Abstract
High‐density electrical mapping of event‐related potentials was used to investigate the neural processes that permit some elderly subjects to preserve high levels of executive functioning. Two possibilities pertain: (1) high‐performance in elderly subjects is underpinned by similar processing mechanisms to those seen in young adults; that is, these individuals display minimal functional decay across the lifespan, or (2) preserved function relies on successfully recruiting and amplifying control processes to compensate for normal sensory‐perceptual decline with age. Fifteen young and nineteen elderly participants, the latter split into groups of high and low performers, regularly alternated between a letter and a number categorization task, switching between tasks every third trial (AAA‐BBB‐AAA…). This allowed for interrogation of performance during switch, repeat, and preparatory pre‐switch trials. Robust effects of age were observed in both frontal and parietal components of the task‐switching network. Greatest differences originated over prefrontal regions, with elderly subjects generating amplified, earlier, and more differentiated patterns of activity. This prefrontal amplification was evident only in high‐performing (HP) elderly, and was strongest on pre‐switch trials when participants prepared for an upcoming task‐switch. Analysis of the early transient and late sustained activity using topographic analyses and source localization collectively supported a unique and elaborated pattern of activity across frontal and parietal scalp in HP‐elderly, wholly different to that seen in both young and low‐performing elderly. On this basis, we propose that preserved executive function in HP‐elderly is driven by large‐scale recruitment and enhancement of prefrontal cortical mechanisms. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: executive control, aging, event‐related potentials, ERP, task‐switching, high‐density electrical mapping, frontal cortex
INTRODUCTION
To switch both rapidly and efficiently from one task to another is a crucial component of human cognitive function, and a core capacity of what are known as “executive control” processes. It entails the ability to actively represent and maintain more than a single task goal and flexibly reallocate attentional resources when task parameters require it. Normal aging has been associated with progressive deterioration in this switching ability [e.g., Cepeda et al., 2001; Paul et al., 2005; Wecker et al., 2005; West and Travers, 2008]. However, in the elderly, there is remarkable variability in the ability to effectively perform executive control tasks, with some showing pronounced deterioration with advancing years and others continuing to perform as well as their younger counterparts [see e.g., Riis et al., 2008]. The neural mechanisms that allow some elderly subjects to preserve high levels of executive control are a matter of acute interest, since the design of effective intervention strategies will ultimately depend on fundamental knowledge regarding the mechanisms that allow for successful aging. For example, if successful maintenance of executive control processes depends on large‐scale reconfiguration/remapping of control processes, this would provide fertile territory for the design of intervention strategies. On the other hand, if those who maintain high levels of function do so by better preserving the same control processes seen in their young counterparts, then a wholly different approach would be prescribed, since intervention would need to target the decay function.
A long‐established model for the neurocognitive effects of aging derives from work in neurological lesion patients [see e.g., Reuter‐Lorenz, 2002]. The notion is that performance deficits are due to diminished contributions of specialized brain regions and that older adults, being atrophic and less able to engage the relevant neural circuits, will show less brain activation than young adults. Neuroimaging studies focusing on patterns of under‐activation in regions associated with executive control are numerous [Gutchess et al., 2007; Milham et al., 2002; Paxton et al., 2008; Grady et al., 1995; Logan et al., 2002]. For example, Milham et al. [2002], using the Stroop paradigm, found evidence of age‐related decreases in the responsiveness of dorsolateral prefrontal and parietal cortical regions, structures well known to support attentional control [e.g., Astafiev et al., 2006; Foxe et al., 2003; Gitelman et al., 1999]. Similarly, Gutchess et al. [2007] showed lower activation of frontal executive control regions in older subjects performing a recognition memory task where they were required to identify novel objects in previously seen background contexts. However, as we will come to below, these authors also found a very interesting dissociation between high‐ and low‐performing elderly subjects.
As plentiful as studies are showing decreases in activation within prefrontal regions, there is also now a large accumulation of studies reporting increases in activation within many of the same regions; that is, greater brain activation in older than young adults. This has been interpreted as indication of a greater need to engage frontally mediated neural circuitry to perform simple tasks that young adults are able to perform without engaging these circuits [Cabeza et al., 2002, 2004]. In one task‐switching study, young subjects activated medial and dorsolateral frontal regions only when required to switch between tasks, whereas, older subjects recruited similar regions while performing each of the constituent tasks in isolation, even though switches were not required [DiGirolamo et al., 2001]. The study of Gutchess et al. [2007], mentioned above, confirmed the association of memory benefits seen in elderly subjects with additional recruitment of frontal regions. Although these authors found a general decrease in the activity of frontal regions for elderly, they also found an important dissociation between high‐performing (HP)‐elderly and low‐performing (LP)‐elderly. In those elderly better able to perform their recognition task, they found recruitment of an additional set of frontal regions. Taken together, these data suggest that an important aspect of successful aging involves recruitment and amplification of activity within executive control circuits.
Although functional neuroimaging studies have provided clear evidence for the involvement of a fronto‐parietal network of regions in task‐switching [e.g., Dias et al., 2003; DiGirolamo et al., 2001; Dove et al., 2000; Dreher and Berman, 2002; Dreher et al., 2002; Gurd et al., 2002; Kimberg et al., 2000; Sohn et al., 2000; Wylie et al., 2004a, 2006], detailed knowledge of the timing of activity within and across the nodes of this network is not nearly as well established [e.g., Wylie et al., 2003a]. One common finding using event‐related potentials (ERP) is the presence of a large positive potential over parietal scalp when subjects engage in typical task‐switching paradigms. A P300‐like component [Kray et al., 2005; Moulden et al., 1998; Nicholson et al., 2006; West, 2004; West and Moore, 2005], or what some have characterized as a late sustained positivity [Karayanidis et al., 2003; Miniussi et al., 2005; Nicholson et al., 2006; Rushworth et al., 2002; Wylie et al., 2003a, 2009], is consistently enhanced during preparation for an upcoming task‐switch, when compared to instances where the subject is preparing for a simple repetition of the same task. For components derived over frontal regions, several studies have reported a stronger negative ERP deflection peaking at about 450 ms after a cue indicates an upcoming switch [Brass et al., 2005; Miniussi et al., 2005; Moulden et al., 1998; Rushworth et al., 2002; Wylie et al., 2009]. When comparing how the elderly deviate from these switch‐related activation patterns, there are mainly data regarding parietal components. Switch‐related effects are typically attenuated or delayed with age and often show a broader topographic distribution over the scalp [Eppinger et al., 2007; Friedman et al., 2008; Kray et al., 2005; West, 2004; West and Moore, 2005]. Very little has been reported regarding age‐related differences for frontal components. Friedman et al. [2008] found increased P3a amplitude for switch compared to repeated trials in young, while elderly subjects showed comparable P3a for both switch and repeat trials. In a series of experiments by West et al. [2004, 2005, 2008], the authors obtained mixed results. In these studies, a cue was used to indicate whether a switch in task was required, and this resulted in a cue‐evoked sustained frontal activity when preparing for a switch of task in young. The frontal activity was absent [West, 2004], attenuated [West and Moore, 2005], or enhanced [West and Travers, 2008] in the elderly.
To our knowledge, there has been no attempt to date to differentiate between HP‐elderly and LP‐elderly adults in terms of the engagement of control circuits for impending switches of task1. Our goal here was to inter‐relate age‐related ERP modulations over parietal regions with those over frontal regions to achieve a more complete understanding of how the aged brain establishes executive control. As mentioned above, we posited two possible activity patterns across this network that might allow some elderly subjects to preserve high levels of executive functioning. (1) HP‐elderly may rely on preserved engagement of very similar brain regions to those of healthy young adults. If so, one would predict minimal deviations from the switch‐related ERP activity pattern established in the young. Elderly subjects showing more decline would simply show less activation of the same circuits. (2) The preservation of executive function in older age relies on the ability to recruit supplementary neural circuits, to successfully reconfigure and amplify control processes to cope with the normal decline of sensory‐perceptual mechanisms. In this case, one would predict an activity pattern across the fronto‐parietal network that deviates substantially from the switch‐related activity seen in young adults. Similarly, a somewhat counterintuitive prediction is that less successful elderly subjects will actually show a more youth‐like ERP pattern, but these activities would be significantly attenuated and/or decay at a faster rate.
We employed the paradigm of Wylie et al. [2003a], a modified version of the classic study of Rogers and Monsell [1995]. Participants regularly alternate between two tasks on every third trial (AAA BBB AAA BBB) in a completely predictable pattern. This design allowed the assessment of performance during switch (the trial immediately following a switch of task), repeated (the second nested trial of each triad), and pre‐switch trials (the final trial of the triad). In using repeated trials (where no switch is required) as a baseline against which to compare pre‐switch trials (after which subjects knew they would have to prepare for a switch of tasks), we are able to detail brain activity related to the preparation for a switch in the forthcoming trial. On the other hand, using repeated trials as a baseline against which to compare switch trials (the trial immediately following a switch) allowed us to detail brain activity related to the execution of a switch of task.
METHODS
Subjects
Fifteen young (eight male, M age = 24.1; 20–35) and nineteen elderly volunteers (nine male, M age = 73.8; 66–82) participated in the experiment. The separation of the elderly cohort into groups of high‐ and low‐ performers was based on a combined measure of task performance. Response time had to be less than 760 ms (median) and d‐prime greater than 2.0 to be classified as a high‐performer. The sample of young participants reported that they had normal hearing and had no known neurological deficits. Elderly participants were screened with Mini‐Mental Status Exam [MMSE, Cockrell and Folstein, 1988], Geriatric Depression Scale [van Marwijk et al., 1995], a short form of the Health Survey [Ware et al., 1996], and the Quick IQ test [Ammons and Ammons, 1969] to exclude clinical variance in our sample (Table I).
Table I.
Demographics of young, LP‐elderly, and HP‐elderly participants
| Young | LP‐Elderly | HP‐Elderly | |
|---|---|---|---|
| Number of subjects | 15 | 9 | 10 |
| Gender | 8 females | 4 females | 6 females |
| Age | 24.1 | 69.7 | 70 |
| Year of education | — | 14.0 | 15.33 |
| MMSE | — | 27.9 | 27.3 |
| GDS | — | 3.1 | 3.5 |
| Quick IQ test | — | 123.5 | 122 |
LP‐ and HP‐elderly were screened using Mini‐Mental Status Exam (MMSE), Geriatric Depression Scale (GDS), and the Quick IQ test in LP‐elderly and HP‐elderly.
Participants were paid a modest fee of $12 per hour for participating in the study. All participants provided written informed consent, and the Institutional Review Board of the Nathan Kline Institute approved all the procedures.
Stimuli
We used letter‐number pairs as stimuli in this experiment. The letters were drawn from a set containing four vowels (A E I U) and four consonants (G K M R). The numbers were drawn from a set containing four even numbers (2 4 6 8) and four odd numbers (3 5 7 9). On every trial, one letter and one number were randomly chosen with the constraint that neither the letter nor the number was the same as on the previous trial. One of these characters was presented 1° to the left of central fixation; the other was presented 1° to the right of fixation (this was randomly determined). All stimuli subtended 1.6° in the horizontal plane and 1° in the vertical plane (duration = 120 ms). The stimulus onset asynchrony was 2,000 ms. The stimuli were colored: for three trials in a row, they were red, for the next three they were purple, for the next three they were red, and so on (see Fig. 1). In total, 150 stimuli were presented in a single block of trials.
Figure 1.
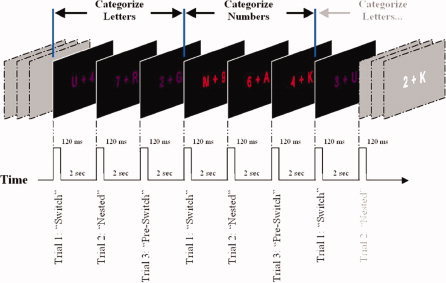
Task‐switching paradigm. Seven successive trials are shown. Subjects were instructed to perform one task (in this example, the letter task) when the stimuli were one color (e.g., purple) and to perform the other task (the number task) when the stimuli were the other color (red). Subjects always preformed three trials of each task before switching to the other task.
It is also important to point out that issues of target congruence and incongruence across tasks are fully balanced in this design. That is, on any given trial, the target (or nontarget) stimulus of the currently relevant task is paired equally often with a target or a nontarget stimulus of the irrelevant task.
Tasks
Participants were instructed to alternate between two tasks, switching from one to the other on every third trial (see Fig. 1). When the stimuli were colored red, subjects were instructed to categorize the letter according to whether it was a vowel or a consonant. When the stimuli were colored purple, they were instructed to categorize the number according to whether it was even or odd. This was a Go/No‐Go paradigm in which subjects only responded during the letter trails if the stimuli contained a vowel. They only responded during the number trials if the stimuli contained an even number. These instructions were counterbalanced across subjects such that half of the subjects performed the letter task when the stimuli were red and the number task when the stimuli were purple, and the rest did the reverse. In all cases, subjects were asked to respond by pressing a button with their right forefinger.
ERP Recording
The electrical activity of the brain was recorded using the Neuroscan Synamp I system from 64 tin scalp electrodes (impedances < 5 kΩ), referenced to the nose, with a passband of 0.05–100 Hz, and digitized at 500 Hz. Epochs of 2,100 ms were employed including a 100 ms prestimulus baseline, and baseline corrected over the prestimulus epoch. All analyses were conducted on individual subject averages that were not digitally filtered. The grand average was subsequently low‐pass digitally filtered off‐line at 45 Hz (12 dB/octave), purely for purposes of illustration. Trials with blinks and eye movements were rejected off‐line on the basis of vertical and horizontal EOG. An automatic artifact rejection criterion of ±70 μVs was used for all other scalp recordings. The average number of accepted trials for young participants was 813 with a range of 601–1,002 trials. The average number for elderly was 906 trials and the range was 798–1,121.
Procedure
Participants were seated in a dimly lit, sound‐attenuated, electrically shielded room 105 cm from a computer monitor. Letter‐number pairs were presented centrally, superimposed on a fixation cross. Subjects were instructed to maintain central fixation throughout each block (150 trials). Five blocks were recorded in which subjects were instructed to passively view the stimuli while maintaining fixation. The aim for recording the visual evoked potential (VEP) in the absence of any active task was to assess age‐related differences in early sensory processing. These data have already been published [De Sanctis et al., 2008] and will not be considered here. After these five passive blocks, participants were instructed about the tasks and completed two mandatory practice blocks. If needed, additional practice blocks were allowed until such time as the participant indicated that they were comfortable with the task and ready to begin the formal testing phase. The number of experimental blocks varied between 20 and 35 blocks (mean = 28.2). Subjects were required to take short breaks between blocks and encouraged to take longer breaks and leave the testing room whenever they felt the need. This was done to prevent fatigue and concentration lapses.
Analysis Strategy
The analyses to be conducted here were guided by our previous study, which used an essentially identical task and exhaustively probed both the early and later componentry of the ERP for switch‐related effects [Wylie et al., 2003a]. Wylie's study revealed a delimited number of components that were sensitive to switch‐related effects and we limited our analyses to these here. These were over frontal scalp sites (P450, Late‐positivity 1, and Late‐positivity 2), and over parietal scalp sites (P340, P450, Late‐positivity 1, and Late‐positivity 2) [see Table I in Wylie et al., 2003a].
As a first step, our analyses detailed the effects of ageing on ERPs associated with switch, repeat, and pre‐switch trials employing analysis of variance (ANOVA) with age (elderly versus young) as a between‐subject factor and trial (switch, repeat, and pre‐switch) as a within‐subject factor. If a significant effect of Age and/or Trial was found, our second step was to run four protected ANOVAs, two for each age group to unpack these main effects. That is, for each age group, we computed an ANOVA comparing the ERP associated with switch and repeat trials to investigate processes underlying the execution of a switch, and an ANOVA comparing the ERP associated with pre‐switch and repeat trials to investigate processes underlying the anticipatory preparation for an upcoming switch of task.
To compare the effect of performance within our elderly sample on the ERP associated with task‐switching, we ran an ANOVA with Performance (high‐ versus low‐performing elderly) as a between‐subject factor and Trial (switch, repeat, and pre‐switch) as a within‐subject factor. If a significant effect of Performance and/or Trial was found, our second step, as above, was to run four protected ANOVAs, two for each performance group. That is, for HP‐elderly and LP‐elderly, we computed an ANOVA comparing the ERP associated with switch and repeat trials to investigate processes underlying the execution of a switch, and an ANOVA comparing the ERP associated with pre‐switch and repeat trials to investigate processes underlying the preparation of a switch of task.
Behavior
We used a two factorial mixed ANOVA with Age (elderly versus young) as a between‐subject factor and Trial (switch, repeat, and pre‐switch) as within‐subject factor to test for differences in both reaction times (RT) and accuracy. The detection sensitivity measure d‐prime (d′) was calculated to aid in classification of the elderly group into high and low performers [Green and Swets, 1988].
ERP‐components
Components were identified over anterior and parieto‐occipital scalp regions at electrodes that best represented the maximal topography of the components of interest. The data at each electrode site were averaged across the appropriate epoch centered on the peak latency, and the area under the waveform (against the 0 μV baseline) during this epoch was calculated. We tested each identified component with a 2 × 3 × 2 × 3 repeated‐measure ANOVA. The factors were Age (young vs. elderly), Trials (switch, repeat, pre‐switch), Hemisphere (left vs. right), and Electrode (three electrodes at homologous locations over each hemisphere). The component structure, latency bins, and topographic regions from which electrodes were chosen were all based on our previous study [Wylie et al., 2003a], which comprehensively described the ERP componentry associated with performing of this exact task.
Topographic voltage maps
Scalp topographic maps in the present study represent interpolated voltage distributions, derived from 64‐scalp measurements. These interpolated potential maps are displayed on the 3D reconstruction of a rendered scalp surface (derived from an anatomical MRI) as implemented in the BESA2000 (Ver. 5.0) multimodal neuroimaging analysis software package (MEGIS Software GmbH, Munich, Germany). The topographical mapping focused on switch‐related effects, that is, processes underlying the preparation of a switch of task (pre‐switch minus repeat trial) and processes underlying the execution of a switch (switch minus repeat trial). The distribution across the scalp combined with the topographic statistical analysis (TANOVA, see below) allowed us to detail changes in the electrical field, which when present are indicative of changes in the underlying generator configuration [Fender, 1987; Lehmann, 1987].
Topographic statistics
Topographical differences were assessed using the so‐called TANOVA technique. To quantify differences in topography between Young, HP‐elderly, and LP‐elderly we computed the global dissimilarity (GD) [Lehmann and Skrandies, 1980]. GD is an index of configuration differences between two scalp distributions, independent of their strength as the data are normalized using the global field power. The GD equals the square root of the mean of the squared differences between the potentials measured at each of the 64‐scalp electrodes. For each pair of participants (stemming from the groups HP‐elderly versus Young, or LP‐elderly versus Young) and time point, the GD indexes a single value, which varies between 0 and 2 (0 = homogeneity, 2 = inversion of topography). To create an empirical probability distribution against which the GD can be tested for statistical significance, the Monte Carlo MANOVA was applied (for a more detailed description, see Manly [1991]).
Dipole source localization
To investigate the generator configuration underlying task‐switch related processes, we performed source modeling using brain electric source analysis [BESA 5.1. software; Scherg and Von Cramon, 1985]. BESA employs a least‐squares fitting algorithm, defining location and orientation of dipoles for which the maximal amount of variance is explained [see Scherg and Picton, 1991; Simpson et al., 1995]. For the purpose of modeling, an idealized four‐shell ellipsoidal head model with a radius of 90 mm and scalp and skull thickness of, respectively, 6 and 7 mm was assumed. Based on results obtained with TANOVA we singled out two time frames for the source analysis, 600–850 and 1,400–2,000 ms, were the topographical comparison between young, LP‐elderly, and HP‐elderly revealed statistically significant differences in scalp electrical field. We assumed bilateral symmetrical sources, an assumption, which considerably reduces the number of independent parameters to be determined [Scherg and Picton, 1991].
RESULTS
Behavioral Results
Figure 2a shows the separation of elderly into high performers (red dots) and low performers (black dots). Each elderly participant is represented by his d‐prime value (accuracy) and RT; both measures are averaged across switch, repeat, and pre‐switch trials. The separation of elderly into groups of high‐ and low‐performers was based on a combined measure of task performance. Response time had to be less than 760 ms (median) and d‐prime greater than 2.0 to be classified as a high‐performer.
Figure 2.
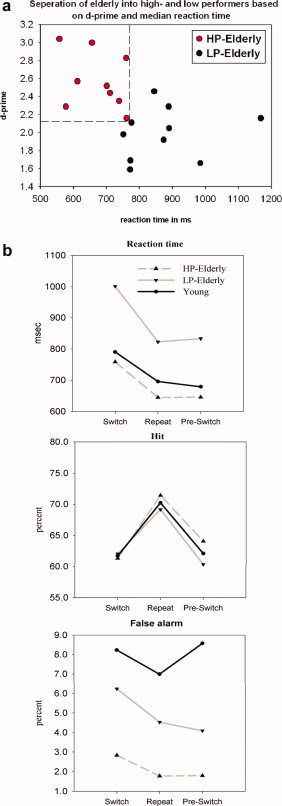
(a) High (red) and low (black) performing elderly identified as such based on their task performance. Each dot represents a participant's speed (reaction time) and accuracy (d‐prime) of response (averaged over switch, repeat, and pre‐switch trials). (b) Mean reaction time (RT), hit rate, and false alarm rate in young, LP‐elderly, and HP‐elderly. All three groups showed the expected increase in RT when changing tasks (i.e., on switch trials), the so‐called switch cost. Young and HP‐elderly showed comparable RTs, while LP‐elderly displayed a general slowing in RT across all three trial types. For the hit rate, all three groups were largely overlapping. For false alarms, the young cohort showed a generally increased rate compared to both elderly groups and the HP‐elderly were also significantly less likely to make false alarms than the LP‐cohort.
Young vs. elderly
RTs, hit rates, and false alarms are shown in Figure 2b. For the RT there was a main effect of Trial (F2, 64 = 111.5, P < 0.0001), and a Trial × Age interaction (F2, 64 = 3.48, P = 0.037), indicating enhanced switching costs in the older group. For the hit rate, a main effect of Trial was found (F2, 64 = 38.8, P < 0.0001). No Age (P = 0.92) or Trial × Age interaction (P = 0.89) was evident. For the false alarm rate, a main effect of Trial (F2, 64 = 9.9, P < 0.0001), Age (F1,32 = 14.1, P < 0.002), and a Trial × Age interaction (F2, 64 = 11.5, P < 0.0004) were found. False alarm rates were generally increased in young, especially so in pre‐switch trials, when compared with older participants.
High‐ vs. low‐performing elderly
Considering performance level within our elderly sample revealed a general slowing in RT for LP‐elderly (F1,17 = 5.21, P = 0.035). However, no difference in switch costs between HP‐elderly and LP‐elderly (Trial × Performance, F < 1) was evident. For the hit rate, there was only an effect of Trial (F2,34 = 18.9, P < 0.001). For the false alarm rate, there was a general increase (F1,17 = 12.9, P = 0.002) seen in LP‐elderly. No difference between HP‐elderly and LP‐elderly × Trial was evident (Trial × Performance, F < 1.5).
Electrophysiological Results
General description of age‐related task‐switching effects on the ERP
Before presenting a detailed statistical analysis of the ERP componentry, we will begin with a general description of the ERP component patterns observed in these data. Figures 3 and 4 show data from a selection of electrodes over frontal, centro‐parietal, and occipital scalp regions that outline the waveform morphologies that were observed in young, elderly, and the group of aged participants divided into HP‐elderly and LP‐elderly. It is also important at the outset to establish that when a comparison of HP‐ and LP‐young subjects was made, the ERP patterns were essentially identical. Thus, as pointed out in the introduction above, any differences between HP‐ and LP‐elderly can be reasonably attributed to the effects of aging and not to pre‐existing baseline differences in executive function. Figure 5 illustrates this issue where ERPs (collapsed across trial‐types) are shown for both young and elderly participants, where both groups are split into groups of HP and LP. That the patterns of activity for HP‐ and LP‐young subjects are essentially the same is evident over this frontal electrode site, in stark contrast to the same comparison in the elderly cohort. A series of exploratory t‐tests at all time points and electrode sites comparing HP‐ and LP‐young yielded no reliable differences between groups. As such, HP‐ and LP‐young were collapsed into a single group for all subsequent statistical analyses.
Figure 3.
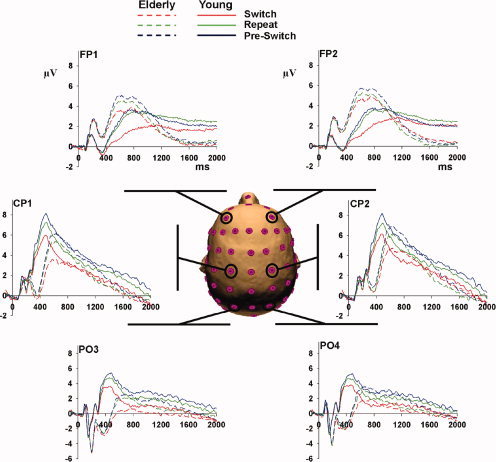
Overview of ERPs at parietal, central, and frontal scalp sites. The three trial types (Switch, Repeat, and Pre‐Switch) are seen for young (solid lines) and for the combined elderly group (broken line) at six electrode sites across the scalp.
Figure 4.
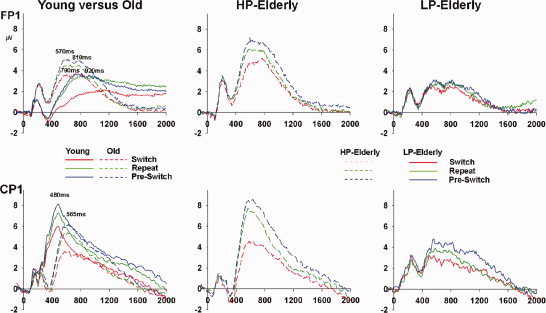
Overview of ERPs at frontal and centro‐parietal sites for Switch, Repeat, and Pre‐switch trials in young and the combined elderly group (left column), for HP‐elderly (middle column), and for LP‐elderly (right column).
Figure 5.
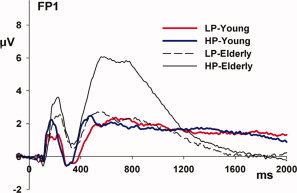
ERP patterns for young and elderly participants classified as high and low performers are illustrated over frontal scalp. These waveforms represent an ERP averaged over trial type (switch, repeat, and pre‐switch). Activity in HP‐young (blue line) and LP‐young (red line) during the early transient and late sustained period is largely overlapping, in contrast to the large differences evident between HP‐ and LP‐elderly.
Looking over occipital scalp regions (see Fig. 3), we see the earliest effect of age for the N1 component of the visual ERP at about 180 ms. The N1 amplitude shows a substantial enhancement of ∼1.5 μV in the combined elderly group. However, within both the young and elderly groups, there is no difference in N1 amplitude between trial type (i.e., switch, repeat (nested) and pre‐switch trial), indicating that the so‐called exogenous N1 component reflecting early sensory‐perceptual processing is not modulated by differences in the degree of executive processes required. In our previous study [De Sanctis et al., 2008], which was based on the identical sample of participants, we measured the VEP in a passive condition, where young and elderly subjects simply viewed alphanumeric stimuli in the absence of any active task. Again, a substantial enhancement of the N1 component in the elderly was evident, a finding that has been frequently reported in the aging literature [e.g., Falkenstein et al., 2006]. For a detailed discussion of age‐related differences seen for the N1 component, we refer the interested reader to De Sanctis et al. [2008].
Over the centro‐parietal regions (Fig. 4, left, bottom panel), the earliest effect of task switching in young was seen at about 330 ms [as in Wylie et al., 2003a] followed by a P3‐like transient activation peaking at about 480 ms. The combined elderly group by comparison showed a less differentiated, delayed, and attenuated activation pattern over centro‐parietal scalp regions. As can be seen in Figure 4 (left, bottom panel), the first effect at about 330 ms appeared to be absent in the elderly and the P3‐like transient activation was delayed by ∼85 ms, peaking instead at 565 ms. However, across both groups of young and elderly participants, we saw that this activation pattern appeared to differentiate with respect to trial‐type in a similar fashion. That is, the response was increased in amplitude to pre‐switch and decreased in amplitude to switch trials relative to the nested repeat trials. A late sustained positivity in young subjects revealed differences between pre‐switch, repeat, and switch trials that persisted throughout the intertrial epoch of 2,000 ms. This pattern was also seen in HP‐elderly (Fig. 4, middle, bottom panel), whereas the differentiation between trials in this late period was not evident in the LP‐elderly (Fig. 4, right, bottom panel).
The effect of age on the ERP pattern over frontal scalp regions (Fig. 4, left, top panel) was in striking contrast to that seen over centro‐parietal scalp regions. Here, it was the activation pattern in the combined elderly group that peaked earlier, was amplified, and showed a stronger degree of differentiation across trials when compared to the pattern seen in young adults. Both young and elderly subjects showed a double‐peaking component structure, at 570 and 810 ms in older adults and 790 and 920 ms in young adults. The differentiation and amplification effects were carried by the HP‐elderly group (Fig. 4, middle, top panel). The late (∼1,200 ms after stimulus onset) sustained activation lasting for the full duration of each trial in young adults is of greatest amplitude on repeat trials (green trace in Fig. 4, left, top panel). For both HP‐elderly and LP‐elderly, we found that the late sustained activity over frontal regions was essentially absent across all three trial types (middle and left, top panels, in Fig. 4).
A detailed description of the statistical analyses conducted on these components follows here and a condensed version is presented in the accompanying table (Table II).
Table II.
Summary of main effects for the trial types Switch versus Repeat and Pre‐switch versus Repeat in young and elderly participants and high performing and low performing elderly participants
| Main effect of trial | ||||||
|---|---|---|---|---|---|---|
| Component | Time (ms) | Switch vs. Repeat | Pre‐Switch vs. Repeat | Switch vs. Repeat | Pre‐Switch vs. Repeat | Electrodes |
| Young | Elderly | |||||
| Anterior Electrodes | ||||||
| P790/570 | Y 790 ± 30 | *** | ns | ** | * | AF3, F3, F1 |
| O 570 ± 30 | AF4, F4, F2 | |||||
| P920/810 | Y 920 ± 50 | *** | ns | ** | * | FP1, AF3, F1 |
| O 810 ± 50 | FP2, AF4, F1 | |||||
| Late‐pos1 | 1,400 ± 200 | ** | ns | ns | ns | F1, F3, AF3 |
| F2, F4, AF4 | ||||||
| Late‐pos2 | 1,800 ± 200 | ** | ns | ns | ns | F1, F3, AF3 |
| F2, F4, AF4 | ||||||
| Posterior Electrodes | ||||||
| P340 | 340 ± 20 | ** | ** | ns | ns | CP1, P1, P3 |
| CP2, P2, P4 | ||||||
| P480/565 | Y480 ± 50 | *** | * | *** | ns | PO5, PO3, P3 |
| O565 ± 50 | PO6, PO4, P4 | |||||
| Late‐pos1 | 1,400 ± 200 | ** | ns | ns | ns | TP7, CP5, CP3 |
| TP8, CP4, CP4 | ||||||
| Late‐pos2 | 1,800 ± 200 | *** | ns | * | ns | PO7, PO5, CP5 |
| PO8, PO4, CP4 | ||||||
| HP‐Elderly | LP‐Elderly | |||||
| Anterior Electrodes | ||||||
| P570 | 570 ± 30 | ** | Trend (P = 0.07) | ns | ns | FP1, AF3, F3 |
| FP2, AF4, F4 | ||||||
| P810 | 810 ± 50 | ns | * | ns | ns | AF3, F3, F1 |
| AF4, F4, F2 | ||||||
| Posterior Electrodes | ||||||
| P630 | 630 ± 30 | *** | * | ns | ns | CP3, P3, P1 |
| CP4, P4, P2 | ||||||
| Late‐pos1 | 1,400 ± 200 | * | ns | ns | * | CP3, P3, P5 |
| CP4, P4, P6 | ||||||
| Late‐pos2 | 1,800 ± 200 | * | ns | ns | ns | CP3, P3, P5 |
| CP4, P4, P6 | ||||||
P = < 0.05;
P = < 0.01;
P = < 0.001;
ns = not significant.
Frontal Scalp Effects, Transient Activation
Visual inspection of the prefrontal scalp effects (Fig. 4, top row) revealed a transient activation that was amplified in HP elderly across the three types of trials. This transient activation was characterized by a double peak at about 790 and 920 ms in young and at about 570 and 810 ms in elderly participants.
Young vs. elderly
P 790/570
For the P790/570 component measured over a ±30 ms epoch centered on the peak latency, we found main effects of Age (f 1,32 = 7.5, P = 0.01), Trial (f 2,64 = 29.3, P < 0.001), and Hemisphere (f 1,32 = 21.3, P < 0.001), and a three‐way Age × Trial × Hemisphere interaction (f 2,64 = 10.3, P < 0.001). Follow‐up protected ANOVAs in the young group revealed only an effect of Switch versus Repeat (f 1,14 = 39.7, P < 0.001). In the combined elderly group, an effect of Switch versus Repeat was also found (f1,18 = 8.3, P = 0.01), as was an effect of Pre‐Switch versus Repeat (f 1,18 = 5.2, P = 0.035) and an interaction of Switch × Hemisphere (f 1,18 = 14.5, P = 0.001).
P 920/810
For the P920/810 component measured over a ±50 ms epoch centered on the peak latency, we found a main effect of Age (f 1,32 = 4.1, P = 0.05), and a main effect of Trial (f 2,64 = 18.6, P < 0.001). Follow‐up protected ANOVAs in the young group revealed an effect of Switch versus Repeat (f 1,14 = 25.5, P < 0.001) and a Switch × Hemisphere interaction (f 1,14 = 5.4, P = 0.036). In the combined elderly group, an effect of Switch × Hemisphere (f 1,18 = 8.2, P = 0.01) and a main effect of Pre‐Switch versus Repeat (f 1,18 = 6.5, P = 0.02) were found.
High‐ vs. low‐performing elderly
The following analysis confirmed that the amplification of the prefrontal activation and the differentiation across trials was driven wholly by the HP‐group within the elderly subjects.
P570
An analysis of a ±30 ms epoch centered on the P570 component revealed a main effect of Performance (f 1,17 = 9.58, P < 0.001), a main effect of Trial (f 2,34 = 8.51, P = 0.001), and a Performance × Trial interaction (f 2,34 = 4.41, P = 0.02) in the elderly group. Follow‐up protected analysis for HP‐elderly showed an effect of Switch versus Repeat (f 1,9 = 9.68, P = 0.012) and a trend toward a Pre‐Switch versus Repeat difference (f 1,9 = 4.23, P = 0.07). For the LP‐group, no effects between trial‐types were significant (P‐values > 0.36).
P810
An analysis of a ±50 ms epoch centered on the P810 component also revealed a main effect of Performance (f 1,17 = 4.99, P = 0.039), and a main effect of Trial (f 2,34 = 6.84, P = 0.003) in the elderly group. The protected analysis for high performers showed an effect of Pre‐Switch versus Repeat (f 1,9 = 8.04, P = 0.02). Again, for LP‐elderly, no effects reached significance (P‐values > 0.30).
Parietal Scalp Effects, Transient Activation
Visual inspection of the parietal scalp effects (Fig. 4, bottom row) revealed a delayed and attenuated P3‐like activation in the combined elderly group. Taking performance differences within the elderly into account revealed that young adults and HP‐elderly show a similar pattern of activation. On the other hand, LP‐elderly differed considerably from both HP‐elderly and young adults.
Young vs. elderly
P340
An analysis of a ±20 ms epoch centered on the P340 component revealed main effects of Age (F 1,32 = 8.7, P = 0.006) and Trial (F 1,32 = 14.2, P < 0.001). Follow‐up ANOVAs in young revealed a main effect of Switch versus Repeat (F 1,14 = 8.3, P = 0.012) and of Pre‐Switch versus Repeat (F 2,64 = 7.7, P = 0.015). For the combined elderly, there was no effect of trial type.
P480/565
An analysis of a ±50 ms epoch centered on the P3‐like component revealed a main effect of Age (f 1,32 = 5.9, P = 0.02), and a main effect of Trial (f 2,64 = 28.5, P < 0.001). The protected analysis for young showed an effect of Switch versus Repeat (f 1,14 = 16.8, P = 0.001) and an effect of Pre‐Switch versus Repeat (f 1,14 = 8.3, P = 0.012). In the combined elderly group, an effect of Switch versus Repeat (f 1,18 = 17.02, P = 0.001), and a Switch × Hemisphere interaction (f 1,18 = 5.1, P = 0.037) were found. In addition a Pre‐Switch × Hemisphere (f 1,18 = 6.04, P = 0.024) interaction reached significance.
High‐ vs. low‐performing elderly
The following analysis confirmed that a similar pattern of activation across trials, to that seen in the young, was also present in HP‐elderly. That is, activity was enhanced in pre‐switch trials and reduced in switch trials, when compared to repeat trials. On the other hand, LP‐elderly showed no evidence of this differentiation across trial types.
P630
An analysis of a ±30 ms epoch centered at 630 ms revealed a main effect of Trial (f 2,34 = 24.4, P < 0.001), and a Trail × Performance interaction (f 2,34 = 8.3, P = 0.001). The protected analysis for high performers revealed an effect of Switch versus Repeat (f 1,9 = 39.5, P < 0.001) an effect of Pre‐switch versus Repeat (f 1,9 = 5.2, P < 0.05). For the LP‐elderly group, no effects reached significance (P‐values > 0.12).
Frontal Scalp Effects, Late Sustained Activation
Visual inspection of the frontal scalp effects (Fig. 4, top row) indicated that the late sustained activity seen in young adults was absent in the combined elderly group. Further, even taking performance differences within the elderly into account, prefrontal late sustained activity was absent in both high‐ and low‐ performing elderly.
Young vs. elderly
Late positive I
An analysis of a ±200 ms epoch centered at 1,400 ms revealed a main effect of Trial (f 2,64 = 4.2, P = 0.019). The protected analysis for young revealed an effect of Switch versus Repeat (f 1,14 = 13.1, P = 0.003) and a Switch × Hemisphere interaction (f 1,14 = 9.7, P = 0.007). In the combined elderly group, there was a significant Switch × Hemisphere interaction (f 1,18 = 17.4, P = 0.001).
Late positive II
An analysis of a ±200 ms epoch centered at 1,800 ms revealed a main effect of Age (f 1,32 = 9.4, P = 0.002), and a main effect of Trial (f 2,64 = 3.3, P < 0.05) and a Trial × Hemisphere interaction (f 2,64 = 11.2, P < 0.001). The protected analysis for young revealed an effect of Switch versus Repeat (f 1,14 = 10.7, P = 0.005) and a Switch × Hemisphere interaction (f 1,14 = 4.9, P = 0.042). In the combined elderly group, a Switch × Hemisphere interaction was found (f 1,18 = 22.6, P < 0.001).
High‐ vs. low‐performing elderly
No effects for Performance, Trial, or Performance × Trial interaction reached significance.
Parietal Scalp Effects, Late Sustained Activation
Visual inspection of the centro‐parietal scalp effects (Fig. 4, bottom row) from ∼1,200 ms onwards in young adults, showed a pattern of late sustained activation that was enhanced for pre‐switch and reduced for switch trials relative to the repeat trials. The combined elderly group showed little or no differentiation between trials. When considering performance within the elderly, a diminished but comparable pattern of activation to that seen in the young was evident in the HP‐elderly group. LP‐elderly showed the strongest sustained activity during pre‐switch trials at around ∼1,500 ms (blue trace, Fig. 4, right, bottom panel), while later on there was no evident differentiation between trials, in contrast to the other two groups.
Young vs. elderly
Late positive I
An analysis of a ±200 ms epoch centered at 1,400 ms revealed a main effect of Age (f 1,32 = 4.4, P < 0.05), Trial (f 2,64 = 11.9, P < 0.001), and a Hemisphere × Trial interaction (f 2,64 = 10.3, P < 0.001). The protected analysis for young revealed an effect of Switch versus Repeat (f 1,14 = 12.6, P = 0.003) and a Switch × Hemisphere interaction (f 1,14 = 11.3, P = 0.005). In elderly, a Switch × Hemisphere interaction (f 1,18 = 8.2, P = 0.01) and a trend toward a Pre‐Switch versus Repeat difference was found (f 1,18 = 3.9, P = 0.062).
Late positive II
An analysis of a ±200 ms epoch centered at 1,800 ms revealed a main effect of Age (f 1,32 = 5.4, P = 0.026), and a main effect of Trial (f 2,64 = 10.8, P < 0.001). In addition, the analysis revealed a Trial × Hemisphere interaction (f 2,64 = 6.8, P = 0.002). The protected analysis for young revealed an effect of Switch versus Repeat (f 1,14 = 18.7, P = 0.001) and a Pre‐Switch × Hemisphere interaction (f 1,14 = 4.6, P = 0.05). In elderly, an effect of Switch versus Repeat (f 1,18 = 4.9, P = 0.04) was found.
High‐ vs. low‐performing elderly
Late positive I
An analysis of a ±200 ms epoch centered at 1,400 ms revealed a main effect of Trial (f 2,34 = 6.6, P = 0.004) and an Age × Trial × Hemisphere × Electrode interaction (f 4,68 = 3.6, P = 0.01). The protected analysis for high performers revealed an effect of Switch versus Repeat (f 1,14 = 9.1, P = 0.015). For low performers, a Switch × Hemisphere × Electrode interaction (f 2,16 = 5.6, P = 0.014) was found and a main effect of Pre‐Switch versus Repeat (f 1,8 = 7.01, P = 0.029).
Late positive II
An analysis of a ±200 ms epoch centered at 1,800 ms revealed a Trial × Hemisphere interaction (f 2,34 = 9.2, P = 0.001). The protected analysis for high performers revealed a Switch × Hemisphere interaction (f 1,9 = 9.5, P = 0.013).
Topographic Mapping, TANOVA, and Source Localization
Pre‐switch related activity
Figure 6 illustrates scalp potential maps, TANOVA, and dipole source estimations of activity when participants prepared for a switch of task (i.e., of differential activity between pre‐switch and repeat trial). The TANOVA results comparing topographies between Young and HP‐elderly (TANOVA HP vs Y) and between Young and LP‐elderly (TANOVA LP vs Y) are shown from 600 to 2,000 ms. Scalp voltage maps and source localization are shown during two time frames, the early transient positivity (600/850 ms) and the late sustained positivity (1,400/2,000 ms) (Table III).
Figure 6.
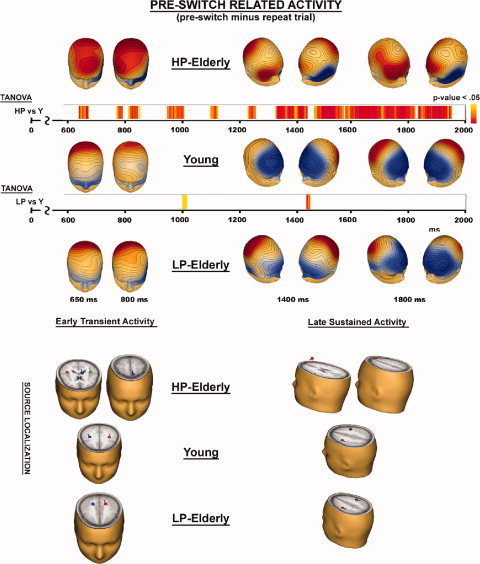
Scalp voltage maps, TANOVA, and estimated dipole source locations of activity when participants prepare for a switch of tasks (pre‐switch minus repeat trial).
Table III.
Source localization analysis of pre‐switch related activity in young, LP‐elderly, and HP‐elderly participants
| LP‐Elderly | Young | HP‐Elderly | |
|---|---|---|---|
| Pre‐switch related Activity | |||
| Early Transient Activity | Right/Left, Parietal lobe, Precuneus, BA 7 | Right/Left Frontal Lobe, Precentral Gyrus, BA 4 | Left/Right Temporal lobe, STG, BA 41 |
| Left SFG, BA 6 | |||
| Late Sustained Activity | Right/Left Frontal Lobe, Precentral Gyrus, BA 4 | Right/Left Frontal Lobe, Middle Frontal Gyrus, BA 6 | Right/Left Parietal Lobe, Postcentral Gyrus, BA 3 |
| Right Frontal Lobe, Superior Frontal Gyrus, BA 8 | |||
Source solutions of switch‐related activity were not stable and therefore not reported.
The early prefrontal amplification was characterized by a clear topographical difference between HP‐elderly and both other groups, Young and LP‐elderly. Figure 6 (left two columns) with the voltage maps during the double‐peak, revealed for all three groups a maximum distribution over parietal scalp region. Furthermore, in HP‐elderly a second maximum over prefrontal scalp region was evident. The TANOVA confirmed differences in topography for the comparison Young vs. HP‐elderly, while no difference was found for the comparison Young vs. LP‐elderly. Bilateral sources localized in the precentral gyrus and the precuneus for young and LP‐elderly. For HP‐elderly, we found bilateral sources in the superior temporal gyrus and in the left superior frontal gyrus.
For the late sustained activity, a very similar pattern of results emerged when comparing the three groups. That is, HP‐elderly differ from both other groups, Young and LP‐elderly. Figure 6 presents the voltage maps at 1,400 ms (middle two columns representing the same map from two different perspectives) and 1,800 ms (right two columns representing the same map from two different perspectives). For young adults, the voltage maps revealed a single maximum with sources localized in the middle frontal gyrus (bottom, right). LP‐elderly showed a similar but less defined topography with a broader maximum spreading over fronto‐parietal scalp regions, and sources localized slightly more posterior in the precentral gyrus of the frontal lobe. The TANOVA comparing Young vs. LP‐elderly revealed no topographical differences. The voltage map of HP‐elderly appeared more complex with two maxima, a first one over the right prefrontal scalp region and a second one bilateral over parietal scalp region. Sources localized in the postcentral gyrus of the parietal lobe and the right superior frontal gyrus. The TANOVA comparing Young vs. HP‐elderly revealed topographical differences for almost the entire period of the late sustained activity.
Switch‐related activity
Figure 7 illustrates scalp voltage maps, TANOVA of activity related to the execution of a switch in task (switch minus repeat trial). Source solution were not stable and therefore not reported. TANOVA revealed topographical differences between HP‐elderly and young from 700 to 800 ms. No further differences were evident from 800 ms on. No differences were found for the comparison between LP‐elderly and young. Topographical mapping revealed a single focus of distribution in all three groups that was shifted anterior in LP‐elderly and young.
Figure 7.
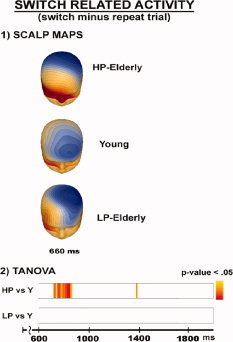
Scalp voltage maps and TANOVA of activity when participants perform a switch of tasks (switch minus repeat trial).
DISCUSSION
Our goal was to test two contrasting hypotheses regarding the neural processes that allow some elderly to preserve high levels of executive functioning. The first possibility is that high‐performance in elderly is marked by preservation of similar processing mechanisms to those found in much younger subjects; that is, these individuals have had little to no decay of function across aging. An alternate account is that successful maintenance of high levels of function relies on a wholesale recruitment and amplification of control processes to cope with the decline of sensory‐perceptual mechanisms that accompanies normal aging. We used a well‐established task‐switching paradigm and high‐density electrical mapping of brain activity to adjudicate between these two possibilities. We have previously detailed a series of frontal and parietal ERP components that index effective switching mechanisms in young adults, and these served as dependent measures here. We detail the pattern of event‐related brain activity in a cohort of elderly subjects, splitting them into a HP and LP group, and compare their patterns of activation to those of young adults.
Behaviorally, we found slower RTs on switch trials (switch costs) across all three groups [Rogers and Monsell, 1995; Wylie et al., 2004b]. Significantly, an examination of task‐switching behavior in the HP‐elderly indicated that these subjects were just as fast and flexible as young. However, the ERP pattern associated with executive control in HP‐elderly was dramatically different to that seen in both young adults and in the LP‐elderly. Detailed analysis of the pattern of ERP effects, significant differences in topographic scalp maps and inverse source modeling, unambiguously distinguished the pattern of brain activations in HP‐elderly from both the young and LP‐elderly groups. The data strongly support the recruitment model, whereby high levels of executive control in HP‐elderly are determined by large‐scale recruitment and perhaps reorganization of the fronto‐parietal task‐switching network. The specifics of the neurophysiological findings that lead to this conclusion are discussed in what follows.
Age and Performance‐Related Effects on the ERP
The effect of aging on the ERP was very different when measured over prefrontal versus parietal scalp. The elderly, when taken as a combined group, showed a less differentiated, delayed, and attenuated activation pattern over parietal scalp. Consistent with Wylie et al. [2003], the first effect that differentiated between trial types (i.e., switch, repeat, and pre‐switch trials) was evident at 340 ms in the young, but this effect was absent in the elderly. The subsequent large‐amplitude P3‐like activation was delayed by 85 ms in the elderly, peaking instead at 565 ms, and was reduced in amplitude by about 2 μV in the elderly as a whole. An altogether different pattern was evident over frontal scalp. Here, activity in the elderly peaked much earlier2, was amplified rather than attenuated, and showed a stronger degree of differentiation between trial‐types. Specifically, the activation pattern was amplified by about 2 μV and started to differentiate between all three trials at about 400 ms in the elderly, whereas for the young group, there was hardly any difference between repeat and pre‐switch trials until a late sustained period of slow‐wave activity (∼1,200 ms). Crucially, when the elderly group was split into HP‐ and LP‐groups, it became evident that this prefrontal amplification and recruitment was entirely driven by the HP‐group, strongly supporting the hypothesis that high levels of executive control in the elderly are dependent on the successful recruitment of additional frontal regions, and apparently also on very early deployment of these mechanisms.
A reduced P3 over centro‐parietal scalp is a well‐documented finding in aging [see Friedman et al., 1997], and here we saw this reduced P3‐like activity replicated in LP‐elderly. On the other hand, the HP‐elderly group showed a robust P3. A number of recent studies that also focused on performance differences within the elderly population have confirmed increased P3 amplitude in HP‐elderly [e.g., Daffner et al., 2005, 2006; Riis et al., 2008].
Significant effects of aging on the ERP were also seen in the current study during the late period of sustained activity. In particular, over prefrontal scalp regions, there was a robust late sustained positivity seen in the young subjects that persisted throughout the entire 2‐s intertrial epoch. This sustained activity was highly attenuated, if not absent, in both HP‐ and LP‐elderly over the last 400–500 ms of the epoch. Within the young group, there were no detectable differences for the sustained activity between pre‐switch (Fig. 4, blue, solid trace) and repeat trials (green, solid trace). This was surprising, as we had expected that a forthcoming change in task would initiate a frontally mediated updating in task goal during pre‐switch trials [e.g., Miller and Cohen, 2001]. A frontal ERP modulation related to a task change has been repeatedly found in designs where a switch of task was cued randomly [e.g., Barcelo et al., 2006; Brass et al., 2005; Miniussi et al., 2005; Moulden et al., 1998; Rushworth et al., 2002], and so it may be in design differences that this difference between studies occurs. Here we used a so‐called “alternating runs” design, which allowed participants to capitalize on the completely predictable sequence of repetitions and alternations of task. Thus, the lack of frontal ERP differentiation in young subjects may result from the fact that the load on frontal regions associated with executive control processes is reduced when participants are able to switch tasks with a predictable rhythm. Nonetheless, clear differences are evident in this sustained activity between pre‐switch and switch trials in the young, and it is also the case that sustained frontal involvement continues to be robust over the full 2‐s interval between trials, regardless of trial type. In stark contrast, none of this was the case for either the HP‐ or LP‐elderly. Thus, while early prefrontal mechanisms were amplified and apparently speeded up in the elderly, especially the HP‐group, late sustained processing was highly attenuated.
Age and Performance‐Related Effects on the Difference‐ERP
We isolated neural processes more specifically related to the preparation for a change in task by subtracting the ERP during the pre‐switch trials (where subjects performed the task and started to get ready for a switch of task) from the ERP during the nested repeat trials (where subjects performed the task and started to get ready to repeat the same task). We used the same logic to isolate neural processes more specifically related to the execution of a task switch by subtracting the ERP during the switch trials (where subjects performed the first instance of the new task) from the ERP during the nested repeat trials.
Pre‐switch related effects
Crucially, when comparing HP‐ and LP‐elderly against young, we found widespread and significant topographical differences that distinguished the HP‐elderly from both other groups, differences that were evident mainly during the preparatory pre‐switch trial (Fig. 6, first bar). There are two conclusions that can be drawn from this finding. First, that there is a significant difference in topography for the HP‐group points to a different generator configuration underlying processing in this timeframe—that is, a different network of brain areas is active in this group to solve the preparation for an upcoming switch. Second, these results suggest that an amplified level of executive control is maintained in HP‐elderly through a higher degree of anticipatory preparation for the change of task [see Sohn et al., 2000; Wylie et al., 2006]. Also notable was the lack of almost any topographic differences between the young adult and LP‐elderly group (Fig. 6, second bar), which we interpret to reflect a failure of compensatory recruitment in this group. That is, this lack of topographic changes points to a similar underlying generator configuration in the LP‐group to that in the young group, and implies that the LP‐group continue to rely on the same neural processes that they employed in their youth, despite the fact that these processes are no longer as effective as they were. This lack of effectiveness is manifest as decreased amplitudes relative to the young group. Clearly, this latter interpretation is speculative and a longitudinal study would be much better suited to assessing this proposal.
In addition to the topographic changes noted, source localization also provided strong support for the recruitment model of executive control in HP‐elderly. Before participants were required to switch tasks, topographical mapping revealed a common maximal distribution over parietal cortex for all three groups during the early transient activity. However, a second prominent focus of activity over frontal cortex was evident only in HP‐elderly (at both P570 and P810), and source modeling suggested that this activity was localized in the vicinity of the superior frontal gyrus (Fig. 6, left, bottom panel). A similar dissociation between groups was also evident for the late sustained activity (i.e., anticipatory pre‐switch processes). That is, the pattern of activity for HP‐elderly was completely different to that of both other groups, with the LP‐ and young groups showing remarkably similar topographies. Topographical mapping in HP‐elderly revealed two foci of distribution over right prefrontal cortex and bilateral parietal cortex during the late sustained activity. In contrast, a single focus over frontal cortex was evident in the young. LP‐elderly showed a similar but considerably less defined topography with a broader maximum spreading over fronto‐parietal scalp regions. For all three groups, sources were localized in the frontal lobe. On the other hand, additional sources in the parietal lobe were necessary to account for the data in the HP‐elderly group in the late sustained period.
Switch‐related effects
There were almost no significant topographic differences evident between groups during switch trials—i.e., new task execution (Fig. 7, first and second bar). The only exception was during the time period between ∼700 and 800 ms, where a significant difference between HP‐elderly and young was observed. Attempts to source‐localize this difference resulted in no stable solution. No differences were found for the comparison of young versus LP‐elderly. If we looked at ERP differences between switching and repeating the task within the young and the combined elderly group, effects started earlier, at 340 ms in young and 565 ms in elderly over parietal scalp regions. Furthermore, ERP effects extended through the early transient and late sustained period of activity in young and HP‐elderly. Generally, the effect represented decreased amplitude for switch compared to repeat trials. For LP‐elderly, there were no ERP differences between switching and repeating the task during either the early transient or late sustained periods of activity.
In sum, these topographic and source‐analysis data point to major differences in the brain‐networks employed during the pre‐switch trials between HP‐elderly and young adults. In contrast, while there are differences in amplitude during the switch trials, it appears that much the same circuits are active for each of the three groups. So, it appears that the effective compensatory mechanisms invoked by HP‐elderly are mainly to do with more effective preparation for a task‐switch than in the actual execution of that task.
An alternative account of the current pattern of results concerns the possible role of practice effects. It is well established that a more extensive brain circuit is activated during the initial stages of task‐acquisition when subjects are expending substantial cognitive resources to acquire proficiency in a new task [e.g., Kelly et al., 2006; Petersen et al., 1998; Raichle et al., 1994]. A consistent finding in this line of work is that once competence has been attained and automated, the active circuit winnows to fewer nodes (regions) [see Chein and Schneider, 2005]. One clear possibility with the present study is that our young adults acquired proficiency much more rapidly in the switching task than did the older adults, and there is certainly evidence for slowing of skill‐acquisition in older adults [e.g., Strayer and Kramer, 1994]. One plausible scenario is that the greater activity we see in HP‐elderly, including the extra medial prefrontal involvement during task‐preparation, might also be evident in our young subjects during the initial stages of skill acquisition. Under this account, the high performance levels seen in our HP‐elderly group would not be expressly due to a recruitment of additional task‐switching circuits, but rather, would be achieved through persistent activation of “normal” task‐acquisition circuits. That is, perhaps our HP‐elderly group simply never automated the task. In a similar vein, it might be possible to show persistent activation of these circuits in young subjects by increasing the complexity of the task‐switching design so that it cannot be readily automatized. It will remain for future work to assess this possibility.
CONCLUSIONS
We found strong evidence for amplified, speeded, and more differentiated patterns of prefrontal activity in HP‐elderly performing a classical task‐switching paradigm. This was especially so immediately preceding a task‐switch, during the anticipatory preparation period. In contrast, the activity pattern in LP‐elderly was highly attenuated relative to both HP‐elderly and young controls, and somewhat paradoxically, this pattern much more closely resembled the pattern seen in healthy young adults. This pattern of results leads us to conclude that maintenance of high levels of executive function across the lifespan is mediated through large‐scale recruitment of relevant neural circuitries that compensate for normal age‐related sensory‐perceptual decline. Thus, successful aging is not simply a function of maintaining processing routines in the same form as seen in youth. It could also be argued that this is very good news for cognitive aging, since it suggests that cognitive resources can be marshaled to compensate for normal decline and that there is a high degree of flexibility in how brain circuits in the frontal lobe are recruited. Thus, even with some inevitable decay of function, there is the real possibility that appropriate rehabilitative strategies and training regimens can be devised to encourage and optimize these recruitment processes.
Acknowledgements
Sincere thanks go to Ms. Beth Higgins for help with data collection. Professor Foxe takes full responsibility for the integrity of the data and attests that all authors had full access to all the data in this study.
Footnotes
An issue with cross‐sectional investigations of successful aging is the difficulty in parsing changes related to aging from pre‐existing differences in cognitive abilities. For example, if one were to break a population of elderly subjects into a high‐performing group and a low‐performing group on the premise that those in the high group have aged more successfully, it is entirely possible that had the investigator classified the same group 40 years earlier, before aging set in, the majority of the individuals in today's high performing elderly group would also have been classified as such decades earlier. The obvious strategy then is to also break one's young sample into low‐ and high‐performing groups to ensure that any effects seen in aging are truly attributable to differences in the aging process as opposed to pre‐existing baseline differences [see Riis et al., 2008].
These latency differences between groups are an interesting subject by themselves but given no a priori predictions about latency differences, they will bear replicating in future work.
REFERENCES
- Ammons R, Ammons C ( 1969): Quick test of intelligence. Psychol Rep 24: 388–390. [Google Scholar]
- Astafiev SV, Shulman GL, Corbetta M ( 2006): Visuospatial reorienting signals in the human temporo‐parietal junction are independent of response selection. Eur J Neurosci 23: 591–596. [DOI] [PubMed] [Google Scholar]
- Barcelo F, Escera C, Corral MJ, Periáñez JA ( 2006): Task switching and novelty processing activate a common neural network for cognitive control. J Cogn Neurosci 18: 1734–1748. [DOI] [PubMed] [Google Scholar]
- Brass M, Ullsperger M, Knoesche TR, von Cramon DY, Phillips NA ( 2005): Who comes first? The role of the prefrontal and parietal cortex in cognitive control. J Cogn Neurosci 17: 1367–1375. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR ( 2002): Aging gracefully: Compensatory brain activity in high‐performing older adults. Neuroimage 17: 1394–1402. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L ( 2004): Task‐independent and task‐specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex 14: 364–375. [DOI] [PubMed] [Google Scholar]
- Cepeda NJ, Kramer AF, Gonzalez de Sather JC ( 2001): Changes in executive control across the life span: Examination of task‐switching performance. Dev Psychol 37: 715–730. [PubMed] [Google Scholar]
- Chein JM, Schneider W ( 2005): Neuroimaging studies of practice‐related change: fMRI and meta‐analytic evidence of a domain‐general control network for learning. Brain Res Cogn Brain Res 25: 607–623. [DOI] [PubMed] [Google Scholar]
- Cockrell JR, Folstein MF ( 1988): Mini‐Mental State Examination (MMSE). Psychopharmacol Bull 24: 689–692. [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Scinto LF, Holcomb PJ ( 2005): Age‐related differences in novelty and target processing among cognitively high performing adults. Neurobiol Aging 26: 1283–1295. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Ryan KK, Williams DM, Budson AE, Rentz DM, Wolk DA, Holcomb PJ ( 2006): Age‐related differences in attention to novelty among cognitively high performing adults. Biol Psychol 72: 67–77. [DOI] [PubMed] [Google Scholar]
- De Sanctis P, Katz R, Wylie GR, Sehatpour P, Alexopoulos GS, Foxe JJ ( 2008): Enhanced and bilateralized visual sensory processing in the ventral stream may be a feature of normal aging. Neurobiol Aging 29: 1576–1586. [DOI] [PubMed] [Google Scholar]
- Dias EC, Foxe JJ, Javitt DC ( 2003): Changing plans: A high density electrical mapping study of cortical control. Cereb Cortex 13: 701–715. [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Kramer AF, Barad V, Cepeda NJ, Weissman DH, Milham MP, Wszalek TM, Cohen NJ, Banich MT, Webb A, Belopolsky AV, McAuley E ( 2001): General and task‐specific frontal lobe recruitment in older adults during executive processes: A fMRI investigation of task‐switching. Neuroreport 12: 2065–2071. [DOI] [PubMed] [Google Scholar]
- Dove A, Pollmann S, Schubert T, Wiggins CJ, von Cramon DY ( 2000): Prefrontal cortex activation in task switching: An event‐related fMRI study. Brain Res Cogn Brain Res 9: 103–109. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Berman KF ( 2002): Fractionating the neural substrate of cognitive control processes. Proc Natl Acad Sci USA 99: 14595–14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Koechlin E, Ali SO, Grafman J ( 2002): The roles of timing and task order during task switching. Neuroimage 17: 95–109. [DOI] [PubMed] [Google Scholar]
- Eppinger B, Kray J, Mecklinger A, John O ( 2007): Age differences in task switching and response monitoring: Evidence from ERPs. Biol Psychol 75: 52–67. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Yordanova J, Kolev V ( 2006): Effects of aging on slowing of motor‐response generation. Int J Psychophysiol 59: 22–29. Epub 2005 Oct 27. [DOI] [PubMed] [Google Scholar]
- Fender DH ( 1987): Source localisation of brain electrical activity In: Gevins AS, Remond A, editors. Handbook of Electroencephalography and Clinical Neurophysiology, Vol. 1. Methods of Analysis of Brain Electrical and Magnetic Signals. Amsterdam: Elsevier; pp 355–399. [Google Scholar]
- Foxe JJ, McCourt ME, Javitt DC ( 2003): Right hemisphere control of visuospatial attention: Line‐bisection judgments evaluated with high‐density electrical mapping and source analysis. Neuroimage 19: 710–726. [DOI] [PubMed] [Google Scholar]
- Friedman D, Kazmerski V, Fabiani M ( 1997): An overview of age‐related changes in the scalp distribution of P3b. Electroencephalogr Clin Neurophysiol 104: 498–513. [DOI] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Johnson R,Jr , Ritter W, Bersick M ( 2008): Age‐related changes in executive function: An event‐related potential (ERP) investigation of task‐switching. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 15: 95–128. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M ( 1999): A large‐scale distributed network for covert spatial attention: Further anatomical delineation based on stringent behavioural and cognitive controls. Brain 122( Part 6): 1093–1106. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, Pietrini P, Schapiro MB, Haxby JV ( 1995): Age‐related reductions in human recognition memory due to impaired encoding. Science 269: 218–221. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA ( 1988): Signal Detection Theory and Psychophysics. Los Altos, CA, USA: Peninsula Publishing. [Google Scholar]
- Gurd JM, Amunts K, Weiss PH, Zafiris O, Zilles K, Marshall JC, Fink GR ( 2002): Posterior parietal cortex is implicated in continuous switching between verbal fluency tasks: An fMRI study with clinical implications. Brain 125( Part 5): 1024–1038. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Hebrank A, Sutton BP, Leshikar E, Chee MW, Tan JC, Goh JO, Park DC ( 2007): Contextual interference in recognition memory with age. Neuroimage 35: 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayanidis F, Coltheart M, Michie PT, Murphy K ( 2003): Electrophysiological correlates of anticipatory and poststimulus components of task switching. Psychophysiology 40: 329–348. [DOI] [PubMed] [Google Scholar]
- Kelly C, Foxe JJ, Garavan H ( 2006): Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Arch Phys Med Rehabil 87( 12 Suppl 2): S20–S29. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Aguirre GK, D'Esposito M ( 2000): Modulation of task‐related neural activity in task‐switching: An fMRI study. Brain Res Cogn Brain Res 10: 189–196. [DOI] [PubMed] [Google Scholar]
- Kray J, Eppinger B, Mecklinger A ( 2005): Age differences in attentional control: An event‐related potential approach. Psychophysiology 42: 407–416. [DOI] [PubMed] [Google Scholar]
- Lehmann D ( 1987): Principles of spatial analysis In: Gevins AS, Remond A, editors. Handbook of Electroencephalography and Clinical Neurophysiology, Vol. 1. Methods of Analysis of Brain Electrical and Magnetic Signals. Amsterdam: Elsevier; pp 309–354. [Google Scholar]
- Lehmann D, Skrandies W ( 1980): Reference‐free identification of components of checkerboard‐evoked multichannel potential fields. Electroencephalogr Clin Neurophysiol 48: 609–621. [DOI] [PubMed] [Google Scholar]
- Logan JM, Sanders AL, Snyder AZ, Morris JC, Buckner RL ( 2002): Underrecruitment and nonselective recruitment: Dissociable neural mechanisms associated with aging. Neuron 33: 827–840. [DOI] [PubMed] [Google Scholar]
- Manly BF ( 1991): Randomization and the Monte Carlo Methods in Biology. London, UK: Chapman and Hall. [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B ( 2003): Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol 462: 144–152. [DOI] [PubMed] [Google Scholar]
- Milham MP, Erickson KI, Banich MT, Kramer AF, Webb A, Wszalek T, Cohen NJ. ( 2002): Attentional control in the aging brain: Insights from an fMRI study of the stroop task. Brain Cogn 49: 277–296. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD ( 2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Miniussi C, Marzi CA, Nobre AC ( 2005): Modulation of brain activity by selective task sets observed using event‐related potentials. Neuropsychologia 43: 1514–1528. [DOI] [PubMed] [Google Scholar]
- Moulden DJA, Picton TW, Meiran N, Stuss DT, Riera JJ, Valdes‐Sosa P ( 1998): Event‐related potentials when switching attention between task‐sets. Brain Cogn 37: 144–201.9693019 [Google Scholar]
- Nicholson R, Karayanidis F, Bumak E, Poboka D, Michie PT ( 2006): ERPs dissociate the effects of switching task sets and task cues. Brain Res 1095: 107–123. [DOI] [PubMed] [Google Scholar]
- Paul RH, Clark CR, Lawrence J, Goldberg E, Williams LM, Cooper N, Cohen RA, Brickman AM, Gordon E. ( 2005): Age‐dependent change in executive function and gamma 40 Hz phase synchrony. J Integr Neurosci 4: 63–76. [DOI] [PubMed] [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS ( 2008): Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex 18: 1010–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME ( 1998): The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA 95: 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, Petersen SE ( 1994): Practice‐related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex 4: 8–26. [DOI] [PubMed] [Google Scholar]
- Reuter‐Lorenz PA ( 2002): New visions of the aging mind and brain. Trends Cogn Sci 6: 394–400. [DOI] [PubMed] [Google Scholar]
- Riis JL, Chong H, Ryan KK, Wolk DA, Rentz DM, Holcomb PJ, Daffner KR ( 2008): Compensatory neural activity distinguishes different patterns of normal cognitive aging. Neuroimage 39: 441–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Monsell S ( 1995): The cost of a predictable switch between simple cognitive tasks. J Exp Psychol Gen 124: 207–231. [Google Scholar]
- Rushworth MF, Passingham RE, Nobre AC ( 2002): Components of switching intentional set. J Cogn Neurosci 14: 1139–1150. [DOI] [PubMed] [Google Scholar]
- Scherg M, Picton TW ( 1991): Separation and identification of event‐related potential components by brain electric source analysis. Electroencephalogr Clin Neurophysiol Suppl 42: 24–37. [PubMed] [Google Scholar]
- Scherg M, Von Cramon D ( 1985): Two bilateral sources of the late AEP as identified by a spatio‐temporal dipole model. Electroencephalogr Clin Neurophysiol 62: 32–44. [DOI] [PubMed] [Google Scholar]
- Simpson GV, Pflieger ME, Foxe JJ, Ahlfors SP, Vaughan HG Jr, Hrabe J, Ilmoniemi RJ, Lantos G ( 1995): Dynamic neuroimaging of brain function. J Clin Neurophysiol 12: 432–449. [DOI] [PubMed] [Google Scholar]
- Sohn MH, Ursu S, Anderson JR, Stenger VA, Carter CS ( 2000): Inaugural article: The role of prefrontal cortex and posterior parietal cortex in task switching. Proc Natl Acad Sci USA 97: 13448–13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer DL, Kramer AF ( 1994): Aging and skill acquisition: Learning‐performance distinctions. Psychol Aging 9: 589–605. [DOI] [PubMed] [Google Scholar]
- van Marwijk HW, Wallace P, de Bock GH, Hermans J, Kaptein AA, Mulder JD ( 1995): Evaluation of the feasibility, reliability and diagnostic value of shortened versions of the geriatric depression scale. Br J Gen Pract 45: 195–199. [PMC free article] [PubMed] [Google Scholar]
- Ware J Jr, Kosinski M, Keller SD ( 1996): A 12‐item short‐form health survey: Construction of scales and preliminary tests of reliability and validity. Med Care 34: 220–233. [DOI] [PubMed] [Google Scholar]
- Wecker NS, Kramer JH, Hallam BJ, Delis DC ( 2005): Mental flexibility: Age effects on switching. Neuropsychology 19: 345–352. [DOI] [PubMed] [Google Scholar]
- West R ( 2004): The effects of aging on controlled attention and conflict processing in the Stroop task. J Cogn Neurosci 16: 103–113. [DOI] [PubMed] [Google Scholar]
- West R, Moore K ( 2005): Adjustments of cognitive control in younger and older adults. Cortex 41: 570–581. [DOI] [PubMed] [Google Scholar]
- West R, Travers S ( 2008): Differential effects of aging on processes underlying task switching. Brain Cogn 68: 67–80. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ ( 2003a): Task switching: A high‐density electrical mapping study. Neuroimage 20: 2322–2342. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ ( 2003b): Cognitive control processes during an anticipated switch of task. Eur J Neurosci 17: 667–672. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ ( 2004a): Don't think of a white bear: An fMRI investigation of the effects of sequential instructional sets on cortical activity in a task‐switching paradigm. Hum Brain Mapp 21: 279–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ ( 2004b): The role of response requirements in task switching: Dissolving the residue. Neuroreport 15: 1079–1087. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Javitt DC, Foxe JJ ( 2006): Jumping the gun: Is effective preparation contingent upon anticipatory activation in task‐relevant neural circuitry? Cereb Cortex 16: 394–404. [DOI] [PubMed] [Google Scholar]
- Wylie GR, Murray MM, Javitt DC, Foxe JJ ( 2009): Distinct neurophysiological mechanisms mediate mixing costs and switch costs. J Cogn Neurosci 21: 105–118. [DOI] [PubMed] [Google Scholar]


