Abstract
The brain mechanisms mediating visuospatial attention were investigated by recording event‐related potentials (ERPs) during a line‐orientation discrimination task. Nonpredictive peripheral cues were used to direct participant's attention involuntarily to a spatial location. The earliest attentional modulation was observed in the P1 component (peak latency about 130 ms), with the valid trials eliciting larger P1 than invalid trials. Moreover, the attentional modulations on both the amplitude and latency of the P1 and N1 components had a different pattern as compared to previous studies with voluntary attention tasks. In contrast, the earliest visual ERP component, C1 (peak latency about 80 ms), was not modulated by attention. Low‐resolution brain electromagnetic tomography (LORETA) showed that the earliest attentional modulation occurred in extrastriate cortex (middle occipital gyrus, BA 19) but not in the primary visual cortex. Later attention‐related reactivations in the primary visual cortex were found at about 110 ms after stimulus onset. The results suggest that involuntary as well as voluntary attention modulates visual processing at the level of extrastriate cortex; however, at least some different processes are involved by involuntary attention compared to voluntary attention. In addition, the possible feedback from higher visual cortex to the primary visual cortex is faster and occurs earlier in involuntary relative to voluntary attention task. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: involuntary attention, event‐related potentials (ERPs), peripheral cues, V1, striate cortex, low‐resolution brain electromagnetic tomography (LORETA)
INTRODUCTION
Attention heightens perception. Covert attention, both voluntary and involuntary, has been shown to enhance the electrical response of cortical neurons to sensory input. Such cortical amplification may underlie the greater perceptual sensitivity to target detection and identification at a precued location as shown in psychophysical studies [Hawkins et al.,1990]. However, where in the visual processing stream this attentional enhancement effect occurs continues to be an active area of research. Studies in humans indicate that attention does not alter the earliest cortical response to stimulation, i.e., in striate cortex [Clark and Hillyard,1996; Gomez Gonzalez et al.,1994]. However, both single unit recording in animals [Motter,1993] and neuroimaging studies in humans [Gandhi et al.,1999; Martinez et al.,1999] have indicated that attention can modulate neural activity in striate cortex, particularly in the presence of distractors [Gilbert et al.,2000]. As neuroimaging methods used in these studies have low temporal resolution, however, the issue of when involuntary attention affects neural activity was not addressed. In order to obtain a fuller understanding of the time course of the effect of involuntary spatial attention on striate and extrastriate cortex activation, the present study capitalizes on the high temporal resolution provided by event‐related potentials (ERPs).
Covert attention can be allocated in either a voluntary or an involuntary manner. Attention is allocated voluntarily by attending to a spatial location in a sustained manner in response to instructions [Hillyard and Muente,1984], or in response to a central, symbolic cue in a trial‐by‐trial manner [Mueller and Rabbitt,1989]. Attention is allocated involuntarily, for example, when abrupt peripherally located stimulus onsets attract attention automatically [Yantis and Jonides,1990]. Although there are debates about whether voluntary and involuntary attention involve similar or different mechanisms [Briand and Klein,1987; Warner et al.,1990], it is generally agreed that involuntary attention as elicited by sudden‐onset peripheral cues is exogenous, automatic, and reflexive—hence faster than endogenous, controlled voluntary attention [Cheal and Lyon,1991a,b; Eimer,2000; Mangun,1995].
ERP studies using voluntary attention tasks (e.g., sustained attention and central cueing paradigms) have consistently reported that stimuli at an attended location elicited enlarged P1 (70–130 ms) and/or N1 (150–200 ms) components at posterior recording sites contralateral to stimulus location [Anllo‐Vento and Hillyard,1996; Bruin et al.,1998; Clark and Hillyard,1996; Eimer,1994a; Fu et al.,2000; Handy and Mangun,2000; Hillyard and Muente,1984; Hillyard and Anllo‐Vento,1998; Luck et al.,1994; Mangun,1995; Mangun and Hillyard,1990; Wijers et al.,1997]. By way of explanation, a sensory gain control mechanism has been proposed, according to which attention amplifies the processing of visual information by increasing the signal/noise ratio at the attended location at an early processing stage, so that the relevant information can be extracted from the stimulus display for further processing [for a review, see Hillyard et al.,1998]. This is supported by ERP evidence that the P1/N1 enhancement for attended stimuli is associated with faster responses or increased sensitivity to the targets [Eimer,1994a; Hawkins et al.,1990; Luck et al.,1994], and that the P1 amplitude enhancement over the posterior region from attention was observed without a change in latency or scalp voltage distribution [e.g., Anllo‐Vento and Hillyard,1996; Wijers et al.,1997; however, see Clark and Hillyard,1996], suggesting that the same, rather than different populations were affected by attention. It has been further observed that this attentional modulation occurred in extrastriate cortex, with only slight location shifts attributed to stimuli and task differences [Corbetta et al.,1993; Gomez et al.,1994; Heinze et al.,1994; Clark et al.,1995; Clark and Hillyard,1996; Mangun et al.,1997,2001; Woldorff et al.,1997].
In contrast to this large literature on the effects of voluntary attention on the ERP response to stimulus input, a few studies have used involuntary attention tasks [Anllo‐Vento,1995; Eimer,1994b,2000; Fu et al.,2001,2004; Hillyard et al.,1994; Hopfinger and Mangun,1998; Van de Lubbe et al.,1997]. An operational definition of involuntary attention task might be that the task involves a peripheral cueing paradigm and has short cue‐to‐stimulus onset asynchrony (SOA), so that attention is automatically attracted to the cued location, and the slower voluntary attention process does not have enough time to take place. The results obtained with peripheral cueing tasks vary [e.g., Hillyard et al.,1994; Eimer,1994b, for long cue‐to‐stimulus SOA; Anllo‐Vento,1995; Fu et al.,2001, for short cue‐to‐stimulus SOA], but a general conclusion is that the earliest attention modulation involves the P1 component, which is thought to reflect extrastriate cortical activity [Hopfinger and Mangun,1998; Van de Lubbe and Woestenburg,1997].
The ERP evidence from voluntary attention tasks to date suggests that P1 is the earliest component to be modulated by attention; in contrast, C1 (peak latency 60–100 ms), the first visual ERP component elicited at posterior sites, has not been shown to be affected by spatial attention in a variety of tasks [Anllo‐Vento and Hillyard,1996; Clark and Hillyard,1996; Gomez et al.,1994; Heinze et al.,1994; Luck et al.,1994; Mangun et al.,2001; Wijers et al.,1997]. Studies combining ERP and PET measures [Heinze et al.,1994; Mangun et al.,1997; Woldorff et al.,1997], ERP and fMRI [Martinez et al.,1999; Di Russo et al.,2003], and ERP, ERMF, and fMRI in humans [Noesselt et al.,2002] have also found that the initial visual processing in primary visual cortex is not modulated by spatial attention. ERP dipole modeling studies have suggested primary visual cortex to be the neural generator of the C1 component [Aine et al.,1996; Clark et al.,1995; Clark and Hillyard,1996; Di Russo et al.,2001]. These results, along with neurophysiological evidence from monkeys [Luck et al.,1997; Moran and Desimone1985], suggest that V1 acts as a passive information receiver and is not subject to attentional control in the initial stages of visual processing.
However, it has been suggested that attention may affect evoked activity in primary visual cortex in monkeys when targets have to be selected under a “cluttered field” conditions [Motter,1993]. Recently, several monkey studies have found that neural activity in primary visual cortex may be modulated by attention in discrimination tasks with competing stimuli under such cluttered conditions [Gilbert et al.,2000; Ito and Gilbert,1999; McAdams and Maunsell,1999; Roelfsema et al.,1998; Vidyasagar,1998]. A number of recent imaging studies in humans have also shown attentional modulation in primary visual cortex [Brefczynski and DeYoe,1999; Gandhi et al.,1999; Martinez et al.,1999; Somers et al.,1999; Tootell et al.,1998; however, see Heinze et al.,1994], and even in the lateral geniculate nucleus [LGN, O'Connor et al.,2002].
Two hypotheses have been offered to account for this attentional modulation in the primary visual cortex. According to Luck's [1997] baseline increase hypothesis, enhancement of neural activity observed with single unit recording or fMRI might result from a top‐down bias to attended stimuli, so that there is an overall bias‐related increase in neural activity to attended stimuli relative to unattended stimuli but without modulation of the specific stimulus‐elicited response. fMRI studies in human did observe such attention effects of a baseline increase [Chawla et al.,1999; Kastner et al.,1999]. However, ERP studies are immune to baseline increases [Somers et al.,1999], and some single‐unit and ERP studies observed no such baseline increase for attended stimuli [McAdams and Maunsell,1999; Mehta et al.,2000a].
A second explanation for the attention‐enhanced activation in the primary visual cortex is the reentrant‐feedback hypothesis, which attributes enhanced V1 activity by attention to feedback from higher visual cortex onto V1 [Di Russo et al.,2003; Martinez et al.,1999,2001b; Mehta et al.,2000a,b; Noesselt et al.,2002]. The role of such feedback may be to reduce neural refractoriness and enhance the perceptual salience of attended stimuli [Mehta et al.,2000a,b], or to enhance the figure‐ground contrast and the salience of attended stimuli [Lamme and Spekreijse,2000; Super et al.,2001]. For example, by using intracortical ERP recordings in monkeys, Mehta et al. [2000a,b] found attentional modulation in V1 which was later than the attentional modulation in V4, and the laminar distribution of this attentional effect strongly suggested a feedback mechanism from higher visual cortex (V4) to lower cortex (V1). Several recent studies on humans also support this feedback hypothesis [Noesselt et al.,2002; Martinez et al.,2001b; Di Russo et al.,2003]. Noesselt et al. [2002] found that the initial response at 60–90 ms localized to primary visual cortex was unaffected by attention; however, later activity in the 150–250 ms range with the same dipole localization as the initial C1 component was strongly modulated by visuospatial attention [see also Martinez et al.,2001b]. In addition to the dipole modeling results, Di Russo et al. [2003] further confirmed that this later attentional effect originated in the primary visual cortex by demonstrating inverted polarity of the C1 component for upper vs. lower stimuli—a hallmark feature of the C1 component which reflects the retino‐topical structure of primary visual cortex [Aine et al.,1996; Clark et al.,1995; Di Russo et al.,2001; Mangun,1995]. Considered together, these results strongly suggest that the enhanced activity in V1 is due to delayed feedback from higher visual cortex.
There are other reasons why early attentional modulation has not been previously observed in C1 (localized to V1). V1 activity might not be time‐locked to the attended stimuli so it does not appear in averaged ERP waveforms, or the V1 activity might occur in stellate neurons that do not produce far‐field electrical or magnetic signals [Hillyard et al.,2004]. It is also possible that the stimuli, tasks, and paradigms used in previous ERP studies were not optimal for eliciting attentional modulation in the primary visual cortex. For example, some studies have used higher‐order discrimination tasks, such as letter discrimination [Handy and Mangun,2000] and letter orientation discrimination tasks [Martinez et al.,2001b; Noesselt et al.,2002; Olson et al.,2001], which might have biased the processing in the ventral stream of the visual cortex [Mangun et al.,2001]. Low‐level stimuli, such as spatial frequency [Clark et al.,1995; Fu et al.,2001], size [Di Russo et al.,2003; Mangun et al.,2001] or height [Hopfinger and Mangun,1998], and luminance discrimination tasks [Mehta et al.,2000a,b] have been used in previous studies, but without the presence of distracting stimuli which may be necessary for observing attentional effects on C1. Moreover, most previous ERP studies looking at C1 modulation used central cueing or a sustained attention task [e.g., Di Russo et al.,2003; Martinez et al.,2001b, Noesselt et al.,2002]. While behavioral work has shown that involuntary attention is faster and more automatic relative to voluntary attention, little electrophysiological work has addressed this issue.
To date, no study has investigated the possibility that reentrant feedback can affect neural activity associated with involuntary attention. Based on our previous work with involuntary attention tasks [Fu et al.,2001], the present study sought to understand the role of attentional modulation of primary visual cortex in visuospatial attention. It was hypothesized that feedback from higher visual cortex to V1 was faster and occurred earlier when attention was allocated to the stimulus location in an automatic and reflexive manner. To test this hypothesis, a line orientation discrimination task selected to activate V1 [Hubel et al.,1977] was presented together with a “cluttered field” stimulus array in order to enhance the neural activity for the stimuli at the attended location [e.g., Motter,1993]. Use of peripheral cueing with a perceptual task allows assessment of the potentially earliest attentional modulation in the visual processing pathway.
SUBJECTS AND METHODS
Participants
Thirteen healthy participants (seven male, six female) took part as paid volunteers. One male participant's data were excluded from analyses because of strong alpha waves. The participants were between 18 and 22 years of age (mean age 21.2 years), right‐handed, and had normal or corrected‐to‐normal vision. They reported no history of neurological illness. Informed consent was obtained from all participants.
Stimuli
A speeded line discrimination task was required. A fixation cross (0.5° × 0.5°) was presented at the center of the monitor (black on white) throughout the entire block. Each stimulus array (3.06° × 3.44°, Fig. 1) appeared randomly in the LVF (left visual field) or RVF (right visual field), with its center 6.02° off and 2.63° above the fixation cross. Each stimulus array consisted of two horizontal lines, one vertical line, and one diagonal line, with one line in each quadrant and the two horizontal lines diagonally displayed. The diagonal line could be backward (“\”) or forward (“/”) with equal probability, and it could appear at any of the four quadrants with equal probability. Prior to the presentation of stimulus, a peripheral cue consisting of four small dots (0.24° × 0.24° each, Fig. 1) was flashed randomly in the left or right visual field. The cued region was defined by dots to minimize the potential cue‐stimulus array interaction. The cue could appear with equal likelihood at the same location as the stimulus or at an analogous location in the opposite visual field, i.e., the cue was nonpredictive of stimulus location. On one‐third of trials, the peripheral cue was presented, followed by a blank screen (cue‐only trial or blank trial). The durations of the cue and stimuli were 50 and 100 ms, respectively. The cue‐to‐stimulus SOA was fixed at 150 ms and the intertrial interval (ITI) ranged randomly between 1,200 and 1,600 ms.
Figure 1.
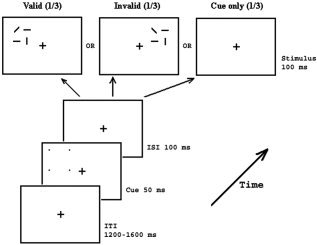
Schematic illustration of the procedure of the present study. Each cue consisted of four small dots in the corner of a virtual square and was presented for 50 ms prior to the stimuli. Each stimulus consisted of two horizontal, one vertical, and one diagonal line and was presented for 100 ms. Cue‐to‐stimulus SOA was fixed at 150 ms. The cue predicted the location of the stimulus in 50% of trials. Participants' task was to respond to the orientation of the only diagonal line in the stimulus display (“/” vs. “\”). 20% of trials are cue‐only trials without presentation of the stimulus array.
Procedure
Participants were required to fixate the cross and minimize eye blinks and body motion during all the experimental blocks. They were instructed to discriminate the orientation of the only diagonal line in the stimulus display, and to respond to the backward line (\) with their right thumb and forward line (/) with their left thumb using a NeuroScan (El Paso, TX) Stimpad. Response accuracy and speed were emphasized equally. In total there were 1,152 trials separated into 29 blocks (40 trials for the first 28 blocks and 32 trials for the last block), leading the theoretical number of trials per condition. Short breaks were allowed between blocks.
EEG Recording
STIM and SCAN software packages (NeuroScan) were used to present stimuli, record, and analyze EEG. Thirty‐two channels of EEG and EOG were recorded from the scalp with an electrode cap. Standard 10–20 sites were FP1, FP2, FZ, F3, F4, F7, F8, CZ, C3, C4, PZ, P3, P4, O1, O2, T3, T4, T5, and T6. EEG from the left mastoid was also recorded, with the right mastoid serving as reference. Additional electrodes were CP1/CP2 (halfway between PZ and C3/C4), OL/OR (halfway between O1/O2 and T5/T6), OZ (occipital midline electrode, at the 2/3 location to PZ on the PZ–inion line), PO1/PO2 (halfway between OZ and PZ‐P3/PZ‐P4), and TP7/TP8 (halfway between T5/T6 and T3/T4). Horizontal eye movements (HEOG) were monitored by placing two electrodes lateral to the left and right orbits. Vertical eye movements (VEOG) and eye blinks were measured by placing two electrodes 1.5 cm below and above the left eye. The EEG from each electrode site was digitized at 500 Hz and was filtered with a bandpass of 0.1 to 40 Hz. A 200‐ms prestimulus epoch of EEG (relative to the onset of the cue) was used as baseline.
Data Analysis
Prior to averaging the EEG, artifact rejection was performed to discard epochs contaminated by eye blinks, body movements, and muscle activity. The rejection criterion was a negative or positive change of more than 75 μV. Both ERPs to cue‐plus‐stimulus trials and cue‐only trials were averaged from onset of the cue.
The advantage of using fixed and short cue‐stimulus SOA is that the potentially small attentional effects in striate and extrastriate cortex might be time‐locked and consistent across trials, and therefore can be detected easily. These might not be seen if a variable SOA was used, since early attentional effects are sensitive to SOA [Cheal and Lyon,1991a,b]. The disadvantage of this method is the ERP overlap which then exists between the cue and the stimulus. To remove the ERP overlap between the cue and the stimulus, a subtraction procedure [Greenwood and Goff,1987; Iragui et al.,1993; Kiss et al.,1998; Simson et al.,1985] was carried out in the present study. For stimuli in the LVF, the ERPs for valid trials were obtained by subtracting ERPs of cue‐only trials in LVF from ERPs of cue‐plus‐stimulus trials when they both appeared in LVF, whereas the ERPs for invalid trials were obtained by subtracting ERPs of the cue‐only trial in RVF from ERPs of cue‐plus‐stimulus trials when the cue appeared in RVF and the stimulus appeared in LVF. With the same rationale, the ERPs of valid trials in RVF were obtained by subtracting ERPs of the cue‐only trials in RVF from ERPs of the cue‐plus‐stimulus trials when both the cue and stimulus appeared in RVF, whereas the ERPs of invalid trials in RVF were obtained by subtracting ERPs of the cue‐only trials in LVF from ERPs of the cue‐plus‐stimulus trials when the cue appeared in LVF and the stimulus appeared in RVF. The peak amplitudes and latencies of the difference waves obtained after subtraction were used for statistical analyses.
Behavioral data were analyzed by means of repeated measures analysis of variance (ANOVA) on the two within factors: cue validity (valid or invalid) an visual field (left or right). Electrophysiological data were also analyzed with a repeated measure ANOVA with the hemisphere (left or right) factor included as a factor. Peak latency of ERP components elicited by the stimulus array was recalculated based on the 150 ms fixed cue‐to‐stimulus SOA. In order to minimize type 1 error, only the electrodes with largest ERP component of interest were used for statistic analysis. Low‐resolution brain electromagnetic tomography (LORETA) [Pascual‐Marqui et al.,1994; Pascual‐Marqui,1999] was used to localize the activation areas related to attentional modulation. The ERPs were filtered using a bandpass of 1.5–30 Hz for LORETA analysis. The LORETA transform matrix was based on 10–20 electrode coordinate system, and the user‐defined over‐smoothness value was set to 1.0 e‐4. Voxel‐wise LORETA (text format) comparisons (paired samples) between the valid and invalid conditions were performed for the early activations in the striate cortex, with “no normalization” and “log‐transform data” setting. All the t and P values of the LORETA comparison were corrected to avoid type I error of multiple comparison. Talairach coordinates of the highest activation was reported according to Talairach and Tournoux [1988] and Talairach Daemon by Lancaster et al. [2000].
RESULTS
Behavioral Measures
The mean error rate was 4.3%. Participants responded faster to valid relative to invalid trials (552 ± 18 ms vs. 570 ± 17 ms, respectively; F(1,11) = 26.03, P < 0.0005). No other main effect or interactions were significant.
ERP Measures
Figure 2a shows the grand average ERPs for valid and invalid cue‐plus‐stimulus trials when the stimuli appeared in the LVF. Note that time zero corresponds to cue onset, with stimulus onset 150 ms later. Because the ERPs for valid and invalid trials overlap with the ERPs elicited by the cues due to the fixed cue‐to‐stimulus SOA, a subtraction procedure was applied to remove the overlap from the cues before comparing ERPs for valid and invalid trials (see Data Analysis for details of subtraction procedure). Figure 2b shows the grand average ERPs for cue‐plus‐stimulus and cue‐only trials when cue and array appeared in the LVF. Difference waves that were obtained by subtracting ERPs for cue‐only trials from cue‐plus‐stimulus trials in Figure 2b are the ERPs for valid trials in LVF.
Figure 2.
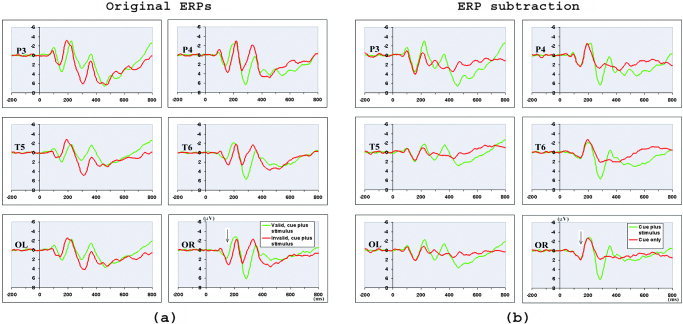
a: Grand average ERPs across 12 participants for valid and invalid cue‐plus‐stimulus trials when the stimuli appeared in the LVF. Note that time zero is the onset of the cues, with the onset of the stimuli (marked by an arrow) 150 ms after cue onset. The ERP to the stimulus was overlapped by the ERP to the cue because of fixed and short cue‐to‐stimulus SOA. b: The ERPs for LVF‐cue‐plus‐LVF‐stimulus trials and LVF‐cue‐only trials. The difference waves obtained by subtracting the ERPs of left‐cue‐only trials from ERPs of left‐cue‐plus‐left‐stimulus trials were the ERPs of valid stimuli in LVF. Similar subtraction procedure was applied to obtain ERPs of invalid trials in LVF, and valid and invalid trials in RVF. See text for details of the subtraction procedure.
The ERPs for valid and invalid trials in LVF and RVF following the subtraction procedure are shown in Figure 3. The success of this subtraction procedure is demonstrated by the relatively flat ERPs before the onset of the stimulus array (150 ms after cue onset, marked by an arrow). It is clear that both valid and invalid trials elicited a C1 (peak latency at about 80 ms), P1 (peak latency at about 130 ms), N1 (peak latency at about 190 ms), and a later P3 component. It is also evident that visuospatial attention modulated stimulus processing in the early stage of processing, as shown by the ERP difference in the P1 and N1 components between the valid and invalid trials.
Figure 3.
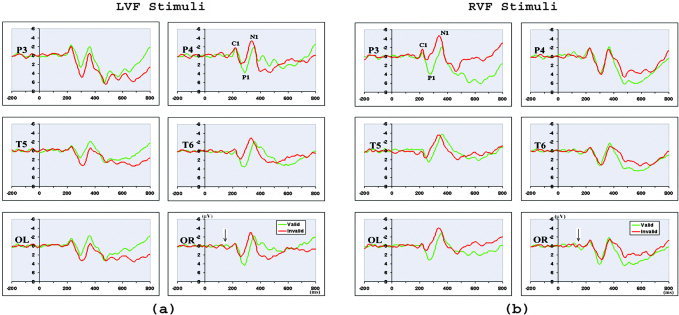
The ERPs of valid and invalid stimuli in LVF (a) and RVF (b) after ERP subtraction. Time zero is the onsets of the cues, and 150 ms is the onsets of the stimuli (marked by an arrow). Attentional modulation on the P1 and N1 components was consistently observed at posterior sites (P3/P4, OL/OR, T5/T6) contralateral to stimulus side.
The earliest attentional modulation was seen in both the amplitude and latency of the contralateral P1 component (Fig. 3). Valid trials elicited a larger contralateral P1 compared to invalid trials, revealed by a significant validity × visual field × hemisphere interaction (P3/P4: F(1,11) = 15.552, P < 0.002)) (Fig. 4a). No other main effects or interactions were significant. The peak latency analysis showed that the contralateral P1 was later for valid than for invalid trials, as suggested by a significant validity main effect (P3/P4: F(1,11) = 6.962, P < 0.023)) and validity × visual field × hemisphere interaction (P3/P4: F(1,11) = 10.25, P < 0.008)) (Fig. 4b). In contrast, the earliest visual ERP component, C1 (peak latency 80 ms), was not modulated by attention at either lateral electrodes or midline electrodes (P3/P4: F(1,11) = 2.342, P < 0.154; Pz: (F(1,11) = 0.534, P < 0.480) (Fig. 3).
Figure 4.
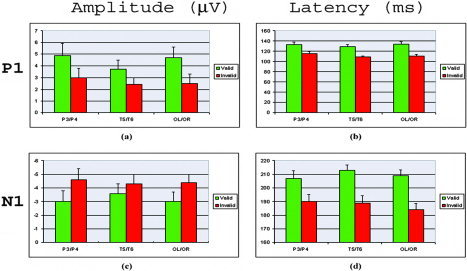
The mean amplitude and latency of the contralateral P1 and N1 components for valid and invalid trials at posterior sites (P3/P4, T5/T6, and OL/OR). Data were averaged across visual field and hemisphere. Valid trials elicited a larger and later contralateral P1, and a smaller and later contralateral N1 than invalid trials.
The amplitude and latency of the contralateral N1 component was also modulated by attention (Fig. 3). Valid trials elicited smaller posterior contralateral N1 relative to invalid trials, as suggested by significant validity × visual field × hemisphere interaction (P3/P4: F(1,11) = 10.48, P < 0.008) (Fig. 4c). The peak latency analysis of posterior N1 showed that valid trials elicited later contralateral N1 relative to invalid trials (F(1,11) = 12.96, P < 0.004) (Fig. 4d). In addition, valid trials elicited larger anterior N1 than invalid trials (C3/C4: F(1,11) = 46.759, P < 0.0005).
LORETA Results
LORETA is a distributed, linear 3D solution to the ERP inverse problem, which yields authentic but “blurred” 3D point sources at the activity location with certain dispersions [Pascual‐Marqui,1999]. Figure 5 shows the LORETA localization results of the C1 component (peak latency 80 ms) for valid and invalid trials when they appeared in LVF (5a) and RVF (5b). Surprisingly, the strongest activation was found at the posterior cingulate gyrus (4, –67, 15, BA 31) for valid LVF stimuli, and at the cuneus (–3, 81, 18, BA 18) for the valid RVF stimulus. However, strong activation in the primary visual cortex (around the calcarine fissure) was also found for valid and invalid stimuli in both the left and right visual fields (Fig. 5, sagittal view of the brain in right‐most panels). The activation strength was comparable and showed no significant difference (all P > 0.05) between valid and invalid trials in the primary cortex by voxel‐wise LORETA comparisons, suggesting that the initial sensory response which peaked at about 80 ms was not modulated by attention.
Figure 5.
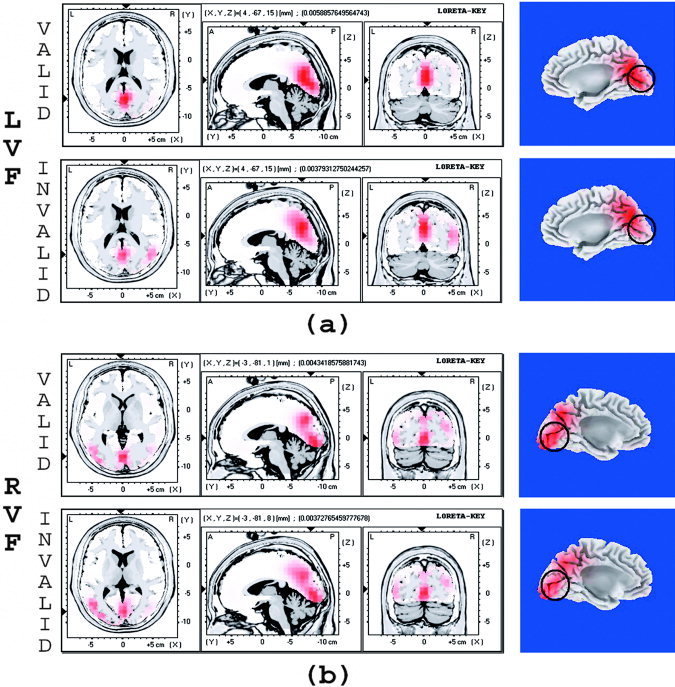
The LORETA localization of the C1 component in response to valid and invalid stimulus in LVF (a) and RVF (b). The activations were compared between valid and invalid trials at the location where strongest activation was found for valid trials. Activation in primary visual cortex (around the calcarine fissure, marked by black circles) was observed in the sagittal view of the brain, and the activation strength was comparable between valid and invalid stimuli for both LVF and RVF stimulus.
Figure 6a shows the attention‐related ERP difference waves obtained by subtracting ERPs of invalid trials from ERPs of valid trials in the LVF (left panel) and RVF (right panel), respectively. A P140 attention‐related difference wave was observed on the sites contralateral to stimulus location. This contralateral distribution of the P140 attention‐related difference wave is shown clearly on the 2D scalp voltage maps in Figure 6a. Figure 6b shows the largest activations observed for attention‐related ERPs averaged from 106–166 ms after stimulus onset. The strongest activation was found in the middle occipital gyrus (46, –67, 8, BA 19/39, for LVF stimuli; and –52, –68, 8, BA 19/39 for RVF stimuli) contralateral to stimulus location. Figure 6c shows the 3D surface view of these attention‐related activations. Again, strong activations in the contralateral middle occipital gyrus were observed in the hemisphere contralateral to stimulus location. Table I shows the brain areas activated by attention during 106–166 ms. The largest attention‐related activations were observed in the occipital (middle occipital gyrus and lingual gyrus) and parietal areas (precuneus/cuneus and inferior parietal lobule).
Figure 6.
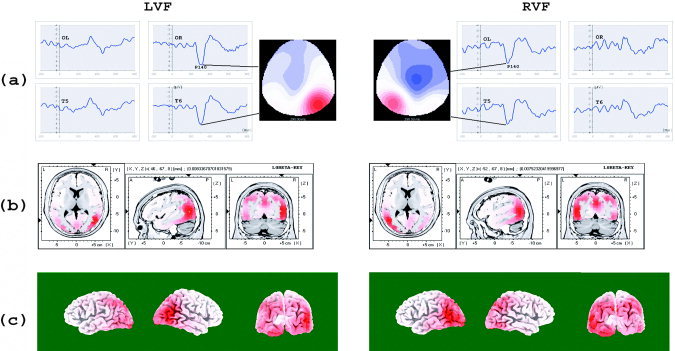
a: The attention‐related waves obtained by subtracting ERPs of invalid trials from ERPs of valid trials in LVF (left column) and RVF (right column). Time zero is the onset of the cue, and 150 ms is the onset of the stimuli. There was a positive going component which peaked at about 140 ms (P140) on the posterior side contralateral to stimulus location. This contralateral distribution of P140 is shown on the scalp voltage distribution maps aside the difference waveforms (color scale, –5 to 5 μV for both LVF and RVF stimuli). b: The strongest attention‐related activation found by LORETA for LVF and RVF stimuli. c: Surface view of the attention‐related brain activations for LVF and RVF stimuli. See Table I for the brain areas activated by visuospatial attention. ERP data were averaged from 106 to 166 ms for (b) and (c).
Table I.
Attention‐related brain activations for LVF and RVF stimuli
| Coordinates | Activation value | Brodmann area | |||
|---|---|---|---|---|---|
| x | y | z | |||
| LVF | |||||
| 46 | −67 | 8 | 8.336709E‐0003 | BA 19, R middle occipital gyrus | |
| −10 | −95 | −13 | 7.290534E‐0003 | BA 17, L lingual gyrus | |
| −3 | −74 | 36 | 6.440516E‐0003 | BA 7, L precuneus | |
| −24 | −88 | 29 | 5.688578E‐0003 | BA 19, L cuneus | |
| −45 | −67 | 43 | 5.427034E‐0003 | BA 40, L inferior parietal lobule | |
| 32 | −88 | 1 | 5.361648E‐0003 | BA 18, R middle occipital gyrus | |
| 32 | −74 | 43 | 5.263569E‐0003 | BA 19, R precuneus | |
| RVF | |||||
| −52 | −67 | 8 | 7.523204E‐0003 | BA 19/39, L middle occipital gyrus | |
| −3 | −74 | 36 | 7.169171E‐0003 | BA 7, L precuneus | |
| −10 | −95 | −13 | 6.815138E‐0003 | BA 17, L lingual gyrus | |
| −38 | −88 | 1 | 6.431602E‐0003 | BA 18, L middle occipital gyrus | |
| 32 | −74 | 43 | 6.107071E‐0003 | BA 19, R precuneus | |
| −24 | −81 | 36 | 5.340000E‐0003 | BA 19, L precuneus | |
| 53 | −60 | 36 | 5.133480E‐0003 | BA 40, R inferior parietal lobule | |
| 39 | −53 | 50 | 5.133480E‐0003 | BA 40, R inferior parietal lobule | |
| 46 | −60 | −13 | 5.103978E‐0003 | BA 37, R fusiform gyrus | |
LVF, left visual field; RVF, right visual field.
Figure 7 shows the time course of the attention‐related brain activations for LVF (7a) and RVF stimuli (7b), starting from 102 ms to 124 ms every 8 ms. The coordinates are shown (by the arrowheads) where the highest activations occurred. Activations were strongest in the extrastriate cortex at about 124 ms (P1 time range) for both LVF (BA 19/39, 46, –67, 15, middle occipital/temporal gyrus) and RVF (BA 19, –52, –67, 8, middle occipital gyrus) stimuli, in the hemisphere contralateral to stimulus visual field. In addition, attention‐related activation in the primary visual cortex (–10, –95, –13, BA 17) was observed at 102 ms for LVF stimuli (Fig. 7a, 102 ms) and at 110 ms for RVF stimuli (Fig. 7b, 110 ms), later than the initial response, which had a peak latency at about 80 ms. Interestingly, this apparent reactivation in the primary visual cortex was more pronounced in the left hemisphere for both LVF and RVF stimuli, i.e., no contralateral activation pattern similar to the attentional modulations in the P1 and N1 time range was observed for this reactivation in the primary visual cortex. The timing and the localization of this reactivation in the primary visual cortex in the present study suggests that a feedback mechanism from the higher visual cortex to primary visual cortex might have been involved.
Figure 7.
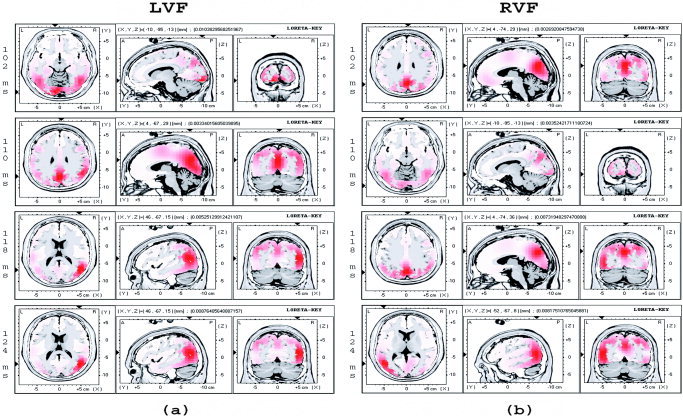
Time course of the attention‐related brain activations from 102 ms to 124 ms every 8 ms when the stimulus appeared in LVF (a) and RVF (b). Activations in the contralateral middle occipital/temporal area (47, –67, 15, BA 19 for LVF stimuli; and –52, –68, 8, BA 19/39 for RVF stimuli) were observed at 124 ms after stimulus onset. Activations in the primary visual cortex (–10, –95, –13, BA 17) were observed at 102 ms (LVF) and 110 ms (RVF) after stimulus onset, along with activations in other visual cortex.
DISCUSSION
The present study provides evidence that involuntary visuospatial attention modulates the electrophysiological response of striate cortex to “cluttered stimuli,” possibly by means of reentrant feedback from other higher visual cortex to primary visual cortex. As such, the present results are consistent with previous research—which mostly examined voluntary or sustained spatial attention [Martinez et al.,1999,2001b; Noesselt et al.,2002; Di Russo et al.,2003]—suggesting the importance of attention even in the early stages of object recognition.
The earliest attentional modulation of the P1 component by peripheral cues peaked between 110–140 ms after stimulus onset, with contralateral P1 being larger on valid relative to invalid trials at posterior sites. Attentional modulation on the amplitude of contralateral N1 was also observed, with the invalid trials eliciting larger contralateral N1 than valid trials. In addition to the amplitude modulation, the peak latency of the contralateral P1 and N1 was earlier on invalid than valid trials. In contrast, the C1 component, the earliest ERP response to visual stimulus, peaked at about 80 ms after stimulus onset and was not modulated by attention.
We chose to use LORETA in the present study, rather than dipole modeling, such as BESA [e.g., Di Russo et al.,2003; Martinez et al.,2001b]. Distributed solution (such as LORETA) and dipole modeling solutions (such as BESA) are two methods among the infinite solutions of the ERP inverse problem. While dipole modeling is a good method for investigating small electrically activated neural areas, LORETA has the advantage of a distributed solution when large brain areas are involved [Pourtois et al.,2004]. LORETA results suggested that the strongest attention modulation occurred in the middle occipital area at about 140 ms after stimulus onset. No primary visual cortex activation in response to attentional modulation was observed at the initial sensory processing stage (peaked at 80 ms); however, attention‐related activation in the primary visual cortex was observed at a later processing stage, starting at about 110 ms after stimulus onset, indicating the existence of a feedback mechanism.
Previous ERP studies using voluntary [Anllo‐Vento and Hillyard,1996; Anllo‐Vento et al.,1998; Clark and Hillyard,1996; Handy and Mangun,2000; Hillyard and Muente,1984; Luck et al.,1994; Mangun,1995; Mangun and Hillyard,1990; Wijers et al.,1997] and involuntary attention tasks [Hopfinger and Mangun,1998; Fu et al.,2001; Van de Lubbe,1997] have similarly found no C1 amplitude change as a function of attention, suggesting that the early sensory response to visual stimuli in primary visual cortex was not modulated by attention. ERP localization and imaging studies of voluntary attention have found that the earliest attentional modulation of P1 can be localized to extrastriate cortex with slight variation in the exact source observed [Heinze et al.,1994; Mangun et al.,1997; Woldorff et al.,1997]. Consistent with these previous studies, the present study did not observe attentional modulation in the C1 component peaking at about 80 ms after stimulus onset. The source for this initial response was localized by LORETA to the brain areas around the calcarine fissure (primary visual cortex) with comparable activation strength between valid and invalid trials for both LVF and RVF stimuli. This suggests that the initial sensory response to visual stimuli in the primary visual cortex is not modulated by visuospatial attention under present experimental manipulations.
Nevertheless, the present results cannot exclude the possibility of attentional modulation in the primary visual cortex under certain experimental conditions. Future studies should use stimuli and tasks selected to reveal the full power of visual attention [Desimone and Duncan,1995]. For example, the locus of selective attention is sensitive to the perceptual load of the stimuli and early selection can only occur when the perceptual load of the stimuli is high [Lavie, 1994; Lavie and Tsal, 1995]. This hypothesis has been tested in an ERP study using voluntary attention task [Handy and Mangun,2000], and we have confirmed Lavie's hypothesis using involuntary attention task [Fu et al.,2004].
The earliest evidence of attentional modulation we observed was in the P1 component. This is consistent with previous results obtained from both voluntary and involuntary attention tasks [Anllo‐Vento and Hillyard,1996; Bruin et al.,1998; Clark and Hillyard,1996; Eimer,1994a; Fu et al.,2001; Handy and Mangun,2000; Heinze et al.,1994; Hopfinger and Mangun,1998; Mangun,1995; Wijers et al.,1997]. This suggests that both involuntary and voluntary visuospatial attention modulate visual stimulus processing early in processing. However, latency as well as amplitude of P1 was modulated by attention in the present study, with the invalid trials eliciting earlier P1 than valid trials; attentional modulations on the N1 component in the present study also showed that invalid trials elicited earlier and larger contralateral N1 than valid trials. Therefore, the present results of attentional modulation on both the amplitude and latency on P1 and N1 components replicated our previous results [Fu et al.,2001], but differ from reports of larger P1 and N1 without peak latency change for valid trials reported in voluntary attention tasks [Anllo‐Vento and Hillyard,1996; Bruin et al.,1998; Clark and Hillyard,1996; Heinze et al.,1994; Mangun,1995; Wijers et al.,1997]. However, it should be noted that the present ERP results, including modulation of N1 latency and amplitude by attention, might have been contaminated by ERP components very close in space and time after the ERP subtraction. This possible explanation cannot be excluded, even though we obtained similar results using both fixed (present study) and jittered [Fu et al.,2001] cue‐stimulus SOA. Additional work is needed to testify this possibility.
The present results are inconsistent with the sensory gain control hypothesis [Hillyard et al.,1998]. According to that hypothesis, attention amplifies processing of attended stimuli without changing the timing of the latency and scalp distribution of the early P1 and N1 components. However, we observed that the peak latency (timing) of the P1 and N1 components were modulated by attention and the amplitude of N1 showed a reversed modulation pattern as compared with previous voluntary attention tasks. This is consistent with the previously expressed view that at least partially distinct mechanisms are involved for producing voluntary and involuntary attentional effects [Kustov and Robinson,1996]. In a voluntary attention task, Clark and Hillyard [1996] also observed clear attention‐related shifts of the current source density (CSD) mapping and the dipole positions of the P1 and N1 components, indicating the possibility that new, attention‐specific neural sources were also being activated. However, they argued that likely both this attention‐specific component and the attention‐nonspecific sensory gating component contributed to the overall ERPs, and hence the sensory gating was still a major mechanism of spatial attention. The present results do not exclude this possibility; however, if this is the case the present results suggest that this new attention‐specific component in involuntary attention task differs from that in voluntary attention tasks.
The present study also observed a P140 attention‐related difference wave over the posterior occipito‐temporal sites contralateral to stimulus location. This attentional effect was localized to the middle occipital gyrus, consistent with the conclusion from previous studies that involuntary attention, as well as voluntary attention, modulates visual processing in extrastriate cortex [Hopfinger and Mangun,1998; Van de Lubbe,1997]. The deviations in the exact location of these attention‐related processes are probably due to different stimulus and task conditions [Heinze et al.,1994; Mangun et al.,1997; Woldorff et al.,1997]. Interestingly, along with the strong activation in extrastriate cortex, attention‐related activation in primary visual cortex was observed about 110 ms after stimulus onset. Because the initial response of primary visual cortex peaked at about 80 ms after stimulus onset, this seems to be a reactivation in the primary visual cortex. This reactivation seems unlikely to be an artifact from concurrent activation in the nearby brain areas such as middle occipital gyrus, because it was observed only in the left hemisphere whether stimuli were presented in LVF or RVF; in contrast, the activity in extrastriate cortex (BA19, middle occipital gyrus) and parietal areas (BA 7/19, precuneus/cuneus) had a strong contralateral hemispheric distribution. The reason for this left hemisphere reactivation in primary visual cortex is unknown; it is may be related to the orientation discrimination task per se. Moreover, this reactivation was more posterior than the initial activation in the primary visual cortex. This is inconsistent with some previous ERP studies which used the same dipole for initial and later reactivations in the primary visual cortex [e.g., Martinez et al.,2001a, 2001b]. However, it is consistent with the idea that different regions in primary visual cortex might be responsible for receptive and reentrant feedback processes, based on the laminar distribution of the primary visual cortex [Mehta et al.,2000b]. Mehta et al. [2000a,b] found that the attentional modulation in V1 was later than that observed in V4 and V2. Moreover, it was focused in the supragranular laminae but not the initial excitation in lamina 4C of V1, suggesting that different parts of the primary visual cortex are responsible for initial receptive response and later reentrant feedback processes. We suggest that this later reactivation in primary visual cortex reflects feedback from higher visual cortex, such as that reported under voluntary attention conditions [Di Russo et al.,2003; Martinez et al.,1999,2001a, 2001b; Noesselt et al.,2002]. By this feedback mechanism, visuospatial attention may enhance the perceptual salience of the target element from the surrounding distractors [Mehta et al.,2000a,b; Lamme and Spekreijse,2000; Super et al.,2001]. Previous studies using voluntary attention tasks have found that such a feedback takes place at 150 ms [Aine et al.,1995; Di Russo et al.,2003; Martinez et al.,1999,2001b; Noesselt et al.,2002] or later than 200 ms after stimulus onset [Olsen et al.,2001], possibly due to different stimuli and tasks. Nevertheless, all these studies observed the reactivation from feedback after attentional modulation in extrastriate cortex, with the ERP or source waveforms at the time range overlapping the descending portion of N1 and P2 component. In contrast, the present reactivation in primary visual cortex was observed along with activations in extrastriate cortex at about 110 ms, at the time range of P1 component. This evidence of earlier reactivation observed in the present study suggests that the feedback mechanism may occur earlier in involuntary relative to voluntary attention tasks; feedforward and feedback might be two mutually interactive processes at the early stages of visual processing, rather than two completely separated processes with the feedback process waiting for the completion of the feedforward process. Therefore, the present results both confirm previous reports of reentrant feedback into striate cortex [Martinez et al.,1999,2001a, 2001b; Noesselt et al.,2002; Di Russo et al.,2003] and extend previous findings by showing earlier feedback in involuntary relative to voluntary attention tasks.
Acknowledgements
We thank Hoa Vo for assistance in data collection and analysis, and two anonymous reviewers for providing helpful comments.
REFERENCES
- Aine CJ, Supek S, George JS (1995): Temporal dynamics of visual‐evoked neuromagnetic sources: effects of stimulus parameters and selective attention. Int J Neurosci 80: 79–104. [DOI] [PubMed] [Google Scholar]
- Aine CJ, Supek S, George JS, Ranken D, Lewine J, Sanders J, Best E, Tiee W, Flynn ER, Wood CC (1996): Retinotopic organization of human visual cortex: departures from the classical model. Cereb Cortex 6: 354–361. [DOI] [PubMed] [Google Scholar]
- Anllo‐Vento L (1995): Shifting attention in visual space: the effects of peripheral cueing on brain cortical potentials. Int J Neurosci 80: 353–370. [DOI] [PubMed] [Google Scholar]
- Anllo‐Vento L, Hillyard SA (1996): Selective attention to the color and direction of moving stimuli: electrophysiological correlates of hierarchical feature selection. Percep Psychophys 58: 191–206. [DOI] [PubMed] [Google Scholar]
- Anllo‐Vento L, Luck SJ, Hillyard SA (1998): Spatial‐temporal dynamics of attention to color: evidences from human electrophysiology. Hum Brain Mapp 6: 216–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brefczynski JA, DeYoe EA (1999): A physiological correlate of the 'spotlight' of visual attention. Nat Neurosci 2: 370–374. [DOI] [PubMed] [Google Scholar]
- Briand KA, Klein RM (1987): Is Posner's “beam” the same as Treisman's “glue”?: On the relation between visual orienting and feature integration theory. J Exp Psychol Hum Percept Perform 13: 228–241. [DOI] [PubMed] [Google Scholar]
- Bruin KJ, Kenemans JL, Verbaten MN, Van der Heijden AH (1998): Localization of spatial attention processes with the aid of a probe technique. Electroencephalogr Clin Neurophys 108: 110–122. [DOI] [PubMed] [Google Scholar]
- Chawla D, Rees G, Friston KJ (1999): The physiological basis of attentional modulation in extrastriate visual areas. Nat Neurosci 2: 671–676. [DOI] [PubMed] [Google Scholar]
- Cheal M, Lyon DR (1991a): Central and peripheral precueing of forced‐choice discrimination. Q J Exp Psychol A 43: 859–880. [DOI] [PubMed] [Google Scholar]
- Cheal M, Lyon DR (1991b): Importance of precue location in directing attention. Acta Psychol 76: 201–211. [DOI] [PubMed] [Google Scholar]
- Clark VP, Hillyard SA (1996): Spatial selective attention affects early extrastriate but not striate components of the visual evoked potential. J Cogn Neurosci 8: 387–402. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fan S, Hillyard SA (1995): Identification of early visual evoked potential generators by retinotopic and topographic analyses. Hum Brain Mapp 2: 170–187. [Google Scholar]
- Corbetta M, Miezin FM, Shulman GL, Petersen SE (1993): A PET study of visuospatial attention. J Neurosci 13: 1202–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J (1995): Neural mechanisms of selective visual attention. Annu Rev Neurosci 18: 193–222. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Sereno MI, Pitzalis S, Hillyard SA (2001): Cortical sources of the early components of the visual evoked potential. Hum Brain Mapp 15: 95–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Martinez A, Hillyard SA (2003): Source analysis of event‐related cortical activity during visuo‐spatial attention. Cereb Cortex 13: 486–499. [DOI] [PubMed] [Google Scholar]
- Eimer M (1993): Spatial cueing, sensory gating and selective response preparation: an ERP study on visuo‐spatial orienting. Electroencephalogr Clin Neurophysiol 88: 408–420. [DOI] [PubMed] [Google Scholar]
- Eimer M (1994a): “Sensory gating” as a mechanism for visuospatial orienting: electrophysiological evidence from trial‐by‐trial cuing experiments. Percept Psychophys 55: 667–675. [DOI] [PubMed] [Google Scholar]
- Eimer M (1994b): An ERP study on visual spatial priming with peripheral onsets. Psychophysiology 31: 154–163. [DOI] [PubMed] [Google Scholar]
- Eimer M (2000): The time course of spatial orienting elicited by central and peripheral cues: evidence from event‐related brain potentials. Biol Psychol 53: 253–258. [DOI] [PubMed] [Google Scholar]
- Fu S, Fan S, Chen L (2000): Selective attention to orientation and closure: An event‐related potential (ERP) study. Sci China (Series E) 43: 232–241. [Google Scholar]
- Fu S, Fan S, Chen L, Zhuo Y (2001): The attentional effects of peripheral cueing as revealed by two event‐related potential studies. Clin Neurophysiol 112: 172–185. [DOI] [PubMed] [Google Scholar]
- Fu S, Huang Y, Luo Y, Greenwood PM, Parasuraman R (2004): The role of perceptual difficulty in visuospatial attention: an event‐related potential study. Annu Meet Cogn Neurosci Soc, San Francisco, 2004. S87.
- Gandhi SP, Heeger DJ, Boynton GM (1999): Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci U S A 96: 3314–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C, Ito M, Kapadia M, Westheimer G (2000): Interactions between attention, context and learning in primary visual cortex. Vis Res 40: 1217–1226. [DOI] [PubMed] [Google Scholar]
- Gomez Gonzalez CM, Clark VP, Fan S, Luck SJ, Hillyard SA (1994): Sources of attention‐sensitive visual event‐related potentials. Brain Topogr 7: 41–51. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Goff WR (1987): Modification of median nerve somatic evoked potentials by prior median nerve, peroneal nerve, and auditory stimulation. Electroencephalogr Clin Neurophysiol 68: 295–302. [DOI] [PubMed] [Google Scholar]
- Handy TC, Mangun GR (2000): Attention and spatial selection: electrophysiological evidence for modulation by perceptual load. Percept Psychophys 62: 175–186. [DOI] [PubMed] [Google Scholar]
- Hawkins HL, Hillyard SA, Luck SJ, Mouloua M, Downing CJ (1990): Visual attention modulates signal detectability. J Exp Psychol Hum Percept Perform 16: 802–811. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Mangun GR, Burchert W, Hinrichs J, Scholz M, Munte TF, Goes A, Scherg M, Johannes S, Hundeshagen J, et al. (1994): Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature 372: 543–546. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Anllo‐Vento L (1998): Event‐related brain potentials in the study of visual selective attention. Proc Natl Acad Sci U S A 95: 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Munte TF (1984): Selective attention to color and location: an analysis with event‐related brain potentials. Percept Psychophys 36: 185–198. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Luck SJ, Mangun GR (1994): The cueing of attention to visual field locations: analysis with ERP recording In: Heinze HJ, Muente TF, Mangun GR, editors. Cognitive electro‐physiology: event‐related brain potentials in basic and clinical research. Boston: Birkhauser. [Google Scholar]
- Hillyard SA, Vogel EK, Luck SJ (1998): Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos Trans R Soc Lond B Biol Sci 353: 1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Di Russo F, Martinez A (2004): The imaging of visual attention In: Kanwish N, Duncan J, editors. Functional neuroimaging of visual cognition (Attention and Performance XX). New York: Oxford University Press; p 381–388. [Google Scholar]
- Hopfinger JB, Mangun GR (1998): Reflexive attention modulates processing of visual stimuli in human extrastriate cortex. Psychol Sci 9: 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN, Stryker MP (1977): Orientation columns in macaque monkey visual cortex demonstrated by the 2‐deoxyglucose autoradiographic technique. Nature 269: 328–330. [DOI] [PubMed] [Google Scholar]
- Iragui VJ, Kutas M, Mitchiner MR, Hillyard SA (1993): Effects of aging on event‐related brain potentials and reaction times in an auditory oddball task. Psychophysiology 30: 10–22. [DOI] [PubMed] [Google Scholar]
- Ito M, Gilbert CD (1999): Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron 22: 593–604. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG (1999): Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron 22: 751–761. [DOI] [PubMed] [Google Scholar]
- Kiss I, Pisio C, Francois A, Schopflocher D (1998): Central executive function in working memory: event‐related brain potential studies. Cogn Brain Res 6: 235–247. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL (1996): Shared neural control of attentional shifts and eye movements. Nature 384: 74–77. [DOI] [PubMed] [Google Scholar]
- LaBerge D (1995): Computational and anatomical models of selective attention in object identification In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge, MA: MIT Press; p 649–661. [Google Scholar]
- Lamme VA, Spekreijse H (2000): Contextual modulation in primary visual cortex and scene perception In: Gazzaniga MS, editor. The new cognitive neuroscience. Cambridge, MA: MIT Press; p 279–290. [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Hillyard SA, Mouloua M, Woldorff MG, Clark VP, Hawkins HL (1994): Effects of spatial cuing on luminance detectability: psychophysical and electrophysiological evidence for early selection. J Exp Psychol Hum Percept Perform 20: 887–904. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R (1997): Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol 77: 24–42. [DOI] [PubMed] [Google Scholar]
- Mangun GR (1995): Neural mechanisms of visual selective attention. Psychophysiology 32: 4–18. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA (1990): Allocation of visual attention to spatial locations: tradeoff functions for event‐related brain potentials and detection performance. Percept Psychophys 47: 532–550. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hopfinger JB, Kussmaul CL, Fletcher E, Heinze HJ (1997): Covariations in ERP and PET measures of spatial selective attention in human extrastriate visual cortex. Hum Brain Mapp 5: 273–279. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hinrichs H, Scholz M, Mueller‐Gaertner HW, Herzog H, Krause BJ, Tellman L, Kemna L, Heinze HJ (2001): Integrating electrophysiology and neuroimaging of spatial selective attention to simple isolated visual stimuli. Vis Res 41: 1423–1435. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo‐Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA (1999): Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat Neurosci 2: 364–369. [DOI] [PubMed] [Google Scholar]
- Martinez A, Di Russo F, Anllo‐Vento L, Hillyard SA (2001a): Electrophysiological analysis of cortical mechanisms of selective attention to high and low spatial frequencies. Clin Neurophysiol 112: 1980–1998. [DOI] [PubMed] [Google Scholar]
- Martinez A, DiRusso F, Anllo‐Vento L, Sereno MI, Buxton RB, Hillyard SA (2001b): Putting spatial attention on the map: timing and localization of stimulus selection processes in striate and extrastriate visual areas. Vis Res 41: 1437–1457. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH (1999): Effects of attention on orientation‐tuning functions of single neurons in macaque cortical area V4. J Neurosci 19: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AD, Ulbert I, Schroeder CE (2000a): Intermodal selective attention in monkeys. I. Distribution and timing of effects across visual areas. Cereb Cortex 10: 343–358. [DOI] [PubMed] [Google Scholar]
- Mehta AD, Ulbert I, Schroeder CE (2000b): Intermodal selective attention in monkeys. II. Physiological mechanisms of modulation. Cereb Cortex 10: 359–370. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R (1985): Selective attention gates visual processing in the extrastriate cortex. Science 229: 782–784. [DOI] [PubMed] [Google Scholar]
- Motter BC (1993): Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol 70: 909–919. [DOI] [PubMed] [Google Scholar]
- Muller HJ, Rabbitt PM (1989): Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. J Exp Psychol Hum Percept Perform 15: 315–330. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jancke L, Tempelmann C, Hinrichs H, Heinze HJ (2002): Delayed striate cortical activation during spatial attention. Neuron 35: 575–587. [DOI] [PubMed] [Google Scholar]
- O'Connor DH, Fukui MM, Pinsk MA, Kastner S (2002): Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci 5: 1203–1209. [DOI] [PubMed] [Google Scholar]
- Olson IR, Chun MM, Allison T (2001): Contextual guidance of attention: human intracranial event‐related potential evidence for feedback modulation in anatomically early temporally late stages of visual processing. Brain 124: 1417–1425. [DOI] [PubMed] [Google Scholar]
- Pascual‐Marqui RD (1999): Review of methods for solving the EEG inverse problem. Int J Bioelectromagn 1: 75–86. [Google Scholar]
- Pascual‐Marqui RD, Michel CM, Lehmann D (1994): Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol 18: 49–65. [DOI] [PubMed] [Google Scholar]
- Posner MI, Gilbert CD (1999): Attention and primary visual cortex. Proc Natl Acad Sci U S A 96: 2585–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD (1987): How do the parietal lobes direct covert attention? Neuropsychologia 25: 135–145. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Grandjean D, Sander D, Vuilleumier P (2004): Electrophysiological correlates of rapid spatial orienting towards fearful faces. Cereb Cortex 14: 619–633. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR, Lamme VA, Spekreijse H (1998): Object‐based attention in the primary visual cortex of the macaque monkey. Nature 395: 376–381. [DOI] [PubMed] [Google Scholar]
- Simson R, Ritter W, Vaughan HG Jr (1985): Effects of expectation on negative potentials during visual processing. Electroencephalogr Clin Neurophysiol 62: 25–31. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB (1999): Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci U S A 96: 1663–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Super H, Spekreijse H, Lamme VA (2001): Two distinct modes of sensory processing observed in monkey primary visual cortex (V1) (see Comment). Nat Neurosci 4: 304–310. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme. [Google Scholar]
- Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM (1998): The retinotopy of visual spatial attention. Neuron 21: 1409–1422. [DOI] [PubMed] [Google Scholar]
- Van de Lubbe RHJ, Woestenburg JC (1997): Modulation of early ERP components with peripheral precues: a trend analysis. Biol Psychol 45: 143–158. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR (1998): Gating of neuronal responses in macaque primary visual cortex by an attentional spotlight. Neuroreport 9: 1947–1952. [DOI] [PubMed] [Google Scholar]
- Warner CB, Juola JF, Koshino H (1990): Voluntary allocation versus automatic capture of visual attention. Percept Psychophys 48: 243–251. [DOI] [PubMed] [Google Scholar]
- Wijers AA, Lange JJ, Mulder G, Mulder LJ (1997): An ERP study of visual spatial attention and letter target detection for isoluminant and nonisoluminant stimuli. Psychophysiology 34: 553–565. [DOI] [PubMed] [Google Scholar]
- Woldorff MG, Fox PT, Matzke M, Lancaster JL, Veeraswamy S, Zamarripa F, Seabolt M, Glass T, Gao J, Martin CC, Jerabek P (1997): Retinotopic organization of early visual spatial attentional effects as revealed by PET and ERPs. Hum Brain Mapp 5: 280–286. [DOI] [PubMed] [Google Scholar]
- Yantis S, Jonides J (1990): Abrupt visual onsets and selective attention: voluntary versus automatic allocation. J Exp Psychol Hum Percept Perform 16: 121–124. [DOI] [PubMed] [Google Scholar]


