Abstract
Discriminating between successively presented odors requires brief storage of the first odor's perceptual trace, which then needs to be subsequently compared to the second odor in the pair. This study explores the cortical areas involved in odor discrimination and compares them with findings from studies of working‐memory, traditionally investigated with n‐back paradigms. Sixteen right‐handed subjects underwent H2 15O positron emission tomography during counterbalanced conditions of odorless sniffing, repeated single odor detection, multiple odor detection, and conscious successive discrimination between odor pairs. Eight odorants were delivered using a computer‐controlled olfactometer through a birhinal nasal cannula. Conscious successive odor discrimination evoked significantly greater activity in the left anterior insula and frontopolar gyrus when compared to reported sensory detection of the identical odors. Additional activation was found in the left lateral orbital/inferior frontal and middle frontal gyri when discrimination was compared to the odorless condition. The left anterior insula is likely involved in the evaluation of odor properties. Consistent with other studies, frontopolar and middle frontal gyrus activation is more likely related to working memory during odor discrimination. Hum Brain Mapp, 2007. © 2006 Wiley‐Liss, Inc.
Keywords: olfaction, successive discrimination, working memory, insula, PET
INTRODUCTION
Discriminating between successively presented odors is considerably simpler and easier than recognizing and identifying odors presented in isolation and devoid of context [Engen, 1982]. Pairwise odor discrimination was first used in multidimensional studies of odor classification to rate odor similarity [Engen, 1982]. An odor space in the observer's perceptual system was then hypothesized that determines odor similarity or dissimilarity. Such a theoretical model is less prone to bias from language [Carroll and Wish, 1974; Wise et al., 2000].
Brain lesion studies suggest poorer odor discrimination in patients with medial temporal and/or frontal lobectomy, despite normal olfactory detection thresholds [Andy et al., 1975; Eichenbaum et al., 1983; Eskenazi et al., 1983; Potter and Butters, 1980; Zatorre and Jones‐Gotman, 1991], with the right orbital area appearing most important to this process [Zatorre and Jones‐Gotman, 1991]. Lesion studies are nevertheless spatially imprecise, as they can include several adjacent cerebral areas and interrupt connection fibers from other brain regions. In contrast, functional neuroimaging permits a clearer picture of more specific neural networks in the healthy human brain.
Only two neuroimaging studies have investigated odor discrimination. Savic et al. [ 2000] studied human cerebral activation during odor perception, discrimination, and recognition, and found that odor discrimination activated the hippocampus, insula, and orbitofrontal cortex. However, these authors derived their findings by subtracting a single‐odor baseline condition image from the images of their more complex tasks, which involved multiple odors. Activation attributed to odor discrimination could have therefore included the effects of perceptually processing multiple different odors, instead of discrimination per se. More recently, Kareken et al. [ 2003] studied odor discrimination in aging using a baseline control condition that was much closer to the odor discrimination task in that multiple different odors were used at the same presentation rate during both conditions. In this case, the predominant difference between the two conditions was the mental task, with the result of significant hippocampal activation when comparing odor discrimination to the baseline sensory condition. These results were nevertheless obtained from the combined data of young and older subjects, and the odors used in the two conditions were not entirely the same.
It is also the case that because two odors cannot be presented simultaneously, odor discrimination requires brief storage of the first odor's perceptual trace, which is then subsequently compared to the second odor in the pair. Successive odor discrimination thus requires working memory. Only one study has directly targeted olfactory working memory per se. Dade et al. [ 2001] compared olfactory and visual working memory using a two‐back task (serial recognition in which the target continuously changes, and where subjects compare each item with targets presented two trials earlier). The authors reported that mid‐dorsolateral and mid‐ventrolateral prefrontal regions were engaged in working memory independently of sensory modality. In this study, however, the odor working memory condition was compared to an odorless baseline. Thus, it is somewhat difficult to interpret these results, as other olfactory processes cannot be easily separated from odor working memory itself.
The aim of the present study was to investigate cerebral regions active during successive (paired) odor discrimination (Disc) in a sample that was larger and more homogeneously young than the group who participated in our previous study [Kareken et al., 2003]. Furthermore, the discrimination condition was compared principally to a baseline condition involving the subjects' reported sensory detection (detection of multiple odors, DetM) of the identical odors used during discrimination. Since subjects might conceivably discriminate between the odors implicitly and automatically during this sensory baseline, a second control detection task was included using a single odor repetitively presented (detection of a single odor, DetS). Finally, a baseline odorless condition (Base) was used, as well. We hypothesized that successive odor discrimination would activate regions previously observed in odor discrimination and working memory: the hippocampus, and mid‐dorsolateral and mid‐ventrolateral prefrontal regions.
SUBJECTS AND METHODS
Subjects
Sixteen healthy, right‐handed subjects (eight female; age = 25.06 ± 3.23; education = 18.19 ± 2.95) were recruited from the community. Before participating, all subjects voluntarily signed informed consents that had been approved by the university's Institutional Review Board. All subjects were screened for olfactory competence using the University of Pennsylvania Smell Identification Test [UPSIT, Doty et al., 1984] (group mean = 37.63 ± 1.67). The sample's olfactory sensory detection threshold (−5.03 ± 0.94 vol/log) was measured using phenyl ethyl alcohol as administered through polypropylene squeeze bottles [Doty, 2000].
Odor Delivery
Odor stimulation used an 8‐channel, computer‐controlled air‐dilution olfactometer modeled after Lorig et al. [ 1999]. Airflow was generated by an oil‐less pump, filtered through a charcoal filter, and humidified. Solenoid valves injected into a continuous stream (1.0 L/min) either air from a control line (1.0 L/min) or odorized air from one of eight odor channels (each divided into odor and air‐dilution lines, summing to 1.0 L/min), making 2 L/min total system flow. An odorant saturated polyethylene disc (see below) was placed in a glass odorant vial, while a control vial contained a disc with only diluent (propylene glycol). Air was delivered to the subject with a small, birhinal Teflon nasal cannula. Switching between control air and odors with this system evokes no change in flow or somatosensory cue. During odorless baseline condition scans, odorless air was alternately shunted through a pair of valves with the same timing as during odor stimulation. A Pentium‐III laptop running Windows98 and DASYLab (IOtech, Cleveland, OH) controlled the system. A personal DAQ/56 module (IOtech) controlled the solenoid valves. A nasal pressure transducer (PTAF2, Protech, Woodinville, WA) was used to monitor and record sniffing patterns during stimulation (Fig. 1). Amplitudes of inspiratory and expiratory wave forms were estimated by integrating the curves from one side of the baseline to the other using routines developed in Matlab (MathWorks, Natick, MA).
Figure 1.
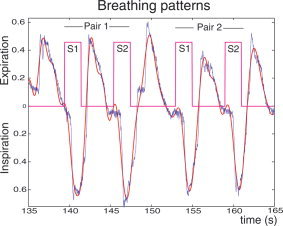
Example of breathing patterns showing inspiratory and expiratory phases synchronized with paired odor stimulations. S1, first stimulus of a pair; S2, second stimulus of a pair; blue line represents respiratory pressure; red line represents the curve modeling the respiratory pressure that thus permitted estimation of the amplitudes of inspiratory and expiratory pressures.
Odorants
Eight relatively familiar odorants (International Flavors & Fragrances, Aldrich, Milwaukee, WI) were used as stimuli (clove, strawberry, Douglas fir, lily, lemon, grass, coconut, and phenyl ethyl alcohol, which has a rose‐like odor). All of the odorants except phenyl ethyl alcohol and grass were diluted with propylene glycol to a 10% concentration. Phenyl ethyl alcohol was used in pure concentration and grass was diluted to a 1% mixture in propylene glycol. Small polyethylene discs (Interstate Specialty Products, Sutton, MA) were placed into the odorants, where they absorbed a constant amount of liquid, and then placed into glass vials with Teflon caps.
During a preliminary experiment, 38 healthy young subjects (age = 24.74 ± 4.51; education = 15.82 ± 2.04; mean UPSIT = 37.08 ± 2.22) rated the intensity and pleasantness of these eight odorants. For intensity, subjects used a mouse to click on digitized versions of the Labeled Magnitude Scale, a psychophysically derived nonlinear scale with 0 falling just below “barely detectable” and 100 representing the “strongest imaginable” [Green et al., 1993, 1996]. For pleasantness, subjects used a digitized linear visual analog scale from 0 to 100 in which the word “neutral” was placed at the midpoint, “unpleasant” at the lower extreme, and “pleasant” at the upper extreme.
Pairwise differences in stimulus quality (intensity, pleasantness) were then quantified according to the odorant pairings used in the Disc task. The average difference between odors in all the item pairs (across all subjects) was 12.6 ± 7.5 in intensity, and 16.6 ± 8.0 in pleasantness.
Imaging Tasks
Subjects underwent a PET imaging session comprised of four task conditions (each task replicated once for a total of eight scans) in a pseudorandomized order (four groups of orders across the 16 subjects). Individual trials in these tasks constituted delivery of either concordant or discordant odor pairs (each duration of odor presentation equaled 2 s) with an odor onset asynchrony of 6 s, a brief question or command with a 5‐s response period, and a 500‐ms pause between trials. Each imaging task involved 16 trials, with two different trial (odor) orders that constituted a second randomization factor (i.e., half the subjects experienced trial order 1 first and trial order 2 on the task replications, while the other half experienced the reverse). Odor (or sham stimulus) delivery began simultaneously with an auditory “sniff” command (delivered through speakers in the PET suite). Details and timing of the stimulus paradigm for the Base, DetS, DetM, and Disc tasks are given in Figure 2.
Figure 2.
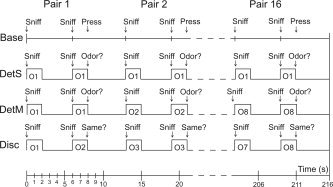
Experimental procedure. Base, baseline condition; DetS, detection of single odor; DetM, detection of multiple odors; Disc, discrimination of paired odors; O1, odor 1; O2, odor 2, etc. See text for details.
For Base, subjects heard the commands “sniff … sniff, press,” whereupon subjects had been trained to sniff gently and press a mouse button (randomly left or right). A sham valve fired at each sniff command. For DetS, subjects heard the command “sniff … sniff, odor?”. At each sniff a single odor was administered repetitively throughout the scan (one scan with lily and one scan with clove). At the question “odor?”, subjects indicated whether or not they were able to detect an odor at either stimulus presentation. The left button of the mouse was used for “yes,” and the right button was used for “no.” Subjects were aware in advance that only a single odor would be repeatedly presented. For DetM, all eight odors were used twice in identical pairs. While only one odorant was administered on any given trial (pair of sniffs), the odor pair changed from trial to trial. Subjects again heard “sniff … sniff, odor?” and answered the “odor?” question in the same manner. Subjects knew in advance that odor pairs would change from trial to trial. However, they were informed that odor quality was not important to the task, and that they had only to indicate whether they were capable of smelling a stimulus. For Disc, subjects heard “sniff … sniff, same?”. Of the 16 trials, half involved paired presentations of the same odor, whereas half involved different pairs. “Same” pairs on trials 1–8 were changed to “different” pairs on trials 9–16, and conversely, “different” pairs on trials 1–8 were changed to “same” pairs on trials 9–16. Subjects indicated whether the odors in the pairs smelled the same or different.
Imaging Parameters
Subjects were imaged in a Siemens Exact HR+ scanner in 2D mode and were positioned using a Versaform pillow (Bissel Healthcare, Jackson, MI) and a cloth head strap to help constrain movement. A 10‐min transmission scan was first conducted using three internal rod sources to correct for attenuation. Fifty mCi of H2 15O water was injected into the antecubital vein of the nondominant arm at the beginning of each functional scan, with scans conducted for 3 min each (arterial sampling was not performed). The 288 × 144 PET image matrix was reconstructed using a 7‐mm wide Hanning filter, integrating over the course of 90 s beginning at 75% of the upward peak.
Image Analysis
SPM99 (Wellcome Department of Cognitive Neurology, London, UK) was used for image processing and analysis. Image volumes were corrected for intrasubject motion using affine, rigid‐body transformations. Each subject's average PET image volumes were then coregistered with each subject's own high‐resolution, whole‐head, T1‐weighted 3D spoiled grass (SPGR) magnetic resonance image (TR = 35 ms; echo time = 12 ms, field of view = 24 × 24 cm; matrix = 256 × 128; 124 contiguous axial slices, slice thickness = 1.1–1.2 mm, receiver bandwidth = 32 kHz, flip angle = 30°). This was accomplished using SPM99's default method of first performing a rigid‐body, affine registration of the images to same‐modality templates, segmenting the images into gray, white, and CSF tissue compartments, and registering the tissue compartments. The anatomical MRIs were then spatially normalized (default 7 × 8 × 7 basis functions, 12 nonlinear iterations) to the Montreal Neurological Institute (MNI) reference brain, and the parameters from this registration used to transform the PET images into MNI stereotactic space. PET images were smoothed using a 12‐mm isotropic Gaussian kernel.
Random effects analysis were performed using a PET model (multi‐subject, condition × subject interaction, no covariates) to first average task conditions into a single contrast image, proportionally scaling the grand mean to a physiologic reference value of 50 mL/100g/min. Contrast images from the PET model were then analyzed in separate “basic model” single sample t‐tests, using an uncorrected height threshold (P < 0.001), and a Z ≥ 3.20 at voxel level. The image contrasts studied in the random effects analyses reflected activation from single odor detection (DetS – Base), multiple odor detection (DetM – Base), and activation due to odor discrimination over and above any implicit discrimination during DetM (Disc – DetM). We also examined the degree to which activation from multiple odor stimulation differed from single odor stimulation (DetM – DetS and DetS – DetM). Duvernoy's [ 1991] anatomic atlas was used to help localize and describe anatomic regions of activation. Activated areas were indicated using the MNI coordinate system. Activation (scaled, normalized signal) across the four conditions was extracted for all significant clusters and used as functional regions of interest (ROIs). Differences in activation between tasks were then tested using a 4 (Task) × 2 (Repetition) repeated measures analysis of variance (ANOVA).
Furthermore, ROI analyses were carried out for the expected regions reported in previous studies [Dade et al., 2001; Kareken et al., 2003; Savic et al., 2000], in the (Disc – DetM) and (Disc – Base) contrasts: the bilateral hippocampus, lateral orbital frontal gyrus (−47, 39, −11), frontopolar gyrus (28, 56, −2 and −34, 51, 5), and middle frontal gyrus (40, 36, 32; −41, 20, 35 and −42, 10, 41). The lateral orbital frontal and middle frontal gyri correspond to regions respectively called mid‐ventrolateral and mid‐dorsolateral cortex by Petrides [ 1996, 2000]. The hippocampal ROIs were anatomical ROIs from the AAL library of MarsBaR, whereas the other ROIs were 8‐mm diameter spheres centered on the coordinates of prefrontal regions [Dade et al., 2001]. The MarsBaR SPM toolbox was used to build the functional and sphere ROIs, extract the activation level, and perform ROI analyses (marsbar.sourceforge.net).
RESULTS
Psychophysical Results
While the average of response accuracy and response time across task replications are of most interest (so as to correspond to the averaged scans per task in the random effects imaging model), any effects of task repetition were examined in the behavioral data. Differences in response accuracy were therefore examined with a 3 (Task) × 2 (Repetition) repeated‐measures ANOVA. This showed significant differences in response accuracy between the tasks (F (2,30) = 4.72, P = 0.02), but no effect between repetitions (F (1,15) = 0.27, P = 0.61), and no significant Task × Repetition interaction (F (2,30) = 0.35, P = 0.71). Post‐hoc comparisons showed greater response accuracy in DetM than DetS and Disc (P < 0.01 and P = 0.02, respectively; Fig. 3, left).
Figure 3.
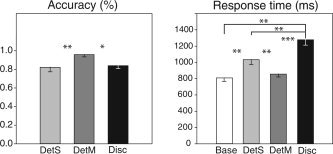
Left, mean response accuracy (%), and right, mean response time (ms), for the different tasks (Base, DetS, DetM, and Disc). *P < 0.05; **P < 0.01; ***P < 0.001.
A similar 4 (Task) × 2 (Repetition) ANOVA was used to analyze differences in response time, which proved significantly different among the four tasks (Base, DetS, DetM, and Disc) (F (3,45) = 16.58, P < 0.0001), but again without a significant effect of repetition (F (1,15) = 0.51, P = 0.49) and without a significant Task × Repetition interaction (F (15,45) = 0.23, P = 0.88). Post‐hoc analyses showed that discriminating between odors took the longest of all the decisions made (Base, P < 0.001; DetS, P < 0.001; DetM, P < 0.001), and that DetS decisions required significantly more time than decisions in Base and DetM (P = 0.004 and P = 0.02, respectively; Fig. 3, right). Judgments about identical odor pairs took significantly longer (1364 ± 79 ms) than judgments about different pairs (1278 ± 74 ms (F (1,15) = 8.24, P = 0.01)), although judgment accuracy was insignificantly different between the two (identical = 91.80 ± 10.13% correct, and different = 84.38 ± 14.61% correct; F (1,15) = 3.13, P = 0.1).
Sniffing Behavior
Since sniffing has been implicated as a potential confound in medial temporal olfactory areas [Sobel et al., 1998, 2000; but see Kareken et al., 2004, for contradictory results using PET], breathing changes were analyzed as a function of the different experimental conditions: tasks, repetitions, and respiratory phases (inspiratory and expiratory). A 4 (Task) × 2 (Repetition) × 2 (Respiratory phase) repeated‐measures ANOVA showed a marginally significant effect of task (F (3,45) = 2.75, P = 0.05), but no significant effect of repetition (F (1,15) = 3.81, P = 0.07) or respiratory phase (F (1,15) = 0.99, P = 0.34), and no significant interaction between the factors. As depicted in Figure 4 (left), mean comparisons indicated that respiratory pressure was slightly higher in DetS than in the Base and DetM conditions (P = 0.04 and P = 0.03, respectively).
Figure 4.
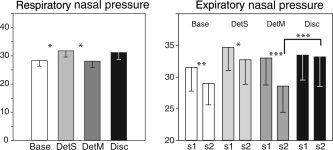
Left, mean respiratory (inspiratory and expiratory) pressures (arbitrary unit) as a function of tasks (Base, DetS, DetM, and Disc). Right, mean expiratory amplitudes as a function of both stimulations (S1 and S2) of a pair. *P < 0.05; **P < 0.01; ***P < 0.001.
Breathing changes were also analyzed as a function of stimulus position (S1 vs. S2) in the pair. When considering inspiratory phases only, a three‐way repeated‐measures ANOVA showed no significant effects of task (F (3,45) = 2.18, P = 0.10), repetition (F (1,15) = 0.79, P = 0.39), or stimulus position in the pair (F (1,15) = 0.30, P = 0.59), and no significant interactions between the factors. When examining expiratory phases, a three‐way repeated‐measures ANOVA did not reveal a significant effect of task (F (3,45) = 2.16, P = 0.11), but a marginally significant effect of repetition (F (1,15) = 4.41, P = 0.053), a significant effect of stimulus position (F (1,15) = 5.58, P = 0.03), and a significant task × stimulus position interaction (F (3,45) = 3.03, P = 0.04). As illustrated in Figure 4 (right), mean comparisons showed that expiratory pressure was lower for the second stimulus in the pair in Base (P = 0.01) and DetM (P < 0.001), and tended to be lower in DetS (P = 0.054) conditions. No significant difference was found in the Disc (P = 0.78) condition. Comparisons also showed that expiratory pressure was higher in Disc than DetM for the second stimulus presentation (P < 0.001), and higher in Disc than Base conditions for the first and second stimulus (P = 0.05 and P < 0.001, respectively; data not reported in figure).
Brain Activation
Odor discrimination
When Disc and DetM conditions were compared (Disc‐DetM contrast), increased activity was evident in the left anterior insula, and the right inferior precentral sulcus, inferior lingual gyrus, and superior temporal gyrus (Table I). Corrected for multiple comparisons, the height of the insula activation was borderline at the voxel level (P = 0.079, corrected), although the cluster size was greater than expected our height threshold (283 voxels, corrected cluster statistic, P = 0.005). In the left anterior insula (−34, 18, 0), as illustrated in Figure 5, regional cerebral blood flow (rCBF) differed significantly between tasks (F (3,45) = 5.22, P = 0.004), with activation being higher in the Disc than Base, DetS, and DetM conditions (P = 0.02, P = 0.02, P < 0.001, respectively).
Table I.
Areas of activation in whole‐brain analysis
| MNI | |||||||
|---|---|---|---|---|---|---|---|
| Contrasts | L/R | Anatomic regions | k | Z | x | y | z |
| Disc – DetM | L | Anterior insula* | 283 | 4.75 | −34 | 18 | 0 |
| R | Inferior precentral sulcus | 62 | 4.08 | 30 | 6 | 26 | |
| R | Inferior lingual gyrus | 80 | 3.89 | 22 | −70 | −12 | |
| R | Superior temporal gyrus | 89 | 3.76 | 46 | −20 | −2 | |
| Disc – Base | L | Lateral orbital gyrus/Inferior frontal gyrus* | 254 | 4.71 | −48 | 44 | 0 |
| R | Cerebellum | 86 | 4.22 | 42 | −70 | −26 | |
| L | Medial orbital gyrus | 107 | 3.52 | −20 | 28 | −18 | |
| L | Medial/posterior orbital gyrus | 3.48 | −20 | 38 | −22 | ||
| L | Medial orbital gyrus | 3.20 | −26 | 32 | −30 | ||
| DetM – Base | L | Lateral orbital gyrus/Inferior frontal gyrus | 113 | 4.10 | −44 | 42 | 4 |
| R | Inferior frontal gyrus | 64 | 3.57 | 34 | 40 | 2 | |
| R | Amygdala | 72 | 3.53 | 26 | −8 | −12 | |
| DetS – Base | R | Lateral orbital gyrus/Inferior frontal gyrus | 135 | 3.56 | 44 | 44 | −2 |
| R | Lateral orbital gyrus | 3.49 | 32 | 36 | −8 | ||
Disc, discrimination; DetM, detection of multiple odors; DetS, detection of a single odor; Base, baseline; L, left; R, right.
Significantly activated (P < 0.05), corrected at cluster level; k, size of the cluster in number of connected voxels; Z, Z‐value; x, y, z, MNI coordinates in mm of the maximum peak.
Figure 5.
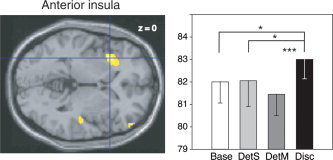
Left, rCBF difference in the left anterior insula (−34, 18, 0) in the Disc‐DetM contrast, superimposed on a horizontal section of the MNI template brain (Left is left). Right, mean rCBFs in this region in the four tasks. Vertical bars, standard errors of the mean; *P < 0.05; **P < 0.01; ***P < 0.001.
When Disc and Base conditions were compared (Disc‐Base contrast), activations were found in a region bordering the left lateral orbital gyrus and the inferior frontal gyrus, in the left medial orbital gyrus and right cerebellum. In the left lateral orbital gyrus (−48, 44, 0; Fig. 6), significant differences between tasks (F (3,45) = 9.87, P < 0.001) were due to significantly higher rCBF in DetS, DetM, and Disc than in the Base condition (P = 0.02, P < 0.001, and P < 0.001, respectively), and significantly higher rCBF in Disc than in DetS (P = 0.006).
Figure 6.
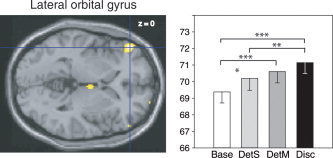
Left, rCBF differences in the left lateral orbital/inferior frontal gyrus (–48, 44, 0) in the Disc‐Base contrast, superimposed on a horizontal section of the MNI template brain. Right, mean rCBFs in this region in the four tasks. See Figure 5 for details.
In the left medial orbital gyrus (−20, 28, −18; Fig. 7), significantly different activation between tasks (F (3,45) = 5,42, P = 0.003) was due to significantly higher rCBF in the DetS, DetM, and Disc conditions (P = 0.03 and P = 0.006, and P = 0.01, respectively) than in the Base condition.
Figure 7.
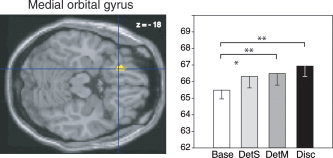
Left, rCBF difference in the left medial orbital gyrus (−20, 28, −18) in the Disc‐Base contrast, superimposed on a horizontal section of the MNI template brain. Right, mean rCBFs in this region in the four tasks. See Figure 5 for details.
Multiple odors and single odor detection
The DetM‐Base contrast evoked significant bilateral inferior frontal gyrus activation, with the left activation bordering the lateral orbital gyrus, as well as activation in the right amygdala. Thus, similar to Disc‐DetM, DetM‐Base also evoked activation in the left lateral orbital/inferior frontal gyrus region (−44, 42, 4; see Fig. 6, left). rCBF in the right inferior frontal gyrus (34, 40, 2) was higher in DetM than in the Base and Disc conditions (P = 0.002 and P = 0.008, respectively) and higher in DetS than Base conditions (P < 0.001). The activated volume in the right amygdala (26, −8, −12) in DetM (Fig. 8) (F (3,45) = 4.82, P = 0.005) also showed significantly higher rCBF in Disc when compared to Base (P = 0.003). Single odor detection (DetS‐Base contrast) resulted in right lateral orbital/inferior frontal gyrus activation, a cluster that also extended into the lateral orbital gyrus at (32, 36, −8). Finally, the differential effect of multiple‐ versus single‐odor detection (DetM‐DetS and DetS‐DetM) did not show statistically significant clusters of greater rCBF.
Figure 8.
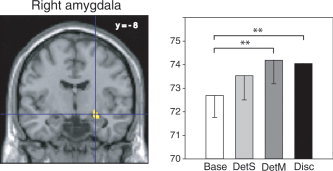
Left, rCBF differences in the right amygdala (26, −8, −12) in the DetM‐Base contrast, superimposed on a coronal section of the MNI template brain. Right, mean rCBFs in this region in the four tasks. See Figure 5 for details.
ROI analyses in the prefrontal and hippocampal regions
In the Disc‐DetM contrast, a significant activation was observed only in the left frontopolar gyrus (Table II). In the Disc‐Base contrast, significant prefrontal activation was found in the left lateral orbital gyrus, the left frontopolar gyrus, and the left middle frontal gyrus, but not in the right lateral orbital gyrus or middle frontal gyrus. ROI analyses did not reveal any significant hippocampal activation for either the Disc‐DetM or the Disc‐Base contrasts.
Table II.
Areas of activation in region of interest analyses
| Contrasts | L/R | Anatomic regions | t | P | MNI | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Disc – DetM | L | Frontopolar gyrus | 2.12 | 0.03 | −34 | 51 | 5 |
| Disc – Base | L | Lateral orbital gyrus | 3.73 | 0.001 | −47 | 39 | −11 |
| L | Frontopolar gyrus | 1.80 | 0.05 | −34 | 51 | 5 | |
| L | Middle frontal gyrus | 2.52 | 0.01 | −41 | 20 | 35 | |
| L | Middle frontal gyrus | 1.84 | 0.04 | −42 | 10 | 41 | |
Disc, discrimination; DetM, detection of multiple odors; Base, baseline; L, left; R, right; t, Student's t‐value; P, result's significance; x, y, z, MNI coordinates in mm of the maximum peak.
DISCUSSION
Our results show that successive discrimination between odors presented pairwise‐induced significantly greater activity in the left anterior insula, the left lateral orbital/inferior frontal and middle frontal gyri, and the left frontopolar area. As elaborated below, both these and other data suggest that the anterior insula is particularly involved in the perceptual processing of odor characteristics. The highly significant, large cluster of activation in the anterior insula is particularly striking insofar as the odors used for both pairwise discrimination and detection were identical, with the principal difference between these tasks being the mental comparison of odor quality within pairs. Furthermore, the insula was not significantly activated in any of the two other olfactory tasks. The detected prefrontal activation more likely reflects executive processes independent of sensory input modality, as previously shown in other studies of working memory.
Left Anterior Insula and Discrimination
In successively discriminating between olfactory stimuli, subjects must compare the odors' perceptual characteristics. Although the perceived intensity and pleasantness of odors in this study were in a relatively homogeneous range, mean scores recorded during the preliminary study indicated some pairwise differences along these dimensions. Perceived intensity and pleasantness could therefore have been used by subjects in their perceptual discriminations. While odor intensity discrimination is considerably less precise than in other sensory domains [Lawless, 1997], multidimensional scaling of odorous notes indisputably points to pleasantness‐unpleasantness as a major facet of olfactory perception [Carrasco and Ridout, 1993; Jones et al., 1978; Moskowitz, 1974; Savic et al., 2002; Schiffman et al., 1977]. These findings are in accord with other data showing that the human response to odors is primarily emotional in nature [Bensafi et al., 2002; Herz and Engen, 1996; Hinton and Henley, 1993; Royet et al., 2000].
The insula, where there was the most significant activity during odor discrimination, functions as a center of limbic integration and visceral/autonomic perception [see Bhaskara and Trimble, 2004, for review]. As such, it is not surprising that the insula also responds to affectively valenced stimuli, both olfactory [Plailly et al., 2006; Royet et al., 2001, 2003] and otherwise [Peyron et al., 2000; Phan et al., 2002; Reiman et al., 1997]. In this context, left anterior insula activation during the odor discrimination task may have therefore had at least some root in perceived differences in pleasantness between odors, or in any corresponding visceral sensations. Nevertheless, when some odors seem equally pleasant, but not at all alike, other dimensions are likely used to distinguish between them [Woskow, 1968]. The extent to which the insula is involved in judgments about these other dimensions, or the extent to which our subjects relied on other features in making their discriminations, remains to be determined. Elsewhere, Gottfried et al. [ 2006] have also recently shown that posterior piriform cortex shows repetition suppression effects to odors of similar quality (but different chemical structure), suggesting that it also plays a significant role in odor quality discrimination.
Working Memory in Successive Discrimination
There is very little extant work regarding the functional anatomy of working memory for olfactory stimuli per se. Dade et al.'s [ 2001] study of an olfactory two‐back paradigm suggested a network that is highly similar to the working memory system for other sensory stimuli. Specifically, there appears to be a mid‐ventrolateral (lateral orbital/inferior frontal) region subserving active encoding and retrieval of information, as well as decision‐making regarding stimuli processed by sensory association cortex, while a mid‐dorsolateral (middle frontal) region appears more involved in ongoing manipulation of information within working memory [Petrides, 1996, 2000; Petrides et al., 2002]. A third, frontopolar region is also related to the executive control of working memory in more complex tasks [Fletcher and Henson, 2001; Koechlin et al., 2000].
We found a similar frontopolar region when subjects discriminated between odors (compared to detecting the same odors), but its strength and spatial extent was considerably less than in the results reported by Dade et al. [ 2001]. We also noted middle frontal gyrus activation, but only when comparing odor discrimination to clean air. We suspect that these differences in the extent and intensity of activation could relate to the much smaller working‐memory demand in paired odor discrimination than in the two‐back working memory task.
Left lateral orbital/inferior frontal gyrus activation (−48, 44, 0) was present in the Disc, but also in the DetS and DetM conditions. This region was thus not specific to successive discrimination, but common to all the olfactory tasks, and could only reflect more complex attention and/or stimulus detection compared to a stimulus‐free baseline. We have previously observed similar lateral frontal and orbital activity during overt judgments of odor hedonicity (36, 48, −6) and familiarity (32, 46, 6), although in these cases the judgments evoked right lateral orbital activation [Royet et al., 2001, 2003].
While the hippocampus is most researched for its clear role in the consolidation and retrieval of long‐term memories [Broadbent et al., 2004; Squire et al., 2004; Yonelinas et al., 2005], some brain imaging studies suggest a possible hippocampal role in short‐term/working memory [Curtis et al., 2000; Ranganath and D'Esposito, 2001; Stern et al., 2001]. There is also a short, disynaptic link between lateral olfactory tract neurons and the hippocampus via entorhinal cortex [Schwerdtfeger et al., 1990; Witter et al., 1989], and patients with surgical resection of the hippocampus/medial temporal area perform poorly on odor discrimination tests [Eichenbaum et al., 1983; Martinez et al., 1993; Zatorre and Jones‐Gotman, 1991]. It might therefore seem plausible that the hippocampus has a role to play in the maintenance of olfactory information in short‐term/working memory, particularly in light of previous data from neuroimaging studies showing odor discrimination to evoke hippocampal activity [Kareken et al., 2003; Savic et al., 2000]. Having said that, Dade et al. [ 2001] did not show a hippocampal response during their olfactory two‐back paradigm. We were also unable to replicate hippocampal activation in the olfactory discrimination task, even at low statistical thresholds.
Confounding Factors
A confound in olfactory‐related activation could potentially come from different respiratory patterns across tasks [Sobel et al., 1998]. In the present study, mean respiratory pressure changes were slightly greater DetS than in the Base and DetM conditions. This likely stems from the observed adaptation to the repetitively administered single odor (i.e., worse detection accuracy in DetS than DetM) in response to which subjects likely increased the amplitude of respiratory airflow to detect it. On the one hand, it should be noted that any comparison involving DetS (i.e., DetS – Base) likely includes significant adaptation from the single repeated stimulus, which could limit the extent of any olfactory activation [Cain, 1970]. At the height threshold employed, however, DetM did not show significantly greater activation than DetS—a result that would have occurred with extensive adaptation. On the other hand, higher respiratory amplitudes could have also increased neural activation. Despite that possibility, we did not find additional activation in DetS compared to DetM, to which the greater respiration could have been attributed.
Respiratory patterns could also differ between both odors of a trial in the Disc and DetM tasks. Comparing only inspiration did not reveal any differences between both odors in any of the tasks. In contrast, mean expiration was higher in Disc than in DetM for the second (but not the first) odor. This higher expiration after the second odor could have been from the longer response time in the Disc task, during which a decision was being made. Whereas inspiration during sniffing might cause differences in cerebral activation related to attention or regulation of odor intake [Sobel et al., 1998, 2000; Zelano et al., 2005; but see also Kareken et al., 2004, for contradictory results], it seems less likely that variation in expiration would itself relate to cerebral activation during odor discrimination.
Since peripheral adaptation can affect brain activation (see above), it could also be a confounding factor in assessing activation differences between the Disc and DetM conditions (i.e., DetM contained identical pairs on all trials, whereas Disc had identical pairs on only 50% of the trials, potentially leading to greater adaptation during DetM). Significant adaptation in this comparison could particularly affect our interpretation of the insula as a region important to odor quality discrimination, especially since the anterior insula is odor‐responsive. However, a more direct opportunity to observe regions affected by adaptation would be in the DetM‐DetS contrast (the latter consisting of 32 presentations of a unique stimulus, the former having eight same‐stimulus pairs presented only twice over 3 min). The lack of differences of activation in this case suggests that adaptation was not a significant factor in the strong insula activation from Disc‐DetM.
Finally, increased attentional demands during the discrimination task could further contribute to some activation differences between tasks. This interpretation seems less likely for the insula, since there was no activation in this area in DetS, a task in which response accuracy was similar to that of the discrimination condition. However, this possibility cannot be ruled out entirely, as reaction time for the discrimination decisions were the longest.
Although no activation was found in piriform cortex in any contrasts comparing the olfactory conditions to the odorless baseline at our chosen threshold, it was observed at a lower height threshold (P = 0.01). In contrast, no piriform activation was found using this lower threshold when contrasting the Disc task to the DetM task. This suggests that piriform cortex activity was not specifically related to conscious odor discrimination. Piriform cortex activity has nevertheless been shown to be related to long‐term odor recognition memory [Dade et al., 2001; Plailly et al., 2005], and to a lesser extent to odor recognition memory judgments about 10 min after stimulus learning [Dade et al., 2001]. This piriform response to learned odors long after rehearsal, and its absence in our working memory paradigm without the need for long‐term consolidation, suggests that piriform cortex activity is more closely related to long‐term explicit odor recognition memory.
CONCLUSION
The current study provides evidence that successively discriminating between odors activates a neural network including several frontal and frontotemporal regions in the left hemisphere. In the context of both this study and the known olfactory network, we believe that the anterior insula plays a prominent role in odor quality discrimination, and that it may relate, in part, to the odors' pleasantness/unpleasantness. Specific manipulation of perceived odorant quality would be required for a more definitive conclusion. The observed left frontal (middle frontal, and frontopolar gyri) activation may relate to the executive function required for working memory. However, the relatively lower working memory load required for successive discrimination (as compared to an n‐back task) likely explains the relative weakness of these frontal activations in comparison to other studies of working memory.
Acknowledgements
We thank Stephen Warrenburg of International Flavors and Fragrances for odorants, Richard Fain, Susan Giger, Kevin Perry, and Sara Sarno for imaging assistance, Adam Tierney for assistance in the psychophysical study, and Samuel Garcia for conceiving with Matlab the software to measure the physiological variations.
Contributor Information
Jane Plailly, Email: plailly@olfac.univ-lyon1.fr.
David A. Kareken, Email: dkareken@iupui.edu.
REFERENCES
- Andy OJ, Jurko MF, Hughes JR( 1975): The amygdala in relation to olfaction. Confin Neurol 37: 215–222. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Rouby C, Farget V, Bertrand B, Vigouroux M, Holley A( 2002): Influence of affective and cognitive judgments on autonomic parameters during inhalation of pleasant and unpleasant odors in humans. Neurosci Lett 319: 162–166. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE( 2004): Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A 101: 14515–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain WS( 1970): Odor intensity after self‐adaptation and cross‐adaptation. Percept Psychophys 7: 271–275. [Google Scholar]
- Carrasco M, Ridout JB( 1993): Olfactory perception and olfactory imagery: a multidimensional analysis. J Exp Psychol Hum Percept Perform 19: 287–301. [DOI] [PubMed] [Google Scholar]
- Carroll JD, Wish MA( 1974): Multidimensional perceptual models and measurement methods In: Carterette EC, Friedman MP, editors. Psychophysical judgment and measurement. New York: Academic Press; p 391–447. [Google Scholar]
- Curtis CE, Zald DH, Lee JT, Pardo JV( 2000): Object and spatial alternation tasks with minimal delays activate the right anterior hippocampus proper in humans. Neuroreport 11: 2203–2207. [DOI] [PubMed] [Google Scholar]
- Dade LA, Zatorre RJ, Evans AC, Jones‐Gotman M( 2001): Working memory in another dimension: functional imaging of human olfactory working memory. Neuroimage 14: 650–660. [DOI] [PubMed] [Google Scholar]
- Doty RL( 2000): The smell threshold test TM administration manual. Haddon Heights, NJ: Sensonics. [Google Scholar]
- Doty RL, Shaman P, Dann M( 1984): Development of the University of Pennsylvania smell identification test: a standardized microencapsulated test of olfactory function. Physiol Behav 32: 489–502. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM( 1991): The human brain‐surface, three dimensional sectional anatomy and MRI. Wien: Springer. [Google Scholar]
- Eichenbaum H, Morton TH, Potter H, Corkin S( 1983): Selective olfactory deficits in case H.M. Brain 106: 459–472. [DOI] [PubMed] [Google Scholar]
- Engen T( 1982): The perception of odors. Toronto: Academic Press. [Google Scholar]
- Eskenazi B, Cain WS, Novelly RA, Friend KB( 1983): Olfactory functioning in temporal lobectomy patients. Neuropsychologia 21: 365–374. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN( 2001): Frontal lobes and human memory: insights from functional neuroimaging. Brain 124: 849–881. [DOI] [PubMed] [Google Scholar]
- Gottfried JA, Winston JS, Dolan RJ( 2006): Dissociable codes of odor quality and odorant structure in human piriform cortex. Neuron 49: 467–479. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM( 1993): A semantically‐labeled magnitude scale of oral sensation with apparent ration properties. Chem Senses 18: 683–702. [Google Scholar]
- Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J( 1996): Evaluating the ‘labeled magnitude scale’ for measuring sensations of taste and smell. Chem Senses 21: 323–334. [DOI] [PubMed] [Google Scholar]
- Herz RS, Engen T( 1996): Odor memory: review and analysis. Psychol Bull Rev 3: 300–313. [DOI] [PubMed] [Google Scholar]
- Hinton PB, Henley TB( 1993): Cognitive and affective components of stimuli presented in thee modes. Bull Psychon Soc 31: 595–598. [Google Scholar]
- Jones FN, Roberts K, Holman EW( 1978): Similarity judgments and recognition memory for some common spices. Percept Psychophys 24: 2–6. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Mosnik DM, Doty RL, Dzemidzic M, Hutchins GD( 2003): Functional anatomy of human odor sensation, discrimination, and identification in health and aging. Neuropsychology 17: 482–495. [DOI] [PubMed] [Google Scholar]
- Kareken DA, Sabri M, Radnovich AJ, Claus E, Foresman B, Hector D, Hutchins GD( 2004): Olfactory system activation from sniffing: effects in piriform and orbitofrontal cortex. Neuroimage 22: 456–465. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Corrado G, Pietrini P, Grafman J( 2000): Dissociating the role of the medial and lateral anterior prefrontal cortex in human planning. Proc Natl Acad Sci U S A 97: 7651–7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless HT( 1997): Olfactory psychophysics In: Beauchamp GK, Bartoshuk L, editors. Tasting and smelling. San Diego: Academic Press; p 125–174. [Google Scholar]
- Lorig TS, Elmes DG, Zald DH, Pardo JV( 1999): A computer‐controlled olfactometer for fMRI and electrophysiological studies of olfaction. Behav Res Methods Instrum Comput 31: 370–375. [DOI] [PubMed] [Google Scholar]
- Martinez BA, Cain WS, de Wijk RA, Spencer DD, Novelly RA, Stass KJ( 1993): Olfactory functioning before and after temporal lobe resection for intractable seizures. Neuropsychology 7: 351–363. [Google Scholar]
- Moskowitz HR( 1974): Combination rules for judgments of odor quality difference. Agric Food Chem 22: 740–743. [Google Scholar]
- Petrides M( 1996): Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc Lond B Biol Sci 351: 1455–1461; discussion 1461–1462. [DOI] [PubMed] [Google Scholar]
- Petrides M( 2000): The role of the mid‐dorsolateral prefrontal cortex in working memory. Exp Brain Res 133: 44–54. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Frey S( 2002): Differential activation of the human orbital, mid‐ventrolateral, and mid‐dorsolateral prefrontal cortex during the processing of visual stimuli. Proc Natl Acad Sci U S A 99: 5649–5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia‐Larrea L( 2000): Functional imaging of brain responses to pain. A review and meta‐analysis. Neurophysiol Clin 30: 263–288. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I( 2002): Functional neuroanatomy of emotion: a meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Plailly J, Bensafi M, Pachot‐Clouard M, Delon‐Martin C, Kareken DA, Rouby C, Segebarth C, Royet JP( 2005): Involvement of right piriform cortex in olfactory familiarity judgments. Neuroimage 24: 1032–1041. [DOI] [PubMed] [Google Scholar]
- Plailly J, d'Amato T, Saoud M, Royet JP( 2006): Left temporo‐limbic and orbital dysfunction in schizophrenia during odor familiarity and hedonicity judgments. Neuroimage 29: 302–313. [DOI] [PubMed] [Google Scholar]
- Potter H, Butters N( 1980): An assessment of olfactory deficits in patients with damage to prefrontal cortex. Neuropsychologia 18: 621–628. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M( 2001): Medial temporal lobe activity associated with active maintenance of novel information. Neuron 31: 865–873. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun LS, Chen K( 1997): Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 154: 918–925. [DOI] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, Gervais R( 2000): Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci 20: 7752–7759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Hudry J, Zald DH, Godinot D, Gregoire MC, Lavenne F, Costes N, Holley A( 2001): Functional neuroanatomy of different olfactory judgments. Neuroimage 13: 506–519. [DOI] [PubMed] [Google Scholar]
- Royet JP, Plailly J, Delon‐Martin C, Kareken DA, Segebarth C( 2003): fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage 20: 713–728. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Larsson M, Roland P( 2000): Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26: 735–745. [DOI] [PubMed] [Google Scholar]
- Savic I, Gulyas B, Berglund H( 2002): Odorant differentiated pattern of cerebral activation: comparison of acetone and vanillin. Hum Brain Mapp 17: 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS, Robinson DE, Erickson RP( 1977): Multidimensional scaling of odorants: examination of psychological and physiochemical dimensions. Chem Sens Flavor 2: 375–390. [Google Scholar]
- Schwerdtfeger WK, Buhl EH, Germroth P( 1990): Disynaptic olfactory input to the hippocampus mediated by stellate cells in the entorhinal cortex. J Comp Neurol 292: 163–177. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD( 1998): Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature 392: 282–286. [DOI] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD( 2000): Time course of odorant‐induced activation in the human primary olfactory cortex. J Neurophysiol 83: 537–551. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE( 2004): The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME( 2001): Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus 11: 337–346. [DOI] [PubMed] [Google Scholar]
- Wise PM, Olsson MJ, Cain WS( 2000): Quantification of odor quality. Chem Senses 25: 429–443. [DOI] [PubMed] [Google Scholar]
- Witter MP, Van Hoesen GW, Amaral DG( 1989): Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci 9: 216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woskow MH( 1968): Multidimensional scaling of odors In: Tanyolaç N, editor. Theories of odor and odor measurement. Istanbul: Robert College; p 147–191. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD( 2005): Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci 25: 3002–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Jones‐Gotman M( 1991): Human olfactory discrimination after unilateral frontal or temporal lobectomy. Brain 114: 71–84. [PubMed] [Google Scholar]
- Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, Telles C, Khan R, Sobel N( 2005): Attentional modulation in human primary olfactory cortex. Nat Neurosci 8: 114–120. [DOI] [PubMed] [Google Scholar]


