Abstract
Endocrine-disrupting chemical (EDC) exposures to the fetus have long-lasting effects on health and disease in adulthood. Such EDC exposure to the F1 fetuses also reaches the germ cells that become the F2 generation. Previously, we demonstrated that adult social and communicative behaviors such as ultrasonic vocalizations and mating behaviors were altered by EDCs in F2 rats, especially males. In the current study, we used the brains of these F2 males to ascertain the underlying molecular changes in the hypothalamus related to these behavioral outcomes. Their progenitors were Sprague-Dawley rat dams, treated on pregnancy days 8 to 18 with one of three treatments: a polychlorinated biphenyl (PCB) mixture, Aroclor 1221, selected because it is weakly estrogenic; the anti-androgenic fungicide vinclozolin (VIN); or the vehicle, 6% dimethylsulfoxide in sesame oil (VEH). In adulthood, F1 male and female offspring were bred with untreated partners to generate paternal or maternal lineages of the F2 offspring, the subjects of molecular work. Quantitative real-time PCR was conducted in the medial preoptic area (POA) and the ventromedial nucleus (VMN) of the hypothalamus, selected for their roles in social and sexual behaviors. Of the genes assessed, steroid hormone receptors (estrogen receptor α, androgen receptor, progesterone receptor) but not dopamine receptors 1 and 2 or DNA methyltransferase 3a expression were altered, particularly in the VIN males. Several significant correlations between behavior and gene expression were also detected. These results suggest that preconceptional exposure of male rats to EDCs at the germ cell stage alters the neuromolecular phenotype in adulthood in a lineage-dependent manner.
Keywords: Endocrine disrupting chemicals (EDCs), germ cell, F2 generation, Polychlorinated biphenyls (PCBs), Aroclor 1221, vinclozolin, gene expression, Preoptic area, Ventromedial nucleus, Hypothalamus, Androgen receptor, Estrogen receptor, Dopamine receptor, ultrasonic vocalization, sexual behavior, lineage
1. Introduction
The health of humans and wildlife has been permanently altered by environmental chemicals from industry, agriculture, manufacturing, and many other sources. Some of these chemicals are categorized as endocrine-disrupting chemicals (EDCs) because they interfere with hormone action [1]. Polychlorinated biphenyls (PCBs) are a class of legacy EDCs, no longer manufactured but with persistent effects from environmental contamination and subsequent bioaccumulation and biomagnification up the food chain. Although banned since the 1970s in the U.S., recent epidemiological data shows that PCBs are still detectable in human tissue [2] and are associated with impaired reproductive and neurological health in humans [3-6]. Contemporary chemicals such as the common-use fungicide vinclozolin (VIN) also cause impairments in human fertility [7-9] as well as physiology and behavior of various species [10-13].
Several brain regions are sexually dimorphic and organized by endogenous steroid hormones during sensitive developmental phases of early postnatal life. These neural circuits are subsequently activated by hormones of puberty and adulthood that lead to the manifestation of sex-appropriate behaviors and physiology [14,15]. The exquisite sensitivity of the developing brain to hormones means that exogenous EDC exposures may perturb these processes, and increase the predisposition for disease and dysfunction later in life [16], including sexually dimorphic behaviors: juvenile play, adult learning, anxiety, and social and sexual behavior [17-20]. Other studies investigating the molecular and neurobiological substrates underlying these behavioral changes caused by EDCs have reported alterations in brain metabolic activity, steroid hormone receptor expression, transcriptional activity, and epigenetic marks in the brain and other tissues [12,21-23].
Prenatal EDCs given to the F1 fetus also exposes the germ cells that become the F2 generation. This means that any observed effects of EDCs on F2 descendants are presumably due to programming effects during the preconceptional period. In a companion study [24], we reported that in F2 rats, EDCs altered adult physiology, sexual behavior, and ultrasonic vocalizations, the latter important for communicating affective state and social/sexual interest [25-27]. Although the effects varied based on sex and lineage (paternal or maternal descent), male rodents descended from the paternal lineage were particularly vulnerable to EDC disruption.
Here, we examined outcomes of preconceptional exposure to two classes of EDCs: Aroclor 1221, a weakly estrogenic PCB mixture, and VIN, an anti-androgenic fungicide, on the F2 generation. These particular EDCs have been the focus of study in our laboratory and were selected because they act via different hormonal pathways (estrogenic vs. anti-androgenic) and represent different classes of EDCs to which humans and wildlife are exposed today (legacy vs. modern). Our choice of the ventromedial nucleus (VMN) and preoptic area (POA) was based on their roles in the control of sociosexual behaviors [28-31], with genes implicated in these functions.
2. Methods
2.1. EDCs
As described [24], Aroclor 1221 (PCB mixture; AccuStandard, New Haven, CT, C-221N-50MG, 083-166) and Vinclozolin (VIN; Chem Service Inc., West Chester, PA N-13745-250MG, 4054200), each at 1 mg/kg, were dissolved in a vehicle (VEH) of 6% dimethylsulfoxide (Sigma number D4540; Sigma, St Louis, Missouri) in sesame oil. The rationale for dosages was detailed in our companion study [24]; in brief, they were selected to model circulating concentrations of these chemicals in humans, and were used at or below the acceptable daily intake level.
2.2. Animal Husbandry and EDC Treatments
All animal protocols were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by The University of Texas at Austin’s Institutional Animal Care and Use Committee (IACUC). Sprague-Dawley rats were purchased from Harlan Laboratories (Houston, Texas & Indianapolis, Indiana) and housed in humidity-controlled rooms on a 10:14 partially reversed light cycle (lights off at 11:30 a.m.) and maintained at 21-23°C. Two to three animals were housed together in polycarbonate cages (43 × 21 × 25 cm) with aspen bedding (PJ Murphy Forest Products, Sani-Chip), with a PVC tube for enrichment. Cages were changed weekly, and rats were fed a low phytoestrogen diet (Harlan-Teklad, Indianapolis, Indiana) ad libitum. These rats were handled once a week for 5 minutes to acclimate them to the experimenters. Mating began two weeks after their arrival.
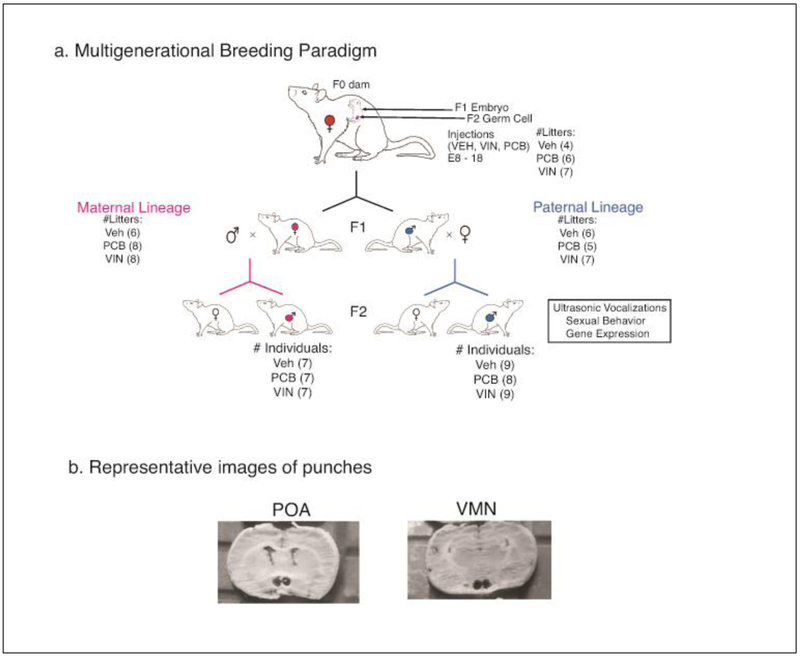
The breeding strategy, numbers of litters, and numbers of F2 experimental subjects, is shown in Figure 1a. Female virgin rats (3-4 months old) were bred with sexually experienced male rats (~6 months old). The day of mating was designated as embryonic day 0 (E0). Following confirmation of the presence of sperm in the vagina, dams were single-housed for the duration of their pregnancy. From embryonic day (E) 8 to E18, dams were weighed daily and injected with ~0.1 mL (based on body weight) of VEH, PCB, or VIN (i.p.). This timeframe encompasses not only the beginning of brain sexual differentiation [32] but also primordial germ cell migration and reprogramming [33].
Fig. 1.
A) The breeding paradigm and experimental design is shown, beginning with pregnant rats injected with either the vehicle (VEH), vinclozolin (VIN) or Aroclor 1221 (PCB) from E8-18. The F1 female offspring were bred with untreated males to generate a maternal F2 lineage. Similarly, F1 male offspring were bred with untreated females to generate a paternal F2 lineage. The F2 generation was behaviorally characterized, and their brains used in the current study. Numbers of litters are indicated in parentheses for F0 and F1 generations, and numbers of F2 male individuals are indicated in parentheses. B) Photographs of representative punches from the medial preoptic area (POA) and the ventromedial nucleus (VMN) of the hypothalamus are shown.
On the day after birth, the F1 litters were culled to 10 pups of equal sex ratio by euthanizing those with extreme anogenital index measures (AGI, anogenital distance / 3√ body weight) [34]. After weaning on P21, individuals were housed two to three per cage with same-sex littermates.
In adulthood (~P80), two F1 females and two F1 males per litter were bred with untreated stimulus animals (purchased from Harlan) to create the F2 generation. The F2 generation male individuals from both the maternal and paternal lineage were the focus of this experimental design based on our observed alterations in behavior and developmental milestones in this sex. These behavioral data have been published [24], and animals’ brains stored as described below. Animals derived from 5-8 litters per group, with 1-2 males per litter used for behavior and brain work (Fig. 1a).
2.3. Tissue Collection
Approximately two weeks after behavioral characterization was completed (between postnatal days (P) 90-120), experimental males were weighed and euthanized by rapid decapitation. Brains were immediately removed and flash-frozen in isopentane, and stored at −80°C. Coronal sections were obtained by slicing the brain on a cryostat at 450 μm. Slices were mounted on slides, placed on a freezing stage and allowed to equilibrate to −16°C. The medial preoptic area (POA) and ventromedial nucleus (VMN) of the hypothalamus were taken bilaterally using a 1 mm punch (Stoelting; Fig. 1b). Tissue punches were placed in 1 mL cold Eppendorf tubes and stored at −80°C until RNA isolation.
2.4. RNA extraction
Frozen tissue punches were lysed and homogenized using 22 gauge needles and syringes. RNA was extracted using the Denville Scientific Inc. SpinSmart Total RNA Mini Purification kit (CM-610-50, CM-610-250) according to manufacturer instructions. RNA was eluted with 50 μl of nuclease-free water (Applied Biosystems Cat No AM9937). Samples were stored at −20°C in 66% ethanol and 0.5 M NaCl for 5-7 days before being concentrated. To pellet the RNA, samples were placed in −80°C for 10 min, then centrifuged at 14000 × g for 20 min at 4°C. The pellet was washed with 70% ethanol and centrifuged again for 10 min, after which the supernatant was discarded. The samples were then dried first by inversion at room temperature for 10 min, then in a speedvac at 43°C for 5 min. The dried pellets were then resuspended in 12 μl nuclease-free water. RNA quantity was determined by the Nanodrop 2000 Spectrophotometer according to manufacturer instructions. 130 – 1200 ng of RNA was isolated, and the quality of our samples was assessed by randomly selecting ~10% of our samples to run on the Bioanalyzer 2100 (Agilent RNA 6000 Nano Kit, Cat 5067-1511, Agilent Technologies, Santa Clara, California); all tested samples had an RNA integrity number of 8.5 and above. The small sizes of the dissections limited us to 4 (VMN) or 5 (POA) genes per region for qPCR.
2.5. Gene expression quantification
Using a high-capacity cDNA reverse transcription kit with RNase inhibitor (Life Technologies, Cat. No. 4368814), 170 ng of RNA per sample were converted to cDNA in 20 μl reactions according to manufacturer instructions. Samples went through the following cycles on the Applied Biosystems 2720 Thermocycler: 25°C for 10 min, then 37°C for 120 min, and finally 85°C for 5 min. cDNA was stored at −20°C for 2-5 days before use. Gene expression primer and probe assays were purchased predesigned from LifeTech to identify genes of interest (FAM/MGB-NFQ, Cat No 4351372) and reference genes (VIC/MGB-NFQ, Cat No 4448489), as shown in Table 1. Assays were prevalidated on a test plate for duplexing to run both target and calibrating genes together. Each sample was run in triplicate, and Taqman Gene expression master mix (Cat No 4369016) was used in a 20μl reaction with 10ng of cDNA.
Table 1.
PCR target and assay information
| Gene Name | Brain region assayed | Life Technologies assay ID |
|---|---|---|
| Glyceraldehyde 3-phosphate dehydrogenase | POA, VMN | Rn01775763_g1 |
| Androgen receptor | POA, VMN | Rn00560747_m1 |
| Estrogen receptor alpha | POA, VMN | Rn01640372_m1 |
| Dopamine receptor D1 | POA | Rn03062203_s1 |
| Dopamine receptor D2 | POA | Rn00561126_m1 |
| DNA methyltransferase 3 alpha | POA, VMN | Rn01027162_g1 |
| Progesterone receptor | VMN | Rn01448227_m1 |
qPCR was conducted on the Applied Biosystems ViiA7 with the following parameters: 50°C for 2 min, 95°C for 10 min, 45 cycles of 95°C for 15 sec, and 60°C for 1 min. Quantification threshold (Ct) was determined automatically by the ViiA7. Gapdh was chosen as a reference gene, based on pilot work showing that this gene is not significantly affected by EDC treatments. Relative expression of targets was calculated using the comparative Ct method: reference Cts were subtracted from target Cts to determine delta Ct within each sample well. Samples were run in triplicate, with triplicate delta Cts averaged together. To normalize the data for each target gene, the median delta Ct (ΔCt) of all VEH males (maternal and paternal lineages) was calculated and subtracted to determine ΔΔCt. From this, fold change in gene expression for each individual was calculated as 2−ΔΔCt.
2.6. Analysis and statistics
Litter was tested as a covariate, and since no effects were found, we used individual rats (n = 7-9 per group) as the unit of statistical analysis (Fig. 1a). The Grubb’s test was used to determine significant outliers, and the number of outliers removed was limited to two per group. Gene expression measures were analyzed using a factorial analysis of variance (ANOVA) to determine main effects of lineage or treatment as well as interactions. Main effects and interactions were investigated using Tukey’s HSD post-hoc tests, since they correct for multiple comparisons. The data were tested initially for normality and homoscedasticity using the Shapiro-Wilk and Bartlett’s test, respectively.
All data presented in this study were normally distributed and homoscedastic. Effect sizes for each factor of the ANOVAs were calculated as partial eta-squared (ηp2), which represents the proportion of variance that is accounted for by the factor being tested. Effect sizes of 0.14 or greater are considered to be large, 0.06 to 0.13 medium, and below 0.06 small. Significance was determined at p < 0.05.
2.6.1. Gene-Behavior-Hormone Correlations
Prior to tissue collection, F2 males had been assessed for ultrasonic vocalizations, mating behavior, and serum hormone levels, reported in [24]. A principal components analysis to determine the underlying factors contributing to the variance within each behavior showed that EDC exposure affected the acoustic properties and number of vocalizations, latency to and frequency of mating behaviors, and concentrations of serum estradiol and testosterone in these males. That study showed that PCB males from the paternal lineage in particular were most affected. These data were used here in Pearson correlations to determine relationships between gene expression with serum estradiol and testosterone, and the principal components of each behavioral outcome. Because a large number of behaviors were scored, a behavior eigenvalue of the principal components was calculated and used for correlations with serum hormones (estradiol, testosterone) and with gene expression. All endpoints were selected for analysis according to a priori hypotheses, and accordingly, significance levels were not adjusted.
3. Results
3.1. POA gene expression
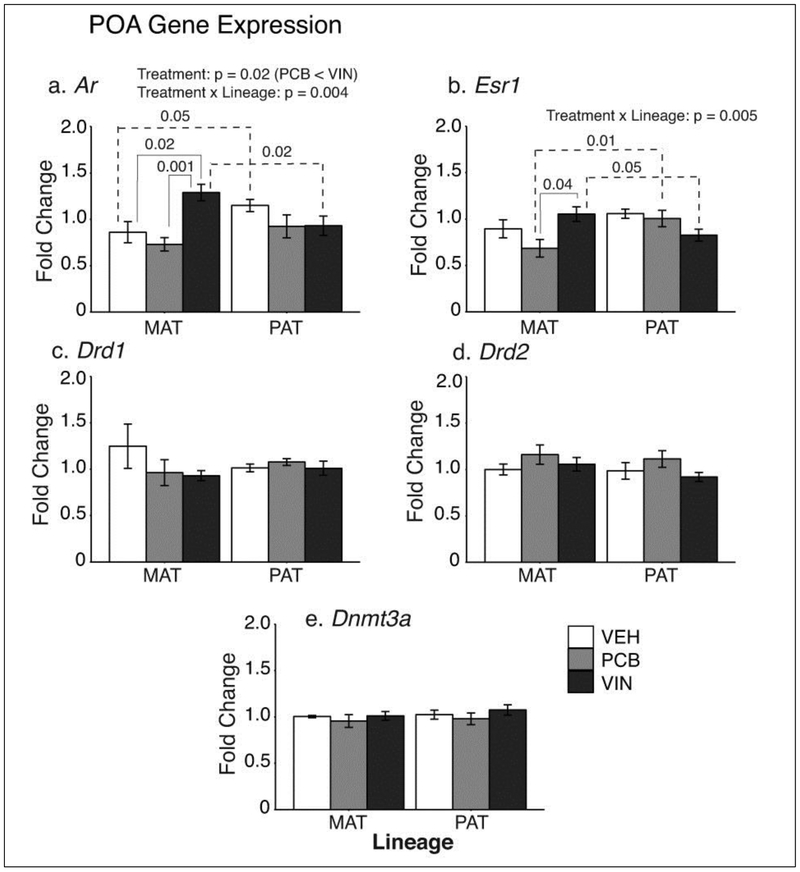
Significant EDC treatment effects were found for the two steroid hormone receptors (Fig. 2). For Ar, a main effect of treatment was found in F2 males (F2, 43 = 4.30, p = 0.02, ηp2 = 0.17; Fig. 2a), with PCB animals significantly lower than VIN animals (p = 0.005). A treatment × lineage interaction was also found for Ar (F2, 43 = 6,44, p = 0.004, ηp2 = 0.23). VEH males from the paternal lineage had higher Ar expression than the maternal lineage (p = 0.05). By contrast, maternal VIN animals had higher Ar expression than paternal lineage VIN males (p = 0.02). Within the maternal lineage, Ar was higher in VIN than VEH (p = 0.02), and lower in PCB than VIN (p < 0.001) rats.
Fig. 2.
POA gene expression results are shown for a) Ar, b) Esr1, c) Drd1, d) Drd2 and e) Dnmt3a. Of these, Ar and Esr1 expression were significantly changed, with main effects and interactions indicated. Significant treatment differences within a lineage are indicated with solid brackets; significant differences between lineages are indicated with dashed brackets. Data are graphed as mean ± SEM.
Esr1 expression had a significant treatment × lineage interaction (F2, 36 = 6.25, p = 0.005, ηp2 = 0.26; Fig 2b). Post-hoc analysis showed that maternal PCB males had significantly lower Esr1 expression than paternal PCB males (p = 0.01). An opposite pattern (MAT > PAT) was found in the VIN males (p = 0.05). Within the maternal lineage, Esr1 was significantly higher in VIN than PCB males (p = 0.04).
The dopamine receptors 1 and 2 (Drd1, Drd2), and DNA methyltransferase 3a (Dnmt3a) genes, were unaffected by treatment or lineage in the POA (Fig. 2c, 2d, 2e).
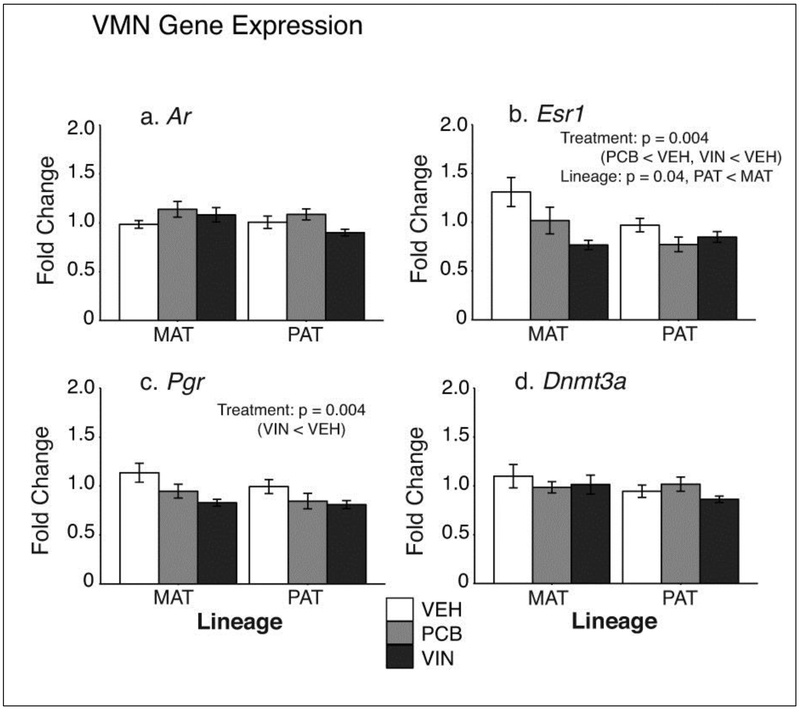
3.2. VMN gene expression
In the VMN, Ar and Dnmt3a were unaffected by treatment or lineage (Fig 3a,3d). Esr1 was significantly affected by treatment (F2, 42 = 6,49, p = 0.004, ηp2 = 0.24; Fig 3b). Post-hoc tests showed that both PCB (p = 0.01) and VIN (p = 0.001) males had significantly lower Esr1 expression than VEH males. There was also a main effect of lineage (F2, 42 = 4.71, p = 0.04, ηp2 = 0.10), with males from the paternal lineage having lower Esr1 than those in the maternal lineage (p = 0.04).
Fig. 3.
VMN gene expression results are shown for a) Ar, b) Esr1, c) Pgr, and d) Dnmt3a. Esr1 had significant main effects of both treatment and lineage, and Pgr had a significant treatment effect. Ar and Dnmt3a were unaffected. Significant treatment differences within a lineage are indicated with solid brackets. Data are graphed as mean ± SEM.
Pgr expression in the VMN was significantly affected by treatment (F2, 41 = 6.43, p = 0.004, ηp2 = 0.23; Fig 3c). Post hoc tests revealed that VIN males had significantly lower Pgr than VEH males (p = 0.02).
3.3. Correlations
In [24], we examined the behavioral and physiological phenotype of these same F2 male rats, investigating serum hormone concentrations (estradiol, testosterone) and communicative and reproductive behaviors. A principal components analysis determined the primary underlying factors in each behavior, and we subsequently analyzed the eigenvalues of the principal components (PC) for treatment differences. The following measures were significantly affected in adult males [24] and included in the Pearson’s correlation analyses: 1) ultrasonic vocalization (USV) acoustic properties, 2) total USV call counts, 3) the latency to engage in sex behavior, 4) female-elicited sex behavior, and 5) circulating serum estradiol and 6) testosterone concentrations.
To determine whether the observed molecular changes in the POA and VMN could be responsible for EDC-induced behavioral and physiological changes in males, we conducted pair-wise Pearson’s correlations between expression of significantly affected genes (POA: Ar, Esr1; VMN: Esr1, Pgr) with serum hormones (estradiol, testosterone) and the eigenvalues of the principal components of the behaviors (Table 2).
Table 2.
F2 Male Gene-Behavior-Hormone Correlation
| Maternal Veh | Behavior | Serum Hormones | |||||
|---|---|---|---|---|---|---|---|
| USV Acoustic Properties |
Total USV Call Counts |
Latency to Sex Behavior |
Female-Elicited Sex Behavior |
Estradiol | Testosterone | ||
| POA | Ar | 0.00 | 0.47 | −0.27 | 0.45 | 0.45 | −0.50 |
| Esr1 | −0.25 | 0.11 | 0.59 | 0.08 | 0.11 | 0.25 | |
| VMN | Esr1 | 0.01 | −0.74 | 0.79* | 0.04 | −0.38 | 0.21 |
| Pgr | 0.13 | −0.19 | 0.26 | 0.75 | 0.20 | −0.38 | |
| Maternal PCB | Behavior | Serum Hormones | |||||
| USV Acoustic Properties |
Total USV Call Counts |
Latency to Sex Behavior |
Female-Elicited Sex Behavior |
Estradiol | Testosterone | ||
| POA | Ar | −0.42 | −0.36 | 0.08 | −0.26 | 0.28 | −0.25 |
| Esr1 | −0.24 | −0.20 | −0.12 | 0.47 | −0.31 | −0.78* | |
| VMN | Esr1 | −0.27 | −0.08 | 0.24 | 0.36 | 0.18 | −0.61 |
| Pgr | −0.15 | 0.03 | 0.42 | 0.31 | 0.35 | −0.16 | |
| Maternal VIN | Behavior | Serum Hormones | |||||
| USV Acoustic Properties |
Total USV Call Counts |
Latency to Sex Behavior |
Female-Elicited Sex Behavior |
Estradiol | Testosterone | ||
| POA | Ar | −0.33 | 0.14 | −0.63 | 0.77* | −0.89** | 0.31 |
| Esr1 | 0.21 | 0.22 | −0.54 | 0.36 | −0.62 | 0.35 | |
| VMN | Esr1 | 0.32 | 0.26 | −0.42 | −0.04 | 0.06 | 0.10 |
| Pgr | 0.03 | 0.44 | −0.70 | 0.75 | −0.82* | 0.18 | |
| Paternal Veh | Behavior | Serum Hormones | |||||
| USV Acoustic Properties |
Total USV Call Counts |
Latency to Sex Behavior |
Female-Elicited Sex Behavior |
Estradiol | Testosterone | ||
| POA | Ar | 0.23 | 0.51 | −0.10 | −0.37 | −0.49 | −0.10 |
| Esr1 | 0.02 | 0.75* | 0.21 | −0.01 | −0.68 | −0.13 | |
| VMN | Esr1 | −0.33 | 0.19 | 0.68 | 0.19 | −0.21 | −0.47 |
| Pgr | 0.39 | 0.65 | −0.23 | −0.02 | −0.74* | −0.56 | |
| Paternal PCB | Behavior | Serum Hormones | |||||
| USV Acoustic Properties |
Total USV Call Counts |
Latency to Sex Behavior |
Female-Elicited Sex Behavior |
Estradiol | Testosterone | ||
| POA | Ar | 0.60 | 0.07 | 0.52 | −0.40 | 0.01 | −0.48 |
| Esr1 | 0.62 | 0.83* | 0.05 | −0.48 | 0.34 | −0.23 | |
| VMN | Esr1 | 0.30 | 0.82* | −0.55 | −0.48 | 0.42 | 0.28 |
| Pgr | 0.48 | 0.33 | 0.34 | −0.36 | 0.10 | −0.74 | |
| Paternal VIN | Behavior | Serum Hormones | |||||
| USV Acoustic Properties |
Total USV Call Counts |
Latency to Sex Behavior |
Female-Elicited Sex Behavior |
Estradiol | Testosterone | ||
| POA | Ar | −0.43 | −0.34 | −0.70 | 0.74* | −0.19 | −0.29 |
| Esr1 | −0.48 | −0.25 | −0.44 | 0.48 | −0.09 | −0.16 | |
| VMN | Esr1 | −0.24 | −0.12 | 0.16 | −0.07 | 0.26 | 0.24 |
| Pgr | 0.13 | −0.51 | 0.20 | −0.03 | −0.10 | −0.09 | |
Correlations were conducted for each treatment and lineage between behaviors (left) and hormones (right) and gene expression in the F2 male rats. The correlation coefficient is shown. Significant correlations (p < 0.05) are indicated with asterisks.
3.3.1. Serum Hormones-Gene expression correlations
Circulating estradiol levels showed a few significant correlations in EDC groups relative to the VEH group (Table 2). In the POA, circulating estradiol concentrations were negatively correlated with Ar in maternal VIN males (r = −0.89, p = 0.008), with no relationship in the VEH group. In the VMN, Pgr and estradiol were negatively correlated in both maternal VIN males (r = −0.82, p = 0.03) and in paternal VEH males (r = −0.74, p = 0.04). For testosterone, only one correlation was found. In the maternal PCB lineage males, testosterone was negatively correlated with Esr1 in the POA (r = −0.78, p = 0.04), with no such relationship observed in the VEH group (Table 2).
3.3.2. Sex Behavior-Gene expression correlations
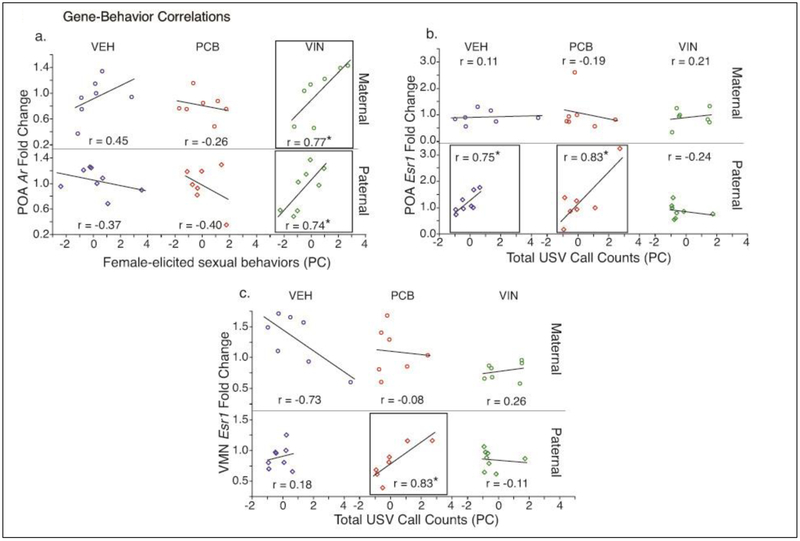
Few significant correlations were found between gene expression and male sexual behavior (Table 2). In paternal VIN males, there was a significant positive correlation between POA Ar and female-elicited sex behavior (r = 0.74, p = 0.04; Fig. 4a). Similarly, maternal VIN males showed a positive correlation in the POA (r = 0.77, p = 0.04, Fig.4a). By contrast, neither paternal nor maternal VEH or PCB males had significant correlations between Ar and these behaviors.
Fig. 4.
Within-animal Pearson r correlations between F2 male behaviors and gene expression for select measures are shown separately for each EDC. Eigenvalues for behaviors that were altered by EDC exposure in F2 males were correlated with POA and VMN gene expression levels. Shown are: (a) POA Ar expression and female-elicited sex behaviors, (b) POA Esr1 expression and total USV call counts, and (c) VMN Esr1 expression and total USV call counts. Note the different y-axis scales between graphs. Those correlations that are significant at p < 0.05 are boxed and indicated by *. Pearson r correlation coefficients are provided for all graphs.
Maternal VEH males had a significant positive correlation between Esr1 in the VMN, and latency to sex behavior (r = 0.79; p = 0.03; Table 2), while the EDC males had no significant relationship.
3.3.3. Ultrasonic Vocalizations-Gene expression correlations
Several correlations were found between USVs and gene expression (Table 2). Esr1 in the POA of paternal lineage VEH and PCB males was significantly positively correlated with total USV call counts (VEH: r = 0.75, p = 0.03; PCB: r = 0.83, p = 0.02; Fig. 4b), while VIN males had no significant relationship. In the VMN, paternal PCB males showed a significant positive correlation between Esr1 and total USV counts (r = 0.83, p = 0.02; Fig 4c), an effect not observed in VEH males.
4. Discussion
The results of this study provide novel information about how preconceptional EDCs reprogram the molecular phenotype of the POA and VMN of F2 male rats, and its relationships to hormones and behaviors that had previously been characterized in these animals. The basis of this present work was that numbers and characteristics of USVs, as well as dyadic sexual interactions between the males with untreated female rats, differed substantially between EDC and vehicle F2 males, especially of the paternal lineage [24], This led us to choose the POA and VMN as regions of interest based on their roles in sociosexual behaviors and as part of the USV neural pathway [35-37]. Here, to determine the underlying molecular phenotype, we selected as our gene targets steroid hormone receptors (Ar, Esr1, Pgr), dopamine receptors 1 and 2, and the epigenetic modifier DNA methylation transferase 3a.
4.1. Lineage is important to intergenerational effects of EDCs
The lineage of the F2 males – maternal or paternal – was revealed as a key factor in determining behavioral and molecular phenotypes in the current and companion study [24]. Lineage is rarely considered as a variable in most other EDC studies due to methodological approaches such as sibling or same-treatment breeding (i.e., EDC animals bred with similarly treated EDC animals), or focus on a single lineage (e.g. paternal [21] or maternal [38-40], but not both).
In our work, we specifically isolated maternal and paternal lineages by breeding our experimental progenitors with untreated partners. Our results showed that expression of Esr1 (POA, VMN) and Ar (POA) differed by lineage, as did certain behaviors in these rats [24]; these data will be discussed in more detail below. Notably, our lab has published evidence for lineage differences in both EDC- and vehicle-treated animals [24,41,42]. These lineage differences are biologically plausible and may be attributable to at least 6 possible factors, none mutually exclusive. First, maternal and paternal F2 lineages differ due to the genotype (XX vs. XY) of the F1 parents. Second, maternal stress may occur in the F0 dams due to the EDC or vehicle injections; this may differentially influence outcomes in F1 male and female offspring. Third, the epigenetic state of the F2 generation germ cells within the F1 embryos differs in the timing of demethylation and remethylation during the exposure period (E8 to 18), with these processes completed in males by birth, but incomplete until puberty in females [43]. Fourth, the gonadal steroid hormone milieu of the F1 progenitors in utero differed quite profoundly with sex (male rat gonads have more active steroidogenesis than those of females), and this influences exposure of the F2 germ cells to differential hormones [32]. Fifth, behaviors of the F1 mothers to their offspring may differ. The F1 maternal lineage dams received exposure to EDCs or vehicle. The F1 paternal lineage dams were untreated, and bred with F1 EDC males. Sixth, information may be imparted by sperm via microRNAs, exosomes, epigenetic factors, and others, that differ between F1 EDC males of the paternal lineage (exposed) vs. F1 male partners (unexposed) to the F1 EDC females [44-46]. With respect to the F2 offspring, these differences in genetics, epigenetic programming, behavior, hormones, and other factors would play out as lineage differences.
4.2. Preconceptional effects of EDCs on gene expression in the POA
In the POA, preconceptional VIN significantly affected expression of Ar, with VIN males of the maternal lineage having higher expression than both VEH and PCB males. These differences were not found in the paternal lineage, illustrating the point raised above that lineage is a key factor in determining outcomes. Furthermore, specific lineage differences in Ar were observed in the VEH and VIN groups, albeit in opposite directions. Other studies on EDCs have demonstrated that the Ar is affected by prenatal EDCs [47], but to our knowledge this has not been studied in the F2 generation. The other steroid hormone receptor measured in the POA, Esr1, was significantly affected by the interaction of treatment and lineage in the POA of F2 males, due to differences between PCB and VIN groups in the maternal lineage, and lineage effects in the PCB and VIN groups. The lack of treatment effect of PCBs on hypothalamic Ar and Esr1 differs from studies on F1 rats showing effects of PCBs [19,47,48]. It is unsurprising that F1 and F2 generations would differ based on their very different life stages during exposure. In addition, the PCB mixture we selected (Aroclor 1221) is mainly weakly estrogenic, but it also has anti-estrogenic and other properties that could affect gene expression outcomes [49].
Neither Drd1 nor Drd2 were altered by EDC exposure in the POA. These gene targets had been selected because D1 and D2 receptor activation in the POA have opposing actions on male sexual behavior ([50]; reviewed in [35]). Furthermore, the mesolimbic dopamine system is heavily implicated in the rewarding and appetitive aspects of 50kHz USV calls [51], which were significantly lower in paternal lineage PCB males (as reported in our companion study [24]).
Early life perturbations can program the brain by modifying DNA methylation patterns [52-55]. It was reported that PCB exposure in rats decreased global DNA methylation [56] and histone modification enzymes in the liver [57], but did not affect methylation of DNA repeats in the liver, spleen or thymus [58]. In our study, we measured mRNA levels of Dnmt3b, DNA methyltransferase involved in de novo DNA methylation, and did not observe any effects of treatment or lineage. Other parts of the DNA methylation machinery, or additional epigenetic processes such as histone modifications to maintain methylation levels, or microRNAs to induce translational repression or degradation [59,60], should be investigated in future work. We reported that prenatal PCB exposure altered microRNA expression in the F1 adult male POA, and that these microRNAs target genes belonging to the nuclear hormone receptor family [47]. In fact, prenatal exposure of F1 male mice to a similar dose of VIN resulted in alterations in microRNA that are involved in F2 primordial germ cell differentiation [61], suggesting that these epigenetic factors could also play a role in the transmission of EDC effects across generations.
4.3. Preconceptional effects of EDCs on gene expression in the VMN
The VMN is a heterogeneous hypothalamic region abundant in steroid hormone receptors, and while best studied for its roles in sexual behavior in females, it also plays important roles in copulation and ultrasonic vocalizations in males [37,62-64]. In this region, there were significant effects of treatment on both Esr1 and Pgr, which were lower in VIN than VEH F2 males. PCB males also had lower Esr1 than VIN males. There was also a main lineage effect observed.
Esr1 in the VMN, especially the ventrolateral compartment, plays important roles in aggressive behaviors [65,66], as well mounting, sniffing, and close investigation behaviors in male mice [66]. Our previous study on F2 male rat behaviors demonstrated treatment- and lineage-specific effects on USV call numbers and frequency, intromission frequency, latency to first ejaculation, and behaviors received from the females [24], behaviors that may relate to the changes in Esr1 in the VMN. Similarly, Pgr in the VMN, which was decreased by VIN, plays behavioral roles. Ablation of Pgr-positive cells in this region reduces mating and aggression of male mice, especially the consummatory aspects [65].
4.4. Relationships among hormones, behaviors and genes
Significant correlations with gene expression were found in a treatment- and lineage-specific manner, especially for USV call counts, female-elicited sex behavior, and serum estradiol concentrations. In the maternal lineage, there were five significant correlations, three in the maternal VIN males (estradiol and Ar in the POA, estradiol and Pgr in the VMN, female-elicited behavior and Ar in the POA). In addition, Esr1 was correlated with latency to sex behavior in the VMN of maternal VEH males, and testosterone was negatively correlated with Esr1 in the POA of maternal PCB males. Two points are notable. First, correlations with genes were limited to Esr1, Pgr, and Ar, the three steroid hormone receptors. Second, most of the correlations were in EDC but not VEH males, and suggestive of the emergence of relationships in F2 descendants of EDC-exposed rats.
The paternal F2 males had five correlations, three of which were between Esr1 and USV call counts. Although previous studies have found that VIN upregulates Esr1 expression across multiple generations [21,67], our study did not observe the same pattern. Since Esr1 expression is necessary for sociosexual behaviors such as ultrasonic vocalizations [68], and the upregulation of Esr1 was not observed in our VIN animals, it would follow that there would be no relationship between the steroid hormone receptor and the USV behavior. However, we are careful not to over-extrapolate these relationships, as the VIN treatment was given to individuals two generations removed from the subjects of the gene expression analysis and the behavioral characterization.
One significant positive correlation, between Ar in the POA and female-elicited sex behaviors, was common to VIN males of both lineages. Based on our previous study [24], we speculate that this decrease could be related to the decreased number of lateral kicks received by the VIN male, which are often interpreted as rejection behaviors, and/or to the increased intromission frequency, compared to the maternal VEH counterparts. Androgen receptor activation in the VMN and POA is necessary for copulatory behaviors [37,62], suggesting that the increased frequency of male sexual behavior (reported in our companion study) and subsequent female-elicited behavior could be driven by VIN mediated changes in Ar expression.
4.5. Conclusion
This study provides novel evidence that hypothalamic gene expression in F2 generation male rats was affected by preconceptional exposure to PCBs or VIN, in a lineage-dependent manner. We were limited to relatively few gene targets due to small sample size and limited RNA; future work should focus on other molecular targets that might underlie the observed changes in behavior. Furthermore, there are many brain regions beyond the POA and VMN that are part of the neural network that regulates sexual behavior, ultrasonic vocalizations, and the integration of rewarding inputs. Therefore, future work should include other brain regions of interest, and take a broader approach to gene expression (e.g. RNA sequencing) to explore the underlying neuromolecular targets of EDC disruption.
Our period of EDC administration was selected because it encompasses that of demethylation and remethylation of DNA in F2 male germ cells. Beyond DNA methylation, other epigenetic mechanisms such as histone modifications that affect chromatin state, and microRNA and other non-coding RNAs, might also be affected by EDCs during this sensitive developmental phase. Differences between maternal- vs. paternal-lineage males indicate that the sex of the F1 fetus, behavior of the F1 parent to the F2 offspring, and other mechanisms are important factors to be considered in future work. As a whole, these results demonstrate the need for toxicological testing to take epigenetic and non-epigenetic mechanisms of EDC transmission into account when considering the health of fut1ure generations of wildlife and humans.
Physiology & Behavior Highlights:
EDC exposure effects are observed in male F2 descendants
Gene expression of steroid hormone receptors were altered in the POA and VMN
Vinclozolin (VIN) F2 males had higher POA Ar and Esr1 expression levels than controls
In the VMN, VIN F2 males had significantly lower Esr1 and Pgr levels than controls
Lineage (maternal, paternal) was a key determinant of the effects of EDCs
Acknowledgements
We thank Dr. Ross Gillette and Dr. Amanda Holley for partnership on rat colony breeding and husbandry.
Disclosure statement: Funding was provided by NIH grant (RO1 ES023254) to A.C.G. and D.C. The authors have nothing to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES:
- [1].Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. , Executive Summary to EDC-2: The Endocrine Society's Second Scientific Statement on Endocrine-Disrupting Chemicals, Endocr. Rev (2015) er20151093. doi: 10.1210/er.2015-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Agency for Toxic Substances and Disease Registry, Toxicological Profile For Polychlorinated Biphenyls (PCBs), (2000) 1–948. [PubMed] [Google Scholar]
- [3].Boucher O, Muckle G, Bastien CH, Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis, Environ. Health Perspect 117 (2009) 7–16. doi: 10.1289/ehp.11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, et al. , Persistent environmental pollutants and couple fecundity: the LIFE study, Environ. Health Perspect 121 (2013) 231–236. doi: 10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buck Louis GM, Barr DB, Kannan K, Chen Z, Kim S, Sundaram R, Paternal exposures to environmental chemicals and time-to-pregnancy: overview of results from the LIFE study, Andrology. 4 (2016) 639–647. doi: 10.1111/andr.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jiang L-G, Cheng L-Y, Kong S-H, Yang Y, Shen Y-J, Chen C, et al. , Toxic effects of polychlorinated biphenyls (Aroclor 1254) on human sperm motility, Asian J. Androl 19 (2017) 561–566. doi: 10.4103/1008-682X.186876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hejmej A, Bilinska B, A role of junction-mediated interactions in cells of the male reproductive tract: impact of prenatal, neonatal, and prepubertal exposure to anti-androgens on adult reproduction, Histol. Histopathol 29 (2014) 815–830. doi: 10.14670/HH-29.815. [DOI] [PubMed] [Google Scholar]
- [8].Rajender S, Avery K, Agarwal A, Epigenetics, spermatogenesis and male infertility, Mutat. Res 727 (2011) 62–71. doi: 10.1016/j.mrrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- [9].Alyea RA, Gollapudi BB, Rasoulpour RJ, Are we ready to consider transgenerational epigenetic effects in human health risk assessment? Environ. Mol. Mutagen 55 (2014) 292–298. doi: 10.1002/em.21831. [DOI] [PubMed] [Google Scholar]
- [10].Söffker M, Tyler CR, Endocrine disrupting chemicals and sexual behaviors in fish – a critical review on effects and possible consequences, Crit. Rev. Toxicol 42 (2012) 653–668. doi: 10.3109/10408444.2012.692114. [DOI] [PubMed] [Google Scholar]
- [11].El Sheikh Saad H, Toullec A, Vacher S, Pocard M, Bieche I, Perrot-Applanat M, In utero and lactational exposure to vinclozolin and genistein induces genomic changes in the rat mammary gland, J. Endocrinol 216 (2013) 245–263. doi: 10.1530/JOE-12-0395. [DOI] [PubMed] [Google Scholar]
- [12].Skinner MK, Bhandari RK, Haque MM, Nilsson EE, Environmentally induced epigenetic transgenerational inheritance of altered SRY genomic binding during gonadal sex determination, Environ. Epigenet 1 (2015) dvv004. doi: 10.1093/eep/dvv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Anway MD, Skinner MK, Epigenetic programming of the germ line: effects of endocrine disruptors on the development of transgenerational disease, Reprod. Biomed. Online 16 (2008) 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Arnold AP, Breedlove SM, Organizational and activational effects of sex steroids on brain and behavior: a reanalysis, Horm. Behav 19 (1985) 469–498. [DOI] [PubMed] [Google Scholar]
- [15].Moore MC, Application of organization-activation theory to alternative male reproductive strategies: a review, Horm. Behav 25 (1991) 154–179. [DOI] [PubMed] [Google Scholar]
- [16].Gore AC, Walker DM, Zama AM, Armenti AE, Uzumcu M, Early life exposure to endocrine-disrupting chemicals causes lifelong molecular reprogramming of the hypothalamus and premature reproductive aging, Mol. Endocrinol 25 (2011)2157–2168. doi: 10.1210/me.2011-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Colciago A, Casati L, Mornati O, Vergoni AV, Santagostino A, Celotti F, et al. , Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring, Toxicol. Appl. Pharmacol 239 (2009) 46–54. doi: 10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- [18].Piedrafita B, Erceg S, Cauli O, Monfort P, Felipo V, Developmental exposure to polychlorinated biphenyls PCB153 or PCB126 impairs learning ability in young but not in adult rats, Eur. J. Neurosci 27 (2008) 177–182. doi: 10.1111/j.1460-9568.2007.5988.X. [DOI] [PubMed] [Google Scholar]
- [19].Bell MR, Thompson LM, Rodriguez K, Gore AC, Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 1. Sexually dimorphic effects on social and anxiety-like behaviors, Horm. Behav 78 (2016) 168–177. doi: 10.1016/j.yhbeh.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Reilly MP, Weeks CD, Topper VY, Thompson LM, Crews D, Gore AC, The effects of prenatal PCBs on adult social behavior in rats, Horm. Behav 73 (2015) 47–55. doi: 10.1016/j.yhbeh.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gillette R, Miller-Crews I, Nilsson EE, Skinner MK, Gore AC, Crews D, Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats, Endocrinology. 155 (2014) 3853–3866. doi: 10.1210/en.2014-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bell MR, Hart BG, Gore AC, Two-hit exposure to polychlorinated biphenyls at gestational and juvenile life stages: 2. Sex-specific neuromolecular effects in the brain, Mol. Cell. Endocrinol 420 (2016) 125–137. doi: 10.1016/j.mce.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tabb MM, Blumberg B, New modes of action for endocrine-disrupting chemicals, Mol. Endocrinol 20 (2006) 475–482. doi: 10.1210/me.2004-0513. [DOI] [PubMed] [Google Scholar]
- [24].Krishnan K, Mittal N, Thompson LM, Rodriguez-Santiago M, Duvauchelle CL, Crews D, et al. , Effects of the Endocrine-Disrupting Chemicals, Vinclozolin and Polychlorinated Biphenyls, on physiological and sociosexual phenotypes in F2 generation Sprague-Dawley rats, Environ. Health Perspect 126 (2018) 97005. doi: 10.1289/EHP3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Knutson B, Burgdorf J, Panksepp J, Anticipation of play elicits high-frequency ultrasonic vocalizations in young rats, J. Comp. Psychol 112 (1998) 65–73. [DOI] [PubMed] [Google Scholar]
- [26].Burgdorf J, Knutson B, Panksepp J, Anticipation of rewarding electrical brain stimulation evokes ultrasonic vocalization in rats, Behav. Neurosci 114 (2000) 320–327. [PubMed] [Google Scholar]
- [27].Maier EY, Ma ST, Ahrens A, Schallert TJ, Duvauchelle CL, Assessment of ultrasonic vocalizations during drug self-administration in rats, J. Vis. Exp (2010) e2041–e2041. doi: 10.3791/2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hull EM, Dominguez JM, Getting his act together: roles of glutamate, nitric oxide, and dopamine in the medial preoptic area, Brain Research. 1126 (2006) 66–75. doi: 10.1016/j.brainres.2006.08.031. [DOI] [PubMed] [Google Scholar]
- [29].Paredes RG, Evaluating the neurobiology of sexual reward, Ilar J. 50 (2009) 15–27. [DOI] [PubMed] [Google Scholar]
- [30].Harding SM, McGinnis MY, Effects of testosterone in the VMN on copulation, partner preference, and vocalizations in male rats, Horm. Behav 43 (2003)327–335. [DOI] [PubMed] [Google Scholar]
- [31].Clancy AN, Zumpe D, Michael RP, Estrogen in the medial preoptic area of male rats facilitates copulatory behavior, Horm. Behav 38 (2000) 86–93. doi: 10.1006/hbeh.2000.1602. [DOI] [PubMed] [Google Scholar]
- [32].Lenz KM, Nugent BM, McCarthy MM, Sexual differentiation of the rodent brain: dogma and beyond, Front. Neurosci 6 (2012) 26. doi: 10.3389/fnins.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Smallwood SBA, Kelsey G, De novo DNA methylation: a germ cell perspective, Trends Genet. 28 (2012) 33–42. doi: 10.1016/j.tig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- [34].Gillette R, Reilly MP, Topper VY, Thompson LM, Crews D, Gore AC, Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero, Horm. Behav 87 (2017) 8–15. doi: 10.1016/j.yhbeh.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Dominguez JM, Hull EM, Dopamine, the medial preoptic area, and male sexual behavior, Physiol. Behav 86 (2005) 356–368. doi: 10.1016/j.physbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- [36].Clark AS, Pfeifle JK, Edwards DA, Ventromedial hypothalamic damage and sexual proceptivity in female rats, Physiol. Behav 27 (1981) 597–602. [DOI] [PubMed] [Google Scholar]
- [37].Harding SM, McGinnis MY, Microlesions of the ventromedial nucleus of the hypothalamus: effects on sociosexual behaviors in male rats, Behav. Neurosci 119 (2005) 1227–1234. doi: 10.1037/0735-7044.119.5.1227. [DOI] [PubMed] [Google Scholar]
- [38].Brehm E, Rattan S, Gao L, Flaws JA, Prenatal exposure to Di(2-ethylhexyl) Phthalate causes long-term transgenerational effects on female reproduction in mice, Endocrinology. 159 (2017) 795–809. doi: 10.1210/en.2017-03004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Drobná Z, Henriksen AD, Wolstenholme JT, Montiel C, Lambeth PS, Shang S, et al. , Transgenerational effects of Bisphenol A on gene expression and DNA methylation of imprinted genes in brain, Endocrinology. 159 (2018) 132–144. doi: 10.1210/en.2017-00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bruner-Tran KL, Osteen KG, Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations, Reprod. Toxicol 31 (2011)344–350. doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Krishnan K, Rahman S, Hasbum A, Morales D, Thompson LM, Crews D, et al. , Maternal care modulates transgenerational effects of endocrine-disrupting chemicals on offspring pup vocalizations and adult behaviors, Horm. Behav 107 (2019) 96–109. doi: 10.1016/j.yhbeh.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mennigen JA, Thompson LM, Bell M, Tellez Santos M, Gore AC, Transgenerational effects of polychlorinated biphenyls: 1. Development and physiology across 3 generations of rats, Environ. Health 17 (2018) 18. doi: 10.1186/s12940-018-0362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Reik W, Dean W, Walter J, Epigenetic reprogramming in mammalian development, Science. 293 (2001) 1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- [44].Dunn GA, Morgan CP, Bale TL, Sex-specificity in transgenerational epigenetic programming, Horm. Behav 59 (2011) 290–295. doi: 10.1016/j.yhbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- [45].Siklenka K, Erkek S, Godmann M, Lambrot R, McGraw S, Lafleur C, et al. , Disruption of histone methylation in developing sperm impairs offspring health transgenerationally, Science. 350 (2015) aab2006–aab2006. doi: 10.1126/science.aab2006. [DOI] [PubMed] [Google Scholar]
- [46].Carone BR, Fauquier L, Habib N, Shea JM, Hart CE, Li R, et al. , Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals, Cell. 143 (2010) 1084–1096. doi: 10.1016/j.cell.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Topper VY, Walker DM, Gore AC, Sexually dimorphic effects of gestational endocrine-disrupting chemicals on microRNA expression in the developing rat hypothalamus, Mol. Cell. Endocrinol 414 (2015) 42–52. doi: 10.1016/j.mce.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC, Endocrine disruption of brain sexual differentiation by developmental PCB exposure, Endocrinology. 152 (2011) 581–594. doi: 10.1210/en.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jansen HT, Cooke PS, Porcelli J, Liu TC, Hansen LG, Estrogenic and antiestrogenic actions of PCBs in the female rat: in vitro and in vivo studies, Reprod. Toxicol 7 (1993) 237–248. [DOI] [PubMed] [Google Scholar]
- [50].Bazzett TJ, Eaton RC, Thompson JT, Markowski VP, Lumley LA, Hull EM, Dose dependent D2 effects on genital reflexes after MPOA injections of quinelorane and apomorphine, Life Sci. 48 (1991) 2309–2315. [DOI] [PubMed] [Google Scholar]
- [51].Brudzynski SM, Communication of adult rats by ultrasonic vocalization: biological, sociobiological, and neuroscience approaches, Ilar J. 50 (2009) 43–50. [DOI] [PubMed] [Google Scholar]
- [52].Champagne FA, Weaver ICG, Diorio J, Dymov S, Szyf M, Meaney MJ, Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring, Endocrinology. 147 (2006) 2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- [53].Xu L, Sun Y, Gao L, Cai Y-Y, Shi S-X, Prenatal restraint stress is associated with demethylation of corticotrophin releasing hormone (CRH) promoter and enhances CRH transcriptional responses to stress in adolescent rats, Neurochem. Res 39 (2014) 1193–1198. doi: 10.1007/s11064-014-1296-0. [DOI] [PubMed] [Google Scholar]
- [54].Maccari S, Krugers HJ, Morley-Fletcher S, Szyf M, Brunton PJ, The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations, J. Neuroendocrinol 26 (2014) 707–723. doi: 10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- [55].Boersma GJ, Lee RS, Cordner ZA, Ewald ER, Purcell RH, Moghadam AA, et al. , Prenatal stress decreases Bdnf expression and increases methylation of Bdnf exon IV in rats, Epigenetics. 9 (2014) 437–447. doi: 10.4161/epi.27558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Desaulniers D, Xiao G-H, Lian H, Feng Y-L, Zhu J, Nakai J, et al. , Effects of mixtures of polychlorinated biphenyls, methylmercury, and organochlorine pesticides on hepatic DNA methylation in prepubertal female Sprague-Dawley rats, Int. J. Toxicol 28 (2009) 294–307. doi: 10.1177/1091581809337918. [DOI] [PubMed] [Google Scholar]
- [57].Casati L, Sendra R, Colciago A, Negri-Cesi P, Berdasco M, Esteller M, et al. , Polychlorinated biphenyls affect histone modification pattern in early development of rats: a role for androgen receptor-dependent modulation? Epigenomics. 4 (2012) 101–112. doi: 10.2217/epi.11.110. [DOI] [PubMed] [Google Scholar]
- [58].Desaulniers D, Cummings-Lorbetskie C, Li N, Xiao G-H, Marro L, Khan N, et al. , Sodium bisulfite pyrosequencing revealed that developmental exposure to environmental contaminant mixtures does not affect DNA methylation of DNA repeats in Sprague-Dawley rats, J. Toxicol. Environ. Health Part A 80 (2017) 32–52. doi: 10.1080/15287394.2016.1231644. [DOI] [PubMed] [Google Scholar]
- [59].Fuks F, DNA methylation and histone modifications: teaming up to silence genes, Curr. Opin. Genet. Dev 15 (2005) 490–495. doi: 10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- [60].Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS, Post-transcriptional gene silencing by siRNAs and miRNAs, Curr. Opin. Struct. Biol 15 (2005) 331–341. doi: 10.1016/j.sbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- [61].Brieño-Enriquez MA, García-Lopez J, Cárdenas DB, Guibert S, Cleroux E, Ded L, et al. , Exposure to endocrine disruptor induces transgenerational epigenetic deregulation of microRNAs in primordial germ cells, PLoS ONE. 10 (2015) 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Harding SM, McGinnis MY, Androgen receptor blockade in the MPOA or VMN: effects on male sociosexual behaviors, Physiol. Behav 81 (2004) 671–680. doi: 10.1016/j.physbeh.2004.03.008. [DOI] [PubMed] [Google Scholar]
- [63].Floody OR, Dissociation of hypothalamic effects on ultrasound production and copulation, Physiol. Behav 46 (1989) 299–307. [DOI] [PubMed] [Google Scholar]
- [64].Gibson BM, Floody OR, Time Course of VMN Lesion Effects on Lordosis and Ultrasound Production in Hamsters, Behav. Neurosci 112 (1998) 1236–1246. [DOI] [PubMed] [Google Scholar]
- [65].Yang CF, Chiang MC, Gray DC, Prabhakaran M, Alvarado M, Juntti SA, et al. , Sexually dimorphic neurons in the ventromedial hypothalamus govern mating in both sexes and aggression in males, Cell. 153 (2013) 896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lee H, Kim D-W, Remedios R, Anthony TE, Chang A, Madisen L, et al. , Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus, Nature. 509 (2014) 627–632. doi: 10.1038/nature13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Crews D, Gillette R, Miller-Crews I, Gore AC, Skinner MK, Nature, nurture and epigenetics, Mol. Cell. Endocrinol 398 (2014) 42–52. doi: 10.1016/j.mce.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Vagell ME, McGinnis MY, The role of gonadal steroid receptor activation in the restoration of sociosexual behavior in adult male rats, Horm. Behav 33 (1998) 163–179. doi: 10.1006/hbeh.1998.1445. [DOI] [PubMed] [Google Scholar]