g. Caplan et al.,1998; Indefrey et al.,2001a,b; Just et al.,1996]. In particular, activation in BA 44 was increased when these structures were hierarchical [Friederici et al.,2006] and contained long‐distance dependencies [Opitz and Friederici,2007], requiring syntactic working memory [Cooke et al.,2002; Fiebach et al.2005]. For example, Opitz and Friederici [2007] compared sentences with long‐distance dependencies. In contrast to violations of long‐distance dependencies, which involved BA 44 more strongly than correct sentences, local syntactic violations did not activate this region. Instead, Opitz and Friederici [2007] observed higher activation in the left premotor cortex for violated versus correct sentences.
In summary, on one hand, BA 44 in Broca's region supports the processing of syntactic information at the word or phrase level, while, on the other hand, it is involved only in the processing of long‐distance dependencies but not of local syntactic dependencies. From this pattern of results, different hypotheses can be derived about the interplay of syntactic violations concerning information at the word level and local versus long‐distance structures. When processing of syntactic information at the word level is combined with the violations of long‐distance relationships, one would expect the involvement of BA 44, because processing of each of the types in isolation recruits this region. Evidence for this notion comes from a recent study by Hammer et al. [2006], who investigated syntactic gender violations in personal pronouns (1a and 1b).
Der Mantel ist schön, weil er warm ist.
| (1a) |
*Der Mantel ist schön, weil sie warm ist.
| (1b) |
The authors observed increased activation in Broca's region when the violated condition was compared to the correct condition, as could be expected from the neuroimaging evidence.
In contrast to this situation in which studies of syntactic processing at the word level and of processing of syntactic relationships make identical predictions about the involvement of BA 44, contradictory hypotheses can be derived for the processing of local violations involving word‐level syntactic information. Consider the case when the syntactic gender of a determiner does or does not match that of the corresponding noun in a determiner phrase (DP; examples from German in 2a and 2b).1
| (2a) |
| (2b) |
The studies on syntactic gender processing suggest that BA 44 is involved in the detection of this violation, because it relates to syntactic gender [cf. Heim,2008], whereas the study by Opitz and Friederici [2007] suggests no such involvement, because it is a local violation that does not involve long distance.
Therefore, the present functional magnetic resonance imaging (fMRI) study was designed to investigate whether syntactic gender violations at the phrase level (i.e. local dependency) only involve the left premotor cortex, or rather both the left premotor cortex and BA 44. To this end, activation for violated DPs was contrasted with that for correct DPs. The overlap of this effect with BA 44 was then assessed on the basis of cytoarchitectonic probability maps. Moreover, to ensure that the observed activation in the study was not related to modality‐specific processing (e.g. phonological working memory, and serial vs. parallel input), grammaticality judgments were performed for visual and for auditory DPs, and the common (i.e. modality‐independent) activation effects were taken as a mask for subsequent analyses.
MATERIALS AND METHODS
Participants
Twenty healthy right‐handed volunteers (mean age, 28 years; range, 20–49 years; 11 women) participated in the experiment. They were native German speakers and had normal or corrected to normal vision. The subjects had no explicit background in linguistics, had normal hearing, and had not been diagnosed for language impairment. All subjects had received at least 10 years of education.
Informed consent was obtained from all participants. All subjects received a reimbursement of 15 EUR for their participation. The experimental standards were approved by the local ethics committee of the University of Aachen.
Stimulus Materials and Procedure
The stimuli consisted of 80 monosyllabic German nouns, 40 with masculine gender (determiner: der), and 40 with neuter gender (determiner: das). Feminine nouns were not included, because the definite determiner for feminine nominative singular (die) is identical with the nominative plural for all three genders. This implies that the combination of “die” with a masculine or neuter noun could in some cases result in (the beginning of) a legal plural and would only be rejected very lately (e.g. der Trieb— themasculine,singular drive vs. die Trieb|e—themasculine,plural drives). Care was taken to create the sets of masculine and neuter nouns as parallel as possible with respect to phonological properties. Any remaining differences between the two sets, however, are of no impact for the purpose of this study, because masculine and neuter nouns were not contrasted against each other. Rather, each noun was presented in each modality, twice with its correct determiner and twice with the incorrect determiner, and only differences between modalities and between violated versus correct DPs were assessed (see below).2
The auditory stimuli were spoken by a female German native speaker, recorded at 44.1 kHz sampling rate, and presented via headphones. The visual stimuli were shown as written strings on a translucent screen in Helvetica font at 48 pts, with always the determiner and the noun being presented simultaneously. Stimulus presentation was controlled by PC using Presentation software (Neurobehavioral Systems, Albany, CA).
Stimuli were presented in an ABCCBA type of design (with A and B representing the visual and auditory modality and C a non‐lexical same/different decision that will not be discussed in this work). The experiment started with visual presentation for one half of the subjects and with auditory presentation for the other half. In each (A or B) block, correct and violated DPs were presented in a pseudo‐randomised order that differed for each individual. The participants performed a grammaticality judgement task. They had to indicate whether the gender of the determiner matched (correct DPs) or mismatched (violated DPs) that of the noun by pressing the left or right button of a response box. The assignment of left or right button presses to the match or mismatch was balanced over subjects.
Each block started with a written instruction for 6 s that reminded the subject of the assignment of match and mismatch decisions to the left and right response button. In the visual session, each experimental trial started with a blank screen that was presented with a variable duration of 250, 500, 750, or 1,000 ms. This temporal jittering ensured that stimuli were presented at different times of the scanner cycle. The blank screen was replaced by the determiner–noun combination presented in the centre of the screen for 1,900 ms. During this time, subjects performed the grammaticality judgement. After the offset of the stimulus, the screen remained blank for a variable time interval, resulting in a total trial duration of 3 s. The auditory blocks were constructed in parallel, but instead of the visual DPs, the auditory recordings were played.
fMRI Data Acquisition and Analysis
The fMRI experiment was carried out on a 3T Siemens Trio scanner (Siemens, Erlangen, Germany). A standard birdcage head coil was used. Foam paddings were used to reduce head motion. Functional images were recorded from 36 slices (thickness = 3 mm; gap = 1 mm) using a gradient‐echo EPI sequence (TE = 30 ms; flip angle = 90°, TR = 3 s; FOV = 200 mm; matrix 64 × 64; voxel size = 3.1 mm × 3.1 mm × 4 mm).
Data processing was performed using MATLAB (The Mathworks, Natick, USA) and SPM5 (Wellcome Department of Cognitive Neurology, UK). Data preprocessing included motion correction, slice‐time acquisition correction to the middle slice, normalisation to the SPM5 single‐subject template, and spatial smoothing (FWHM = 8 mm). The normalisation to the SPM5 single‐subject template by means of linear and non‐linear (using discrete cosine transform) registration transformed each subject's data into standard MNI space. The MNI space allows comparing the localisation of the fMRI effects with cytoarchitectonic probability maps [Amunts et al.,1999,2004], which were normalised into the same stereotactic space (see below).
An event‐related general linear model analysis was conducted at the individual level. The δ‐functions of the stimulus onsets for each condition (i.e. auditory correct, auditory violated, visual correct, and visual violated) were convolved with the canonical haemodynamic response function and its temporal derivative to account for minor latency differences [Friston et al.,1998]. Each condition was contrasted against the implicit (resting) baseline, yielding the beta estimates for each condition in each participant.
At the group level, the individual contrast images were entered into a repeated‐measure ANOVA, affecting a random effects analysis. The main effect of VIOLATION (correct vs. violated DP), the main effect of MODALITY (visual vs. auditory), and the interaction term were calculated. In this ANOVA, the main effect of VIOLATION is of particular importance, because it reveals which brain regions are involved more strongly in the processing of violated versus correct gender information in DPs. To include only those areas in the analysis that were not related to modality‐specific processing (e.g. phonological working memory and serial vs. parallel input), grammaticality judgements were performed for visual and for auditory DPs. The main effects for visual and for auditory processing were then subjected to a conjunction analysis [Price and Friston,1997], thus removing modality‐specific processing, and this conjunction analysis was taken as a mask for the main effect of VIOLATION described earlier. Importantly, masking the VIOLATION effect with this conjunction will give regions involved in processing the gender agreement violation, but not button presses. This is because the motor responses for button presses cancel out in the VIOLATION contrast in which both correct and violated DPs require button press responses.
For the anatomical localisation of the effects, we used cytoarchitectonic probability maps [Amunts et al.,1999,2004]. These maps are based on an observer‐independent analysis of the cytoarchitecture in a sample of 10 post‐mortem brains [Schleicher et al.,1999; Zilles et al.,2002]. They provide information about the location and variability of cortical regions in a standard reference space (the MNI space). For the assignment of MNI coordinates to the cytoarchitectonically defined areas, we used the SPM Anatomy Toolbox ([Eickhoff et al.,2005]; available with all published cytoarchitectonic maps from http://www.fz-juelich.de/ime/spm_anatomy_toolbox). This toolbox allows assessing the percent overlap of the fMRI activation with a cytoarchitectonic area as well as the position of the local maxima relative to all cytoarchitectonic areas.
Acquisition of Reaction Times
Piloting the fMRI experiment revealed that the participants' button press responses were recorded, including reaction times. Only after finishing all fMRI data recordings, we realised that due to some technical failure the PRESENTATION log‐files of 17 of 20 subjects did not contain the button press and reaction time information that should have been available there. Therefore, to relate the imaging findings to behavioral effects, additional data were collected in a second experiment outside the scanner. The sample was matched for age, total number of participants, and number of women (N = 20; mean age, 30 years; range, 20–53 years; 11 women). The reaction times were analysed in a 2 × 2 repeated‐measures ANOVA with factors MODALITY (auditory vs. visual) and VIOLATION (violated vs. correct).
RESULTS
fMRI Study
The results of the fMRI data analyses (all P corr < 0.05 FWE‐corrected at cluster level) are presented in Figure 1 and in Tables I, II, III, IV.
Figure 1.
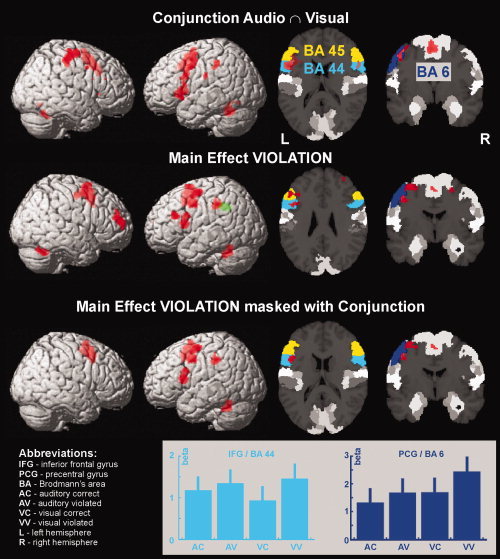
The fMRI results of this study are rendered on the surface of the right and left hemisphere of the SPM5 single subject template brain. Additionally, axial and coronal brain sections are shown in which the maximum probability maps of cytoarchitectonic BA 44 (light blue), BA 45 (yellow), and BA 6 in the precentral gyrus (dark blue) are highlighted. Top row: Main effect of VIOLATION. Brain activation for violated > correct gender is depicted in red; the reverse contrast is shown in green. Second row from top: Conjunction of auditory and visual grammaticality judgments. Bottom row: Main effect of VIOLATION masked with the conjunction analysis. In all activated brain regions violated gender evoked higher activation than correct gender (depicted in red). All effects are presented at a FWE‐corrected threshold of P corr < 0.05. The bar graphs indicate the activation strength (beta values) in each of the four conditions at the local maxima in BA 44 and in BA 6.
Table I.
Activations in the main effect VIOLATION (p < .05 FWE‐corrected at cluster level)
| Region | BAcyto | x | y | z | t max |
|---|---|---|---|---|---|
| VIOLATED > CORRECT | |||||
| L Inferior frontal gyrus | 44 | −48 | 12 | 15 | 4.77 |
| L Inferior frontal gyrus | 45 | −50 | 26 | 25 | 4.69 |
| L Precentral gyrus | 6 | −34 | −4 | 59 | 4.95 |
| L Inferior parietal lobule | n.a. | −36 | −30 | 39 | 4.78 |
| Supplementary motor area | 6 | 4 | 12 | 47 | 4.98 |
| L Cerebellum | n.a. | −16 | −52 | −21 | 4.57 |
| R Cerebellum | n.a. | 36 | −62 | −29 | 4.41 |
| R Middle frontal gyrus | n.a. | 34 | 50 | 19 | 5.04 |
| CORRECT > VIOLATED | |||||
| L Middle cingulate cortex | n.a. | −10 | −42 | 37 | 4.84 |
Abbreviations: L, left; R, right; t max, t value at local maximum; BAcyto, Brodmann's area according to the cytoarchitectonic probability maps; n.a., not assigned (includes probabilities of less than 20% for any cytoarchitectonical region).
Table II.
Activations in the main effect MODALITY (p < .05 FWE‐corrected at cluster level)
| Region | BAcyto | x | y | z | t max |
|---|---|---|---|---|---|
| AUDIO > VISUAL | |||||
| L Superior temporal gyrus | n.a. | −60 | −20 | 5 | 13.78 |
| R Superior temporal gyrus | TE3 | 66 | −8 | 1 | 14.73 |
| VISUAL > AUDIO | |||||
| L Middle occipital gyrus | n.a. | −36 | −88 | −3 | 13.80 |
| R Middle occipital gyrus | n.a. | 30 | −68 | 29 | 7.48 |
| R Inferior temporal gyrus | n.a. | 48 | −72 | −11 | 12.26 |
| L Superior occipital gyrus | n.a. | −22 | −72 | 31 | 6.96 |
| R Superior occipital gyrus | n.a. | 28 | −68 | 49 | 6.21 |
For further details, compare the legend of Table I.
Table III.
Activations in the conjunction analysis of visual and auditory gender verifications (P < 0.05 FWE‐corrected at voxel level)
| Region | BAcyto | x | y | z | t max |
|---|---|---|---|---|---|
| L Inferior frontal gyrus | 44 | −50 | 12 | 15 | 6.33 |
| R Inferior frontal gyrus | 44 | 46 | 12 | 25 | 5.38 |
| L Insula | n.a. | −34 | 18 | 5 | 8.17 |
| R Insula | n.a. | 38 | 22 | 3 | 5.35 |
| L Postcentral gyrus | 2 | −42 | −34 | 47 | 6.37 |
| L Inferior parietal lobule | PFt | −54 | −18 | 27 | 6.41 |
| Supplementary motor area | 6 | 0 | 8 | 49 | 9.28 |
| R Precentral gyrus | 6 | 42 | −20 | 63 | 7.96 |
| R Precentral gyrus | 6 | 54 | 2 | 43 | 5.80 |
| L Cerebellum | n.a. | −20 | −52 | −21 | 8.17 |
| R Cerebellum | n.a. | 32 | −60 | −25 | 6.69 |
For further details, compare the legend of Table I.
Table IV.
Activations in the main effect VIOLATION (P < 0.05 FWE‐corrected at cluster level) masked with the conjunction (P < 0.001 uncorrected)
| Region | BAcyto | x | y | z | t max |
|---|---|---|---|---|---|
| VIOLATED > CORRECT | |||||
| L Inferior frontal gyrus | 44 | −48 | 12 | 15 | 4.77 |
| L Precentral gyrus | 6 | −34 | −4 | 59 | 4.95 |
| L Inferior parietal lobule | n.a. | −36 | −30 | 39 | 4.78 |
| L Cerebellum | n.a. | −16 | −52 | −21 | 4.57 |
| Supplementary motor area | 6 | 4 | 12 | 47 | 4.98 |
For further details, compare the legend of Table I.
ANOVA
In the ANOVA, the main effect of VIOLATION (Table I) revealed higher activation for gender violations than for correct gender in BA 44 [Amunts et al.,2004] in the left inferior frontal gyri (IFG), BA 6 in the left precentral gyrus [Geyer,2003], left cerebellum, right SMA, and right middle frontal gyrus. Higher activation for correct gender processing than for gender violations was restricted to the left middle cingulate cortex.
The main effect for MODALITY (Table II) yielded higher activation for visual than for auditory gender verifications in bilateral visual cortex. Conversely, auditory gender verifications activated bilateral superior temporal gyri more strongly than visual gender verifications.
The interaction term was significant only in the left postcentral gyrus (MNI coordinates −30, −40, and 53; t max = 4.86).
Conjunction of Visual and Auditory Gender Verifications
The results from the conjunction of visual and auditory gender processing are given in Table III. Common activation was observed in bilateral IFG including left BA 44, bilateral insulae, left postcentral gyrus, left inferior parietal lobule, SMA, right precentral gyrus, and the cerebellum.
Modality‐Independent Effects of Gender Violations
To assess whether processing of gender violations (main effect of VIOLATION) was located in regions involved in modality‐unspecific language processing, the main effect of VIOLATION was masked with the conjunction analysis. Significant common activations were observed in BA 44 in the left IFG, BA 6 in the left precentral gyrus, and the right SMA (Table IV).
Reaction Time Study
The average reaction times per condition are given in Table V. We found a significant main effect of VIOLATION (F 1,19 = 21.33; P < 0.001) with longer reaction times for violated than for correct gender. On average, it took the participants 53 ms longer to detect violated DPs than correct DPs. Moreover, there was a significant main effect of MODALITY (F 1,19 = 78.50; P < 0.001) with longer reaction times for auditory than for visual stimuli. The interaction MODALITY × VIOLATION was not significant (F 1,19 = 1.14; P = 0.299).
Table V.
Average reaction times (± standard error of mean) for the grammaticality judgements in the four experimental conditions in Experiment 2
| Auditory | Visual | |
|---|---|---|
| Correct | 1316 ± 29 | 1007 ± 20 |
| Violated | 1353 ± 18 | 1076 ± 21 |
DISCUSSION
This study investigated three related research questions. First, we assessed whether violations of syntactic gender correspondence in DPs are processed in BA 44, in the premotor cortex, or both. The neuroimaging data revealed higher activation in both regions for violated when compared with correct DPs. This effect was also reflected in the reaction times, which were longer for violated DPs. Second, we verified the hypotheses that left BA 44 supports grammaticality judgments about DPs independent of stimulus modality. Finally, a combination of these two analyses revealed that the processing of gender violations is performed by the same brain regions that supported the grammaticality judgements in general.
The central finding in the fMRI data was the main effect of VIOLATION, revealing which brain regions responded in particular to a local mismatch between the gender of the determiner and that of the noun in the DP. From the literature, contradictory expectations about the localisation of the fMRI effects could be derived, predicting the involvement the left premotor cortex alone or in concert with BA 44. The results clearly showed activation in both regions, left BA 44 and left premotor cortex (BA 6).
The involvement of BA 6 was expected in any case and is in line with earlier work by Opitz and Friederici [2007] who demonstrated the involvement of the premotor cortex when local syntactic violations in an artificial grammar were processed. It is also in accordance with findings from a picture‐naming study [Heim et al.,2009b] in which DPs were produced while a distractor word with matching or mismatching gender was presented. A mismatching distractor may be regarded as a kind of violation, interfering with the errorless production of the intended DP. As in the work by Opitz and Friederici [2007] and in this study, the left premotor cortex showed higher activation for the mismatch (i.e. violated) condition than for the match condition. Overall, a functional interpretation of this activation points to the detection of local syntactic mismatch in language production and comprehension.
The situation is less unequivocal with respect to the effect in left BA 44 in this study. As mentioned earlier, no clear prediction could be made with respect to the activation of BA 44 for local syntactic gender mismatch, because BA 44 was involved in syntactic processing at the word level [e.g. Longoni et al.,2005] but not for local syntactic dependencies [Opitz and Friederici,2007]. To reconcile these findings conclusively, alternative interpretations about the role of BA 44 in processing local syntactic violations must be considered.
This study was designed such that the results are modality‐independent, thus excluding potential confounds of only visual or only auditory processing3. However, this study differed from that by Opitz and Friederici [2007] in two critical respects. First, while the latter investigated pseudo‐words in an (artificial) grammar, the former used DPs from a real language (German) implying lexical content. Second, while in the latter, the local violations involved morphological processing, the former study featured word category violations. Thus, there are two potential reasons for the differential effect in BA 44, one being the lexical status of the stimulus, and the other the type of violation (morphological vs. word category).
Considering lexical status (word/pseudo‐word), a number of neuroimaging studies related decisions about the lexical status of a stimulus to Broca's region [e.g. Binder et al.,2003; Carreiras et al.,2006; Hagoort et al.,1999; Nakic et al.,2006], and in particular to BA 44 [Fiebach et al.,2002; Heim et al.,2007; Kotz et al.,2002]. On the other hand, Indefrey et al. [2001a] demonstrated the involvement of BA 44 in syntactic processing also in pseudoword sentences and phrases, that is, without lexical entries. Thus, the existence of a lexical entry is not necessary for the recruitment of Broca's region, in particular, in syntactic tasks.
The alternative explanation for the presence of an fMRI effect in BA 44 in this study in contrast to the one by Opitz and Friederici [2007] relates to the type of violation. This argument implies that morphological processing should recruit BA 44, whereas word‐category violations should be processed without the involvement of BA 44. This is exactly the pattern of data found in the literature. A number of studies demonstrated that the processing of morphemes such as determiners or word endings relies on the contribution of BA 44 [Heim et al.,2009b; Huber et al., 2005; Indefrey et al., 2004; Longoni et al.,2005; Marangolo et al.,2006; Miceli et al.,2002; Padovani et al.,2005]. Furthermore, a recent study from language production [Heim et al.,2009b] suggests that the processing of morphological violations recruits the same brain region as morphological processing in general, implying that morphological violations are quantitatively rather than qualitatively different from correct morphological forms. Again, this is what the present data reveal. Processing of morphological information recruits, among others, left BA 44, and does so more strongly in case of violations.
Finally, and also in line with the distinction between morphological processing but not word category processing in BA 44, neuroimaging studies on word category violations reported no differences between violated and correct conditions in Broca's region, independent of whether the stimuli were visual or auditory [Rüschemeyer et al.,2005]. The seemingly contradictory fact that word‐category decisions do recruit BA 44 [Friederici et al.,2000; Heim et al.,2003] may be explained by differential processing demands for explicit metalinguistic decisions in the latter studies on one hand and rather automatized syntactic structure building in the former studies on the other hand [for further evidence, cf. [Opitz and Friederici,2004]).
To summarise, under specific conditions, BA 44 in Broca's region is involved in the processing of local syntactic violations. This study demonstrated that this can be the case when morphological violations (i.e. a violation of the gender‐marked determiner) are encountered. Thus, in this context, the function of BA 44 is best characterised as morphological processing, whereas the detection of local phrase structure violations appears to be supported by the posteriorly adjacent premotor cortex (BA 6). Evidence from an ERP study that directly compared word‐category violations to morphological (verb–subject agreement) violations suggests that the processing of these two types of violations occurs independently [Rossi et al.,2005]. Future neuroimaging research may thus focus on the functional interplay of BA 6 and BA 44 during morphological and syntactic processing. Moreover, whereas the focus of this study was only on BA 44 and BA 6, that is, two prefrontal regions, future research should additionally consider wider networks. It has been argued by many authors [e.g. Eickhoff et al.,2009; Hagoort, 2005; Heim et al.,2003; Marshall and Fink,2003; Mechelli et al.,2005] that Broca's region is not a module functioning in isolation, but rather a part of different functional networks. Consequently, the functional interplay of Broca's region with other parts of the language network may contribute additional insights into the nature of the processing mechanisms of the brain when encountering local or global syntactic violations. One consistent finding in the literature on effective connectivity in the brain is the functional interplay of Broca's region with temporal lobe structures, for example, with different aspects of the left fusiform gyrus [Mechelli et al.,2005] or the left inferior temporal gyrus [Heim et al.,2009a,b]. In addition, the same studies reported a functional subdivision of Broca's region on the basis of the pattern of effective connectivity, relating lexical processing to its anterior portion (if cytoarchitectonic information was available, BA 45) and phonological processing to its posterior portion (BA 44). Effective connectivity has also been used to distinguish linguistic processing in Broca's region from non‐linguistic articulatory processing in a subcortico–cerebello–cortical network involving motor regions [Eickhoff et al.,2009]. Therefore, it is plausible that the functional segregation of Broca's region for semantic, phonological, and syntactic processing [Friederici,2002; Hagoort, 2005] together with the growing body of evidence for the functional and effective connectivity of Broca's region with other brain regions (and in particular the temporal lobe) will provide a new view on the interplay of these areas during syntactic processing, for example, the violation of phrasal structures.
The argument about the processing of syntactic and morphological violations presented above is in line with the literature, whereas it does not yet explain how such processing takes places, that is, how exactly violation is detected. This study was not designed to answer this question; moreover, fMRI is presumably not the ideal method to investigate such processes occurring in the range of milliseconds [cf. Friederici,2002]. In the behavioural and electrophysiological literature, there are (at least) two different accounts for gender priming effects in language comprehension, one pre‐lexical and one post‐lexical (cf. [Friederici and Jacobsen,1999] for a review). According to the pre‐lexical position, a gender‐marked determiner serves as a prime for the subsequent nouns with respect to their gender, that is, gender information is primed in the mental lexicon at the lemma level. Evidence for gender priming comes from the previous studies of language production [e.g. Akhutina et al.,2001; Deutsch et al.,1999; Schriefers et al.,1998] and comprehension [e.g. Boelte and Connine,2004; Colé and Segui,1994; Friederici and Jacobsen,1999; Grosjean et al.,1994; Hartmann,2004]. For example, in the study by Grojean et al. [1994], lexical decision times were shorter and recognition points in gating earlier when nouns were preceded by a gender‐marked article when compared with a non‐marked article. Hartmann [2004] performed an eye‐movement study with a sentence–picture matching task. The noun in the sentence was preceded by a gender‐marked German determiner and adjective. When the target picture had a competitor with the same gender, the necessary amount of fixations increased. According to syntax‐first models like the neurocognitive model by Friederici [2002], the first information processed after acoustic and phonological analysis is the category of the word. In the case of this study, it is always a noun that is encountered after the determiner, and so this syntactic information becomes available and the system detects that a noun is a legal word after a determiner. As Colé et al. [2003] argued, the gender prime then activates a cohort of lexical entries (nouns) sharing the same gender [see also Schriefers et al. (1998)]. If the target noun indeed matches with the prime with respect to its gender, its lexical entry is selected, and the DP can be closed. However, if there is a mismatch, the noun's gender node will be activated in addition to a gender node pre‐activated by the determiner. Thus, in the case of a mismatch, reanalysis is required, leading to longer reaction times.
According to Friederici and Jacobsen [1999], this reanalysis is in fact due to a post‐lexical‐checking mechanism. As a consequence, longer reaction times in the violated DPs in this study would not result from the lack of pre‐activation of the noun's gender node, but rather from the fact that the noun's gender is retrieved after the noun's lexical entry has been accessed and that, at this point, a mismatch is detected. Boelte and Connine [2004] also favoured the notion of a post‐lexical checking mechanism, which, however, is not related to the processing of gender information at all. They argued that a preceding gender‐matching article increases the familiarity of a word rather than priming a gender node at the lemma level. In their study, they found gender priming from articles to nouns, but only during lexical decisions (Experiments 1 and 3) but not during phoneme monitoring (Experiment 2). These findings demonstrate that gender congruency does not always facilitate word recognition but only when required by a task, and perhaps not immediately but only with some delay [cf. Van Berkum et al.,1999]. However, with respect to the experimental particularities of this study, the alternative view by Boelte and Connine [2004] does not necessarily hold, because in this study an explicit gender‐processing task was performed. It is a well‐known fact and acknowledged by Boelte and Connine [2004] as well as, for example, by Colé et al. [2003] that the experimental task has a substantial influence on language processing. Similar to the findings by Boelte and Connine [2004] who compared lexical decisions with phoneme monitoring during gender priming, we demonstrated in a previous study [Heim et al.,2005] that reaction time differences in word and pseudo‐word processing are present in a lexical‐decision task but disappear in a phonological‐monitoring task. Therefore, in the case of this study, it is likely that the explicit gender‐matching task administered here elicited a focus on syntactic gender processing and thus the well‐documented gender priming effect (like a lexical decision task for words and pseudo‐words draws the subjects' attention to the lexical status of the stimulus), even if such gender priming does not necessarily occur under other experimental circumstances. Further research is required to elucidate the mechanisms of gender priming, for example, under which circumstances gender priming occurs, and whether perhaps gender‐matching articles prime subsequent nouns at a pre‐lexical level, whereas gender mismatch is processed at a post‐lexical level. For the purpose of this study, it is important to note that the observed effect of gender‐congruency versus gender‐violation in the reaction times and in the fMRI data is in line with a wealth of previous literature.
CONCLUSION
We demonstrated that the detection of local syntactic gender violations in DPs is supported by both left BA 44 and BA 6. This effect is independent of stimulus modality. It appears that BA 44 and BA 6 have a functional specialisation for morphological analysis and for the detection of local syntactic/agreement violations, respectively. The neural mechanisms underlying this functional specialisation need to be further investigated.
INSTRUCTION TEXT FOR THE GRAMMATICALITY JUDGEMENT TASK
In dieser Studie geht es um die Verarbeitung von geschriebener und gesprochener Sprache. Sie bekommen in kurzen Blöcken Substantive mit Artikel gezeigt oder vorgespielt, in denen der Artikel entweder zum Substantiv passt („der Baum“) oder nicht passt („das Baum”). Ihre Aufgabe ist es, per Tastenklick mit der linken Hand anzugeben, ob der Artikel richtig oder falsch war. Welche Taste Sie für „richtig“ oder „falsch” drücken sollen, wird Ihnen vor dem Experiment auf dem Bildschirm angezeigt. Bitte drücken Sie die entsprechende Taste immer so schnell wie möglich.
Footnotes
Violations as in (1b) have been previously investigated with event‐related brain potentials [ERPs; e.g., Barber and Carreiras, 2003, 2005; Gunter et al.2000] and may be encountered outside the laboratory in communication with speakers of foreign languages and with aphasic patients [Neumann,2000; see also Vigliocco and Zilli,1999].
It should be noted here that the German determiner for nominative masculine singular is ‘der’, which is also the determiner form for feminine genitive and dative singular, and for genitive and dative plural for all three genders. In this study, only nominative singular forms were used, thus limiting the processing to one instead of four cases. Nonetheless, to make sure that in no instance the subjects would get the idea that a correct genitive/dative plural form was encountered when a violated nominative singular neuter was intended by the experimenters, all neuter stimuli were selected such that their plural stems differed from their singular stems, for example, ‘das Haus’ (singular) but ‘die Häuser’ (nominative plural) and ‘der Häuser’ (genitive and dative plural).
The analysis of the reaction time data revealed a main effect for MODALITY, demonstrating longer RTs for auditory than for visual stimuli. This effect is possibly due to the fact that the relevant gender information came in later for spoken words. However, the absence of an interaction between MODALITY and VIOLATION shows that the processing of violated DPs was not affected by this main effect.
REFERENCES
- Akhutina T, Kurgansky A, Kurganskaya M, Polinsky M, Polonskaya N, Larina O, Bates E, Appelbaum M ( 2001): Processing of grammatical gender in normal and aphasic speakers of Russian. Cortex 37: 295–326. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K ( 1999): Broca's region revisited: Cytoarchitecture and intersubject variability. J CompNeurol 412: 319–341. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, Pieperhoff P, Eickhoff S, Gurd JM, Marshall JC, Shah NJ, Fink GR, Zilles K ( 2004): Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space ‐ The roles of Brodmann areas 44 and 45. Neuroimage 22: 42–56. [DOI] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L ( 2003): Neural correlates of lexical access during visual word recognition. J Cogn Neurosci 15: 372–393. [DOI] [PubMed] [Google Scholar]
- Boelte J, Connine CM ( 2004): Grammatical gender in spoken word recognition in German. Percept Psychophys 66: 1018–1032. [DOI] [PubMed] [Google Scholar]
- Caplan D, Alpert N, Waters G ( 1998): Effects of syntactic structure and propositional number on patterns of regional cerebral blood flow. J Cogn Neurosci 10: 541–552. [DOI] [PubMed] [Google Scholar]
- Carreiras M, Mechelli A, Price CJ ( 2006): Effect of word and syllable frequency on activation during lexical decision and reading aloud. Hum Brain Mapp 27: 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colé P, Segui J ( 1994): Grammatical incongruency and vocabulary types. Mem Cogn 22: 387–394. [DOI] [PubMed] [Google Scholar]
- Cooke A, Zurif EB, DeVita C, Alsop D, Koenig P, Detre J, Gee J, Pinango M, Balogh J, Grossman M ( 2002): Neural basis for sentence comprehension: Grammatical and short‐term memory components. Hum Brain Mapp 15: 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch A, Bentin S, Katz L ( 1999): Semantic influence on processing gender agreement: Evidence from Hebrew. J Psycholinguist Res 28: 515–535. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K ( 2009): A systems perspective on the effective connectivity of overt speech production. Philos Trans R Soc A 367: 2399–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Müller K, von Cramon DY ( 2002): FMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci 14: 11–23. [DOI] [PubMed] [Google Scholar]
- Fiebach CJ, Schlesewsky M, Lohmann G, von Cramon DY, Friederici AD ( 2005): Revisiting the role of Broca's area in sentence processing: syntactic integration versus syntactic working memory. Hum Brain Mapp 24: 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD ( 2002): Towards a neural basis of auditory sentence processing. Trends Cogn Sci 6: 78–84. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Jacobsen T ( 1999): Processing grammatical gender during language comprehension. J Psycholing Res 28: 467–484. [Google Scholar]
- Friederici AD, Bahlmann J, Heim S, Schubotz RI, Anwander A ( 2006): The brain differentiates human and non‐human grammar. Proc Natl Acad Sci USA 103: 2458–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Opitz B, von Cramon DY ( 2000): Segregating semantic and syntactic aspects of processing in the human brain: A fMRI investigation of different word types. Cereb Cortex 10: 698–705. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R ( 1998): Event‐related fMRI: Characterizing differential responses. Neuroimage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Geyer S ( 2003): The Microstructural Border Between the Motor and the Cognitive Domain in the Human Cerebral Cortex. Wien: Springer. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y ( 2000): The neurology of syntax: Language use without Broca's area. Behav Brain Sci 23: 1–71. [DOI] [PubMed] [Google Scholar]
- Grosjean F, Dommergues JY, Cornu E, Guillelmon D, Besson C ( 1994): The gender‐marking effect in spoken word recognition. Percept Psychophys 56: 590–598. [DOI] [PubMed] [Google Scholar]
- Gunter TC, Friederici AD, Schriefers H ( 2000): Syntactic gender and semantic expectancy: ERPs reveal early autonomy and late interaction. J Cogn Neurosci 12: 556–568. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ ( 1999): The neural circuitry involved in the reading of German words and pseudowords: A PET study. J Cogn Neurosci 11: 383–398. [DOI] [PubMed] [Google Scholar]
- Hammer A, Goebel R, Schwarzbach J, Münte TF, Jansma BM ( 2006): When sex meets syntactic gender on a neural basis during pronoun processing. Brain Res 1146: 185–198. [DOI] [PubMed] [Google Scholar]
- Hartmann N ( 2004): Processing grammatical gender in German. An Eye‐Tracking Study on Spoken‐Word Recognition. Bachelor's Thesis in Cognitive Science, University of Osnabrück, Germany (Available from http://www.cogsci.uniosnabrueck.de/~CL/download/Hartmann_GramGender.pdf).
- Heim S ( 2008): Syntactic gender processing in the human brain: A review and a model. Brain Lang 106: 55–64. [DOI] [PubMed] [Google Scholar]
- Heim S, Opitz B, Friederici AD ( 2003): Distributed cortical networks for syntax processing: Broca's area as the common denominator. Brain Lang 85: 402–408. [DOI] [PubMed] [Google Scholar]
- Heim S, Alter K, Ischebeck AK, Amunts K, Eickhoff S, Mohlberg H, Zilles K, von Cramon DY, Friederici AD ( 2005): The role of the left Brodmann's Areas 44 and 45 in reading words and pseudowords. Brain Res Cogn Brain Res 25: 982–993. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Ischebeck AK, Supp G, Amunts K ( 2007): Modality‐independent involvement of the left BA 44 during lexical decision making. Brain Struct Funct 212: 95–106. [DOI] [PubMed] [Google Scholar]
- Heim S, Eickhoff SB, Ischebeck AK, Friederici AD, Stephan, KE , Amunts K ( 2009a) Effective connectivity of the left BA 44, BA 45, and inferior temporal gyrus during lexical and phonological decisions identified with DCM. Hum Brain Mapp 30: 392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S, Friederici AD, Schiller NO, Rüschemeyer SA, Amunts K ( 2009b) The determiner congruency effect in language production investigated with functional MRI. Hum Brain Mapp 30: 928–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AE, Hofmann J, Kotz SA ( 2007): Age of acquisition modulates neural activity for both regular and irregular syntactic functions. Neuroimage 36: 912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Brown CM, Hellwig F, Amunts K, Herzog H, Seitz RJ, Hagoort P ( 2001a) A neural correlate of syntactic encoding during speech production. Proc Natl Acad Sci USA 98: 5933–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indefrey P, Hagoort P, Herzog H, Seitz RJ, Brown C ( 2001b) Syntactic processing in left prefrontal cortex is independent of lexical meaning. Neuroimage 14: 546–555. [DOI] [PubMed] [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR ( 1996): Brain activation modulated by sentence comprehension. Science 274: 114–116. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD ( 2002): Modulation of the lexical‐semantic network by auditory semantic priming: An event‐related functional MRI study. Neuroimage 17: 1761–1772. [DOI] [PubMed] [Google Scholar]
- Longoni F, Grande M, Hendrich V, Kastrau F, Huber W ( 2005): An fMRI study on conceptual, grammatical, and morpho‐syntactic processing. Brain Cogn 57: 131–134. [DOI] [PubMed] [Google Scholar]
- Marangolo P, Piras F, Galati G, Burani C ( 2006): Functional anatomy of derivational morphology. Cortex 42: 1093–1106. [DOI] [PubMed] [Google Scholar]
- Marshall JC, Fink GR ( 2003): Cerebral localization, then and now. NeuroImage 20: S2–S7. [DOI] [PubMed] [Google Scholar]
- Miceli G, Turriziani P, Caltagirone C, Capasso R, Tomaiuolo F, Caramazza A ( 2002): The neural correlates of grammatical gender: An fMRI investigation. J Cogn Neurosci 14: 618–628. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, McClelland JL, Price CJ ( 2005): Dissociating reading processes on the basis of neuronal interactions. J Cogn Neurosci 17: 1753–1765. [DOI] [PubMed] [Google Scholar]
- Nakic M, Smith BW, Busis S, Vythilingam M, Blair RJ ( 2006): The impact of affect and frequency on lexical decision: The role of the amygdala and inferior frontal cortex. Neuroimage 31: 1752–1761. [DOI] [PubMed] [Google Scholar]
- Neumann A ( 2000): Sprachverarbeitung, Genus und Aphasie. Der Einfluss von Genustransparenz auf den Abruf von Genusinformation, PhD Dissertation, Humboldt‐Universität. URL: http://edoc.hu-berlin.de/neumann-annette-2001-04-09/HTML/front.html.
- Opitz B, Friederici AD ( 2004): Brain correlates of language learning: The neuronal dissociation of rule‐based versus similarity‐based learning. J Neurosci 24: 8436–8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B, Friederici AD ( 2007): Neural basis of processing sequential and hierarchical syntactic structures. Hum Brain Mapp 28: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovani R, Calandra‐Buonaura G, Cacciari C, Benuzzi F, Nichelli P ( 2005): Grammatical gender in the brain: Evidence from an fMRI study on Italian. Brain Res Bull 65: 301–308. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ ( 1997): Cognitive conjunction: A new approach to brain activation experiments. Neuroimage 5: 261–270. [DOI] [PubMed] [Google Scholar]
- Rossi S, Gugler MF, Hahne A, Friederici AD ( 2005): When word category information encounters morphosyntax: An ERP study. Neurosci Lett 384: 228–233. [DOI] [PubMed] [Google Scholar]
- Rüschemeyer SA, Fiebach CJ, Kempe V, Friederici AD ( 2005): Processing lexical semantic and syntactic information in first and second language: fMRI evidence from German and Russian. Hum Brain Mapp 25: 266–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher A, Amunts K, Geyer S, Morosan P, Zilles K ( 1999): Observer‐independent method for microstructural parcellation of cerebral cortex: A quantitative approach to cytoarchitectonics. Neuroimage 9: 165–177. [DOI] [PubMed] [Google Scholar]
- Schriefers H, Friederici AD, Rose U ( 1998): Context effects in visual word recognition: Lexical relatedness and syntactic context. Mem Cogn 26: 1292–1303. [DOI] [PubMed] [Google Scholar]
- Van Berkum JJA, Brown CM, Hagoort P ( 1999): When does gender constrain parsing? Evidence from ERPs. J Psycholing Res 28: 555–571. [Google Scholar]
- Vigliocco G, Zilli T ( 1999): Syntactic accuracy in sentence production: The case of gender disagreement in Italian language‐impaired and unimpaired speakers. J Psycholinguist Res 28: 623–648. [DOI] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Palomero‐Gallagher N, Amunts K ( 2002): Quantitative analysis of cyto‐ and receptor architecture of the human brain In: Mazziotta J, Toga A, editors. Brain Mapping, the Methods, 2nd ed. San Diego: Academic Press; pp. 573–602. [Google Scholar]


