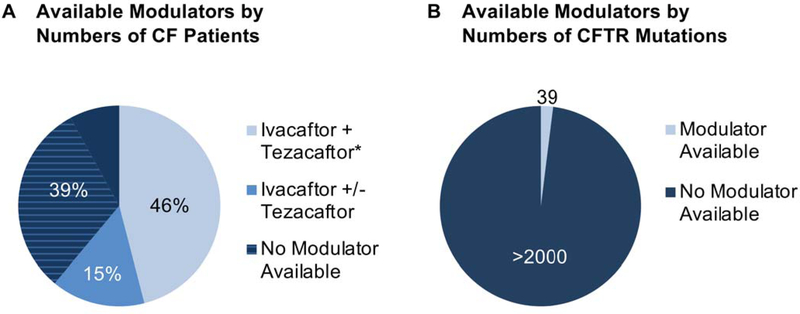
Figure 2.
(A) Subset of individuals with an approved modulator from among ~30,000 patients in the U.S. Approximately 15% of individuals with CF are approved for treatment using ivacaftor alone, with a subset of these (below) also approved for ivacaftor/tezacaftor. The (*) describes F508del/F508del genotype, and denotes approval for ivacaftor together with either lumacaftor or tezacaftor (46% of patients with CF). For another ~ 39% of patients, no modulator drugs are yet available. The majority of these individuals carry a copy of F508del and are anticipated to benefit from triple combination therapy (TCTs, hatched detail of diagram, see text). (B) Subset of CF variants with an approved modulator. Among > 2000 disease-associated mutations30, thirty-nine currently are “on label.” Ivacaftor as a single agent is available for the following mutations (~ 15% of patients): E56K, P67L, R74W, D110E, D110H, R117C, E193K, L206W, R347H, R352Q, A455E, D579G, E831X, S945L, S977F, F1052V, K1060T, A1067T, R1070W, F1074L, D1152H, D1270N, 711+3A->G, 2789+5G->A, 3272–26A->G, 3849+10kb C->T, G551D, R117H, G178R, S549N, S549R, G551S, G1069R, R1070Q, G1244E, S1251N, S1255P, and G1349D. Combination treatment with ivacaftor and tezacaftor is also approved when any of the first 26 ivacaftor-responsive variants from the list above is present at either CFTR locus, or for individuals homozygous with F508del. Additional studies will be required to estimate numbers of patients with CF who encode variants not formally approved for modulator treatment, but likely to show clinical benefit following drug administration.