Abstract
We examined developmental differences, in location and extent of fMRI language activation maps, between adults and children while performing a semantic fluency task. We studied 29 adults and 16 children with echo planar imaging BOLD fMRI at 1.5 T using covert semantic verbal fluency (generation of words to categories compared to rest) using a block design. Post task testing was administered to assess performance. Individual data were analyzed with an a priori region of interest approach from t maps (t = 4) and asymmetry indices (AI). Group studies were analyzed using SPM 99 (Wellcome, UK; fixed effect, corrected P < 0.0001). We found no significant differences in location or laterality of activation between adults and children for a semantic verbal fluency task. Adults activated more pixels than children in left inferior frontal gyrus and left middle frontal gyrus, but AIs were the similar across ages (r2 < 0.09). Extent or laterality of activation was not affected by performance (r2 < 0.15). The brain areas that process semantic verbal fluency are similar in children and adults. The laterality of activation does not change appreciably with age and appears to be strongly lateralized by age 7 years. Hum. Brain Mapping 18:176–185, 2003. © 2003 Wiley‐Liss, Inc. Published 2003 Wiley‐Liss, Inc.
Keywords: fMRI, language, verbal fluency, children, development
INTRODUCTION
Verbal fluency paradigms readily identify dorsolateral prefrontal networks involved in language processing [Cuenod et al., 1995; Frith et al., 1991; Paulesu et al., 1997; Peterson et al., 1989; Pujol et al., 1999]. These tasks identify hemispheric dominance for language, an observation corroborated by the intra‐carotid amobarbital procedure [adults: Bahn et al., 1997; Benson et al., 1999; Lehericy et al., 2000; Yetkin et al., 1998; children: Harvey et al., 1999; Hertz‐Pannier et al., 1997; Keene et al., 2000; Stapleton et al., 1997] and electrocorticography [Fitzgerald et al., 1997, Rutten et al., 2002] in adult, older school‐aged children (10–12 years), and adolescent epilepsy patients. As a consequence of their ease to implement, and reproducibility of activation maps, verbal fluency paradigms have also been used to explore developmental differences between adults and children and gain insight into maturation of language neural networks [Gaillard et al., 2000, Schlaggar et al., 2002]. Evidence from previous studies suggests that younger children may have less consolidated and more bilateral representation of language processing areas, which derives mostly from increased activation in right frontal lobe [Gaillard et al., 2000, Holland et al., 2001]. A more diffuse, less consolidated distribution of language networks has been proposed as an explanation for recovery from dominant hemisphere brain injury in children that extends, with diminishing capacity, into adolescence [Boatman et al., 1999; Gaillard et al., 2000; Müller and Courchesne, 2000; Varga‐Kahdem et al., 1992]. Yet evidence from other studies emphasizing language comprehension suggests that language networks in children are as regionally restricted and lateralized as in adults [Gaillard et al., 2001a, 2002a, b, 2003].
The most commonly employed verbal fluency paradigms use word generation to letters (phonemic fluency) [Cuenod et al., 1995; Friedman et al., 1998; Frith et al., 1991; Lurito et al., 2000; Paulesu et al., 1997; Phelps et al., 1997; Pihlajamaki et al., 2000; Pujol et al., 1999; Rueckert et al., 1994; Schlosser et al., 1998; Wood et al., 2001] or verb generation to nouns [Peterson et al., 1989; Schlaggar et al., 2002; Seyer et al., 1999; Warburton et al., 1996; Wise et al., 1991]. Word generation to categories, or semantic fluency, has also been employed [Hugdahl et al., 1999, Paulesu et al., 1997, Pihlajamaki et al., 2000]. Although covert and evoking either semantic or phonologic strategies, these three verbal fluency tasks are consistently reported to activate similar left hemispheric areas, principally inferior frontal gyrus (IFG); Brodmann Area (BA) 45, 44; middle frontal gyrus (MFG), BA 46, 9; cingulate and supplementary motor areas (SMA). For younger school‐age children, semantic verbal fluency (to categories) may be amenable to studying younger school‐aged children (5–9 years) because it is easier to generate an ample number of words during a limited period of time [Korkman et al., 1998]. We aimed to compare activation patterns using a semantic fluency task between adults and children in order to investigate age‐dependent consolidation of frontal language processing networks and to establish normal parameters in order to identify language dominance in epilepsy patients.
SUBJECTS AND METHODS
Subjects
Twenty‐nine adults and 16 children participated in the protocol after obtaining parental consent and child assent as approved by the National Institutes of Neurological Disorders and Stroke Institutional Review Board and in accord with the Declaration of Helsinki. All children and adults had normal development, school achievement, neurological exam, and structural MRI. Participants with a history of learning or attention deficit disorders were excluded. The children's ages ranged from 7 to 14 years (9 girls, 7 boys; mean age 10.2 years). Adults ages ranged from 22 to 44 years (14 women, 15 men; mean age 29.2 years). All were right handed as assessed by a modified version of the Edinburgh Handedness Inventory [Oldfield, 1971]. Prior to experimental studies, all children had tours of the MRI facilities.
MR imaging parameters and experimental paradigm
Details of imaging parameters and analysis have been reported previously and are briefly summarized here [Gaillard et al., 2001a]. Images were collected with a 1.5 T General Electric (Milwaukee, WI) Signa scanner. Functional images were acquired with a gradient‐echo echo‐planar sequence (TE = 40 msec, FOV = 22 × 22 cm, acquisition matrix = 64 × 64). Ninety‐six sequential echo‐planar volumes were collected with an inter‐scan interval (TR) of 4 sec (total scanning duration = 6 min 24 sec). Anatomical images were acquired using a 3‐D fast spin‐echo gradient (SPGR) sequence. For both the functional and structural images, the whole brain was imaged with the collection of 20 contiguous axial images parallel to the anterior–posterior commissure plane. The paradigm comprised six cycles in a boxcar design. There were 12 epoch hemi‐cycles during which two conditions alternated between an experimental and control task each lasting 32 sec. Eight functional images were collected in each hemi‐cycle.
For the experimental task subjects silently generated words to the following categories: animals, food, cloths, furniture, toys, and TV shows. One category was presented over the scanner intercom at the beginning of each cycle. Silent rest served as the control condition. The tasks were unmonitored, but participants completed semantic verbal fluency tasks (animals and food) 2 weeks remote from the study to assess subject performance of the task. We used a covert task to minimize head motion.
Image data analysis
Data were analyzed using two methods following data reconstruction and motion correction [Ostuni et al., 1997]. Group comparisons utilizing a standard stereotactic atlas were performed for overall activation patterns; and a region of interest (ROI) analysis from individual activation maps for laterlality of activation and correlation with age and performance.
Group analysis
Group data were processed and analyzed with SPM99 (Wellcome Department of Cognitive Neurology, London, UK), using the general linear model [Friston et al., 1991]. Images were reconstructed, realigned, and then normalized into a standard stereotactic space conforming to Talairach and Tournoux [1988] to allow inter‐subject data averaging and cross‐task comparisons [Friston et al., 1991]. Motion was less than 1.5 mm. Data were also smoothed through an isotropic Gaussian kernel filter of 8 mm. Statistical contrasts between active and control conditions were made using averaged intensity of pixels using the t statistic, which generated statistical parametric maps of t‐values (SPM{t} maps) then transformed to z distribution (SPM{z} maps) reflecting differences between two conditions at each pixel location. Data were analyzed using a fixed effect model, P < 0.0001 (corrected). For the adult SPM analysis, the subjects were randomly selected from the adult database to match the same number of males and females as in the pediatric group. Differences in activation maps between the two age groups were examined with a two‐sample t‐test (P < 0.05). This comparison was performed using the contrast images (active‐control) of adults and those of children [Friston et al., 1995].
Individual analysis
Individual analyses were performed using a region of interest (ROI) approach. Individual t maps were generated with a semi‐automated analysis program that compared the control and task conditions on a pixel‐by‐pixel basis. In each region, pixels exceeding a threshold of t = 4.0 were automatically counted [Gaillard et al., 2001a, HB:, 2002a]. Regions included IFG (BA 44, 45, 47), MFG (BA 9, 46), and a region encompassing the receptive language processing areas, which included the middle temporal gyrus, superior temporal gyrus, and inferior parietal lobule (BA 21, 22, 37, 39, 40, 41, 42). We also examined a frontal lobe region encompassing IFG, MFG, and SFG [Gaillard et al., 2001a]. Regions are based on historical anatomical data and imaging data implicating these regions in language processing. These regions are supported by previous results from verbal fluency paradigms that were not based on a priori assumptions, in addition to intracarotid amobarbital test data in patients [Benson et al., 1999; Gaillard et al., 2002a; Geschwind, 1970; Hertz‐Pannier et al., 1997; Lehericy et al., 2000]. An asymmetry index (AI) for each region was calculated, (L−R)/(L+R), with L and R being the number of activated pixels in the left and right hemisphere, respectively. Activation was considered to be left hemisphere dominant if the AI ≥ 0.20, and right hemisphere dominant if the AI ≤ −0.20. An AI between 0.20 and −0.20 was considered to represent a bilateral activation pattern [Binder et al., 1996; Gaillard et al., 2001a, 2002a; Springer et al., 1999]. For purposes of lateralization at least four pixels had to be present in a region, and there had to be at least a four‐pixel difference between homologous regions [Gaillard et al., 2001a, 2002a]. Correlations between the number of regional pixels activated and the laterality of regional activation for task with cognitive measures were explored with Pearson's r. Comparisons between groups were made with t‐tests. Motion was assessed by generating a histogram of voxel signal variance for the experimental run [Weinberger et al., 1996]. The resulting distribution of the standard error of the mean was examined to report the mean, median, and standard deviation. Motion assessed with this method is derived primarily from pixels along gray matter/CSF, white matter/CSF, and white/gray matter edges where motion results in the greatest signal change.
RESULTS
Group comparison of activation maps in a standardized space showed a similar location of activation for adults and children (Fig. 1). Activation was primarily found in left IFG, MFG, and mesial frontal areas, including SMA, as well as in the thalamus and left parietal lobe (Table I); cluster sizes for activation were larger in the adults and overlapped activation clusters and maxima seen in children. Small areas of homologous activation were seen in right IFG for adults and children. Adults demonstrated activation in left temporal, bilateral occipital regions, and right MFG, which was not seen in children. However, there were no differences between age groups in SPM activation map patterns (two‐sample t‐test).
Figure 1.
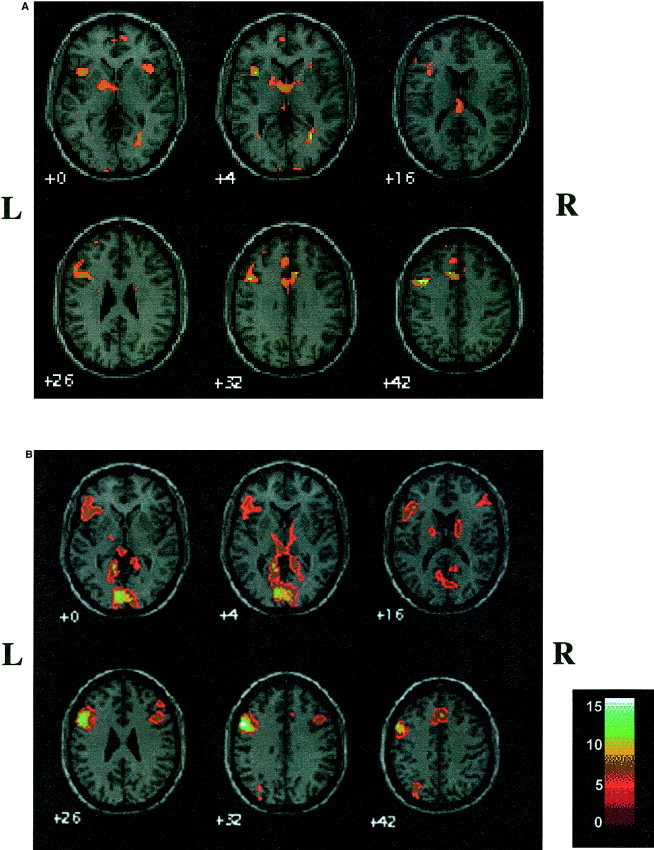
SPM maps for semantic verbal fluency in children (a) and adults (b), corrected P < 0.0001.
Table I.
SPM analysis, fixed effect: semantic verbal fluency
| Brain region | Brodmann's Area | Talairach coordinates | Pixels activated | Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Children | ||||||
| L SFG | 6/8 | −2 | 14 | 52 | 1,035 | > 8 |
| L MFG | 9 | −44 | 10 | 34 | 986 | > 8 |
| L IFG | 47 | −56 | 14 | −6 | 419 | > 8 |
| L SFG | 10 | −12 | 56 | −8 | 265 | > 8 |
| R IFG | 47 | 34 | 22 | −4 | 172 | > 8 |
| L Cuneus | 17 | −18 | −102 | −2 | 142 | > 8 |
| L Caudate | −12 | 2 | 6 | 116 | > 8 | |
| R IOG | 17/18 | 18 | −98 | 0 | 95 | 7.18 |
| L Thalamus | −2 | −24 | 16 | 79 | 7.13 | |
| R Thalamus | 10 | 0 | 6 | 14 | 6.40 | |
| L Parietal (superior) | 7 | −52 | −74 | 52 | 15 | 6.92 |
| L SFG | 8 | −4 | 34 | 56 | 7 | 6.45 |
| L IFG | 44/45 | −54 | 36 | 0 | 5 | 6.27 |
| Adults | ||||||
| L Lingual gyrus | 17/18 | −6 | −86 | 2 | 5,586 | > 8 |
| L MFG | 9 | −52 | 10 | 34 | 3,698 | > 8 |
| L SFG | 6 | −28 | 12 | 60 | * | > 8 |
| L IFG | 47 | −48 | 22 | −6 | * | > 8 |
| L SFG | 6/8 | −2 | 18 | 52 | 1,249 | > 8 |
| L Precuneus | 19 | −30 | −70 | 40 | 503 | > 8 |
| L Fusiform | 20/37 | −30 | −42 | −22 | 486 | > 8 |
| L Parietal (superior) | 7 | −26 | −76 | 54 | 503 | > 8 |
| R MFG | 46 | 54 | 36 | 20 | 290 | > 8 |
| R IFG/MFG | 44/9 | 46 | 16 | 30 | 262 | > 8 |
| R IFG | 47 | 42 | 28 | −18 | 54 | 6.14 |
| L Medial frontal gyrus | 11 | −6 | 54 | −14 | 46 | 6.86 |
| R MFG | 6 | 30 | 20 | 56 | 43 | 6.27 |
| L MFG | 11 | −34 | 48 | −12 | 10 | 6.44 |
Submaxima within LMGF cluster.
In children, height threshold P < 0.0001 (corrected).
SFG = superior frontal gyrus; MFG = midfrontal gyrus; IFG = inferior frontal gyrus; IOG = inferior occipital gyrus.
The ROI data from all participants showed that adults activated more pixels than children in LIFG (P < 0.003) and LMFG (P < 0.0005). There were also more pixels observed in RMFG (P < 0.05) but this finding was not significant when corrected for multiple comparisons (Table II). There were no significant differences for other brain regions (Table II). Age was not correlated with the number of activated regional pixels (r2 < 0.09) for any region. AI was neither significantly different between groups nor did the AI of any region correlate with age (r2 < 0.04; Table II; Fig. 2). Children generated significantly fewer words than adults on the test of semantic fluency than adults (28.9 ± 7.2 vs. 46.5 ± 8.1). However, there was no significant correlation between performance and either number of regional pixels activated or AI (r2 < 0.15) for any region. The variance attributable to performance was 10–15% for left‐sided regions and less than 1% for right sided regions (Fig. 3). There were no significant gender differences in either regional pixel counts or AI. Children, as a group, moved more than adults (P < 0.02, for the three motion measures; Table III). Children had greater variability than adults for motion measures. Eight children moved within one standard deviation of adult motion measures, five moved less than the adult mean, and two children moved more than any adult.
Table II.
Region of interest results
| Adults | Children | |
|---|---|---|
| Number | ||
| Frontal L | 44.7 (35.3) | 19.7 (21.2) |
| Frontal R | 19.4 (33.0) | 7.7 (12.1) |
| IFG L | 9.70 (8.8)* | 3.6 (4.1)* |
| IFG R | 3.5 (5.5) | 1.6 (2.6) |
| MFG L | 19.9 (13.7)** | 6.5 (9.8)** |
| MFG R | 4.8 (6.8) | 1.7 (3.3) |
| T‐P L | 12.2 (17.6) | 7.9 (9.7) |
| T‐P R | 4.7 (7.6) | 5.2 (7.9) |
| AI | ||
| Frontal | 0.55 (0.28) | 0.50 (0.42) |
| IFG | 0.57 (0.51) | 0.40 (0.71) |
| MFG | 0.70 (0.31) | 0.76 (0.25) |
| T‐P | 0.52 (0.46) | 0.45 (0.52) |
Number = voxel counts (t = 4) for left (L) and right (R) regions in adults and children for frontal, inferior frontal gyrus (IFG), middle frontal gyrus (MFG), and Temporal‐Parietal (T‐P) regions. The Asymmetry Index (AI) for all regions is also shown. Standard deviations are shown in parentheses.
P < 0.003.
P < 0.0005.
Figure 2.
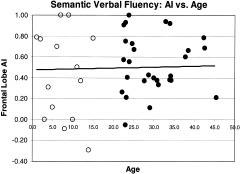
Asymmetry Index (AI) for frontal region across ages. There was no correlation with age, r2 = 0.0012. Open circles, children; solid circles, adults.
Figure 3.
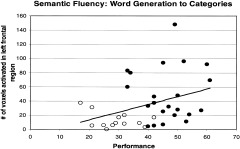
Relationship between performance and pixel number in the left frontal lobe across all ages, r2 = 0.13. Open circles, children; solid circles, adults.
Table III.
Motion measures. mean, median, and standard deviation (SD) of the standard error of the mean of voxel intensity (SD for each measure in parentheses)
| Mean | Median | SD peak | |
|---|---|---|---|
| Adults | 4.65 (0.72) | 3.33 (0.40) | 0.95 (0.12) |
| Children | 5.42 (0.95) | 3.70 (0.48) | 1.08 (0.17) |
| P | 0.01 | 0.02 | 0.01 |
Higher numbers indicate greater motion.
DISCUSSION
We found activation maps to be fundamentally similar in location and laterality for children and adults using a covert semantic verbal fluency task. Principal activation occurred in left inferior frontal lobe (BA 44, 45, 47) and in mid frontal lobe (BA 9, 46) for both age groups. The left IFG has been long implicated in word retrieval and expressive language [Geschwind, 1970; Thompson‐Schill et al., 1997]. Left MFG activation may reflect evocation of verbal working memory necessary for the task [Gabrieli et al., 1998]. We also found activation in the mesial frontal region, including the supplementary motor area, which is commonly attributed to attention and motor planning common to these tasks. We observed inconsistent activation in temporal lobe in individual activation maps, which is likely due to the low burden required of comprehension for this paradigm. These findings are similar to previous imaging studies that employed a variety of verbal fluency paradigms (word generation to letters: Cuenod et al., 1995; Friedman et al., 1998; Frith et al., 1991; Paulesu et al., 1997; Pihlajamaki et al., 2000; Pujol et al., 1999; Rueckert et al., 1994; Schlosser et al., 1998, Wood et al., 2001; word generation to nouns: Peterson et al., 1989; Schlaggar et al., 2002; Warburton et al., 1996; Wise et al., 1991; word generation to categories: Hugdahl et al., 1999, Paulesu et al., 1997, Pihlajamaki et al., 2000].
We also found some apparent differences between children and adults. For example, the adult group reached statistical threshold in left fusiform, lingual gyrus, occipital regions, and right MFG. The activation in posterior brain areas suggests visual imagination and naming of objects recalled during the task. Several frontal lobe maxima, though not identical, are similar given the resolution of imaging techniques, threshold effects, and overlap of large activated clusters. Our group results for children and adults are analyzed and displayed in a standard adult based anatomic space, though the distortion for this transformation in children older than six years is small [Chugani et al., 2001; Muzik et al., 2000; Schlaggar et al., 2002]. Furthermore, comparison of activation maps did not show differences between the groups. This observation is similar to a recent report by Schlagger et al. [2002]. When they collapsed lexical phonologic and semantic fluency task analysis, they found general similarities between children and adults in broad areas encompassing left IFG and MFG in contrast to a smaller region in BA 45/7 that showed greater activation in adults.
Tasks of phonologic and semantic fluency result in activation in the same brain regions and with the same degree of laterality [Grandin et al., 1998; Paulesu et al., 1997; Pujol et al., 1999; Wood et al., 2001]. These findings are supported by other verbal fluency tasks in adults using noun–verb generation [Peterson et al., 1989; Wise et al., 1991; Schlaggar et al., 2002]. Inspection of adult fluency studies suggests the activated brain areas reflect word retrieval and decision processes rather than phonologic or semantic processing per se [Poldrack et al., 1999; Thompson‐Schill et al., 1997]. However, the semantic weighted task may additionally activate BA 30, 31, and 47, whereas the phonologic version may involve greater activation in BA 44 [Paulesu et al., 1997; Poldrack et al., 1999]. The observation is important for pediatric studies as children younger than 8 or 9 years are more facile with semantic than phonologic fluency tasks. From a clinical point of view, semantic fluency tasks may be employed to determine language dominance in preparation for planning epilepsy surgery in younger children in lieu of the phonologic fluency task, which is more commonly reported in older age groups [Bahn et al., 1997; Benson et al., 1999; Harvey et al., 1999; Hertz‐Pannier et al., 1997; Keene et al., 2000; Lehericy et al., 2000; Stapleton et al., 1997; Yetkin et al., 1998].
We found a similar laterality of activation in all selected brain regions between children and adults. There was no correlation with age and laterality of activation with individuals older than 7 years. This finding is in agreement with other studies of auditory comprehension [Balsamo et al., 2002, Gaillard et al., 2002b] and reading comprehension [Gaillard et al., 2001a, 2002a, 2003]. For these tasks the networks underlying language processing in temporal areas, mostly along the middle and posterior aspects of the left superior temporal sulcus, appear to be as regionally specific and as strongly lateralized by age 5 to 6 years as they are in adults. As may be expected, these observations are more pronounced for auditory‐based tasks. In contrast, these same comprehension tasks yield inconsistent frontal activation in children younger than 8 or 9 years. Listening to stories and auditory response naming (naming an object described by a five‐ to six‐word sentence) in children younger than 10 yields variable location of left frontal activation for individual and, as a consequence, less pronounced frontal activation in group analysis [Balsamo et al., 2002, Gaillard et al., 2002b]. Reading tasks, such as reading stories and a reading version of the auditory response naming task described above, show the same degree and laterality of activation in IFG and MFG in adults and children 8 years and older [Gaillard et al., 2001a, 2002a]. The same reading tasks show inconsistent presence or location of frontal activation in children 5 to 7 years old [Gaillard et al., 2003].
The variability in regionally localized activation patterns, especially in frontal regions and regardless of modality of stimulus presentation, in the younger children may reflect the use of different cognitive strategies and differing burdens on working memory and grammatical processing [Gaillard et al., 2003]. Results from the present study suggest that activation in dominant hemisphere frontal regions involved in verbal fluency language processing tasks are strongly lateralized by 7 years. Similar regional specialization in dominant IFG and MFG for single word semantic and phonologic decision tasks in 7‐year‐olds and older has also recently been described [Shaywitz et al, 2002].
Our present findings show activation to be similar in location as earlier studies using verbal fluency in similarly aged children [Gaillard et al., 2000; Holland et al., 2001; Schlaggar et al., 2002]. Yet they also differ from our previous study of verbal fluency [Gaillard et al., 2000], and a more recent study by Holland et al. [2001], which found greater bilateral activation in (younger) children. They differ, too, from our earlier study where we observed a greater extent of activation in children than in adults. These differences in laterality derive from greater activation in right inferior frontal lobe found in prior studies. The investigators proposed that the observed developmental differences may be a consequence of the later physiologic and functional maturation in frontal lobe association cortex, as reflected in regional myelination, compared to motor and sensory regions [Giedd et al., 1996, 1999; Paus et al., 1999; Yakolev and Lecours 1967]. Rather than reflect developmental differences, the observed frontal lobe differences may be a consequence of technical differences such as coil characteristics, head motion, paradigm design, and physiologic age dependent differences in resting cerebral blood flow upon which the BOLD signal is based [Gaillard et al., 2001b]. In our study, there was a small but discernable difference in motion between groups. Our measures cannot distinguish between in phase and out of phase motion: out of phase motion will increase noise and thus decrease activated pixel detection and in phase motion will increase activation bilaterally and may reduce AI. The coil we used was designed for adults; children with shorter necks do not sit in the center of the coil, which may account for diminished signal detection and hence activation in some children. Our previous study used surface coils, which were likely to identify more activated voxels at a lower threshold in children because their thinner skulls and scalp place the coils closer to the brain surface than in adults, in contrast to the methods used here that may favor signal detection in adults. Holland et al. [2001] used the 3 Tesla scanner, which may enhance the capacity to identify smaller signal change relative to noise and may have identified more subtle activation in the right. Studies involving a larger cohort, including younger children, and possibly employing quantitative measures of activation, may be necessary to resolve the differences in experimental observations. The observations from these studies raise a cautionary note regarding technical and developmental factors that may affect signal and noise detection that, in turn, may influence an interpretation of age‐based differences in activation patterns.
We did not find a correlation between performance assessed out of scanner and regional activation, albeit out of scanner performance may differ from experimental performance. Previous investigators describe a similar degree of correlation between performance and activated voxels in 20 adults during word generation to letters [Wood et al., 2001]. This is likely because the task usually results in optimal individual performance. Although performance may be different, effort is likely to be similar. Our choice of paradigm design, a 32‐sec block to generate as many words as possible, may also account, in part, for the differences described by Holland et al. [2001] who employed a noun–verb generation task [Peterson et al., 1989] where stimuli were presented every 5 sec, which may under‐emphasize effort. Parametric tasks have been proposed as a means of controlling for developmental differences in performance speed and accuracy across ages by comparing age group based on optimal performance for task [Bookheimer, 2000].
We found regionally specific areas of activation in inferior and mid frontal lobe in children performing a semantic verbal fluency task. Our findings add to the growing evidence that language processing is strongly lateralized and regionally consolidated to the dominant hemisphere dorsolateral prefrontal cortex and temporal lobe in children seven and older [Gaillard et al., 2001a, 2002b, 2003; Holland et al., 2001; Schlaggar et al., 2002]. Activation patterns are fundamentally similar to those seen in adults in location and laterality. Relatively minor differences described here and by other investigators with this and other language tasks may reflect anatomic maturation, age‐dependent differences in cerebral blood flow, technical factors of image acquisition and analysis, or different language processing strategies. Networks evoked by other, perhaps less mature or efficient, processing approaches appear equally regionally localized and may be utilized in the setting of brain injury or learning disability. Our results do not exclude the possibility of greater bilateral frontal activation in younger age groups, especially children younger than 5 or 6 years. Like adults, children older than 7 exhibit the same degree of modest activation in homologous regions in the non‐dominant hemisphere, especially in IFG and MFG, which may serve as a reserve for functional recovery following injury to the left hemisphere [Booth et al., 2000; Gaillard et al., 2001a; Staudt et al., 2001]. Verbal fluency tasks may be adapted for implementation in younger children and may serve as a probe to identify frontal language areas during normal development and in disease states, such as epilepsy.
Acknowledgements
We thank Christine Kennedy for assistance in preparing the manuscript.
This article is a US Government work and, as such, is in the public domain in the United States of America.
REFERENCES
- Bahn MM, Lin W, Silbergeld DL, Miller JW, Kuppusamy K, Cook RJ, Hammer, G , Wetzel R, Cross D (1997): Localization of language cortices by functional imaging compared with intracarotid amobarbital hemispheric sedation. AJR 169: 575–579. [DOI] [PubMed] [Google Scholar]
- Balsamo L, Grandin C, Xu B, Elliott T, Petrella J, Basso G, Braniecki S, Theodore W, Gaillard WD (2002): An fMRI study of auditory responsive naming in children. Arch Neurol 59: 1168–1174.. [DOI] [PubMed] [Google Scholar]
- Benson RR, FitzGerald DB, LeSueur LL, Kennedy DN, Kwong KK, Buchbinder BR, Davis TL, Weisskoff RM, Talavage TM, Logan WJ, Cosgrove GR, Belliveau JW, Rosen BR (1999): Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology 52: 798–809. [DOI] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, Benbadis S, Frost JA, Rao SM, Haughton VM (1996): Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology 46: 978–984. [DOI] [PubMed] [Google Scholar]
- Boatman D, Freeman J, Vining E, Pulsifer M, Miglioretti D, Minahan R, Carson B, Brandt J, McKhann G (1999): Language recovery after left hemispherectomy in children with late‐onset seizures. Ann Neurol 46: 579–586. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY (2000): Methodological issues in pediatric neuroimaging. Dev Med Mental Retard Rev 6: 161–165. [DOI] [PubMed] [Google Scholar]
- Booth JR, MacWhinney B, Thulborn KR, Sacco K, Voyvodic JT, Feldman HM (2000): Developmental and lesion effects in brain activation during sentence comprehension and mental rotation. Dev Neuropsychol 18: 139–69. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC (2001): Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage 14: 1290–301 [DOI] [PubMed] [Google Scholar]
- Cuenod CA, Bookheimer SY, Hertz‐Pannier L, Zeffiro TA, Theodore WH, Le Bihan D (1995): Functional MRI during word generation, using conventional equipment. Neurology 45: 1821–1827. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DB, Cosgrove GR, Ronner S, Jiang H, Buchbinder BR, Belliveau JW, Rosen BR, Benson RR (1997): Location of language in the cortex: a comparison between functional MR imaging and electrocortical stimulation. AJNR 18: 1529–1539. [PMC free article] [PubMed] [Google Scholar]
- Friedman L, Kenny JT, Wise AL, Wu D, Stuve TA, Miller DA, Jesberger JA, Lewin JS (1998): Brain activation during silent word generation evaluated with functional MRI. Brain Lang 64: 231–256. [DOI] [PubMed] [Google Scholar]
- Friston K, Frith C, Liddle P, Frackowiak R (1991): Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab 11: 690–699. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Homes AP, Worsley KJ, Poline J‐B, Frith CD, Frackowiak RSJ (1995): Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2: 189–210. [Google Scholar]
- Frith CD, Friston KJ, Liddle PF, Frackowiak RSJ (1991): A PET study of word finding. Neuropsychologia 29: 1137–1148. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE (1998): The role of the prefrontal cortex in language and memory. Proc Nat Acad Sci USA 95: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Hertz‐Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH (2000): Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology 54: 180–185. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Pugliese M, Grandin CB, Braniecki SH, Kondapaneni P, Hunter K, Xu B, Petrella JR, Balsamo L, Basso G (2001a): Cortical localization of reading in normal children: A fMRI language study. Neurology 57: 47–54. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Grandin CB, Xu B (2001b): Developmental aspects of pediatric fmri: considerations for image acquisition, analysis, and interpretation. Neuroimage 13: 239–249. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero P, Weinstein S, Conry J, Pearl PL, Sato S, Jabbari B, Vezina LG, Frattali C, Theodore WH (2002a): Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology 59: 256–265. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Ahmad Z, Balsamo LM, Sachs BC, Xu B (2002b): Language networks underlying auditory comprehension in young children identified with fMRI. Ann Neurol 52(Suppl 1): S114. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Ibrahim Z, Xu B (2003): fMRI identifies regional specialization of neural networks for reading in young children. Neurology 60: 94–99. [DOI] [PubMed] [Google Scholar]
- Geschwind N (1970): The organization of language and the brain. Science 170: 940–944. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey Bj, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL (1996): Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex 6: 551–560. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX (1999): Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry 23: 571–588. [DOI] [PubMed] [Google Scholar]
- Grandin CB, Gaillard WD, Whitnah JR, Petrella JR, Braniecki S, Hunter K, Theodore WH (1998): Comparison of phonological and semantic verbal fluency tasks: an fMRI study. Neuroimage 7: S133. [Google Scholar]
- Harvey AS, Anderson D (1999): Functional MRI lateralization of expressive language in children with partial epilepsy and left hemisphere lesions. Epilepsia 40: 183. [Google Scholar]
- Hertz‐Pannier L, Gaillard WD, Mott S, Cuenod CA, Bookheimer S, Weinstein S, Conry J, Papero PH, LeBihan D, Theodore WH (1997): Assessment of language hemispheric dominance in children with epilepsy using functional MRI. Neurology 48: 1003–1012. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS Jr ( 2001): Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage 14: 837–843. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Lundervold A, Ersland L, Smievoll AI, Sundberg H, Barndon R, Roscher BE (1999): Left frontal activation during a semantic categorization task: an fMRI‐study. Intern J Neurosci 99: 49–58. [DOI] [PubMed] [Google Scholar]
- Keene DL, Logan WJ, McAndrews MP, Crawley AP, Mikulius DJ (2000): A comparison of three functional MRI language paradigms in children. Epilepsia 41(Suppl 7): 193.10691116 [Google Scholar]
- Korkman, M. , Kirk, U. , & Kemp, S (1998): NEPSY, a developmental neuropsychological assessment manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Lehericy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, Hertz‐Pannier L, Le Bihan D, Marsault C, Baulac M (2000): Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology 54: 1625–1633. [DOI] [PubMed] [Google Scholar]
- Lurito JT, Kareken DA, Lowe MJ, Chen SHA, Mathews VP (2000): Comparison of rhyming and word generation with FMRI. Hum Brain Mapp 10: 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller RA, Courchesne E (2000): The duplicity of plasticity: A conceptual approach to the study of early lesions and developmental disorders In Ernst M, Rumsey J, editors. The foundation and future of functional imaging in child psychiatry. New York: Cambridge University Press. [Google Scholar]
- Muzik O, Chugani DC, Juhasz C, Shen C, Chugani HT (2000): Statistical parametric mapping: assessment of application in children. Neuroimage 12: 538–549. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Ostuni JL, Levin RL, Frank JA, DeCarli C (1997): Correspondence of closest gradient voxels: A robust registration algorithm. J Magn Reson Imag 7: 410–415. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Goldacre B, Scifo P, Cappa S, Gilardi MC, Castiglioni DP, Fazio F (1997): Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport 8: 2011–2016. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC (1999): Structural maturation of neural pathways in children and adolescents: in vivo study. Science 283: 1908–1911 [DOI] [PubMed] [Google Scholar]
- Peterson S, Fox, P , Posner M, Mintun M, Raichle M (1989): Positron emission tomographic Studies of processing of single words. J Cogn Neurosci 1: 153–170. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Hyder F, Blamire AM, Shulman RG (1997): fMRI of the prefrontal cortex during overt verbal fluency. Neuroreport 8: 561–565. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Laakso M, Partanen K, Soininen H, Aronen H (2000): Verbal fluency activates the left medial temporal lobe: a functional magnetic resonance imaging study. Ann Neurol 47: 470–476. [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD (1999): Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage 10: 15–35. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A (1999): Cerebral lateralization of language in normal left‐handed people studied by functional MRI. Neurology 52: 1038–1043. [DOI] [PubMed] [Google Scholar]
- Rueckert L, Appollonio I, Grafman J, Jezzard P, Johnson R Jr, Le Bihan D, Turner R (1994): Magnetic resonance imaging functional activation of left frontal cortex during covert word production. J Neuroimag 4: 67–70. [DOI] [PubMed] [Google Scholar]
- Rutten GJ, Ramsey NF, van Rijen PC, Noordmans HJ, van Veelen CW (2002): Development of a functional magnetic resonance imaging protocol for intraoperative localization of critical temporoparietal language areas. Ann Neurol 51: 350–60. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE (2002): Functional neuroanatomical differences between adults and school‐age children in the processing of single words. Science 296: 1476–1479. [DOI] [PubMed] [Google Scholar]
- Schlosser R, Hutchinson M, Joseffer S, Rusinek H, Saarimaki A, Stevenson J, Dewey SL, Brodie JD (1998): Functional magnetic resonance imaging of human brain activity in a verbal fluency task. J Neurol Neurosurg Psychiatry 64: 492–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA, Rabin LA, Desmond JE, Gabrieli JDE (1999): Verb generation priming involves conceptual implicit memory. Brain Cognit 41: 150–177. [DOI] [PubMed] [Google Scholar]
- Shaywitz B, A Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, Constable RT, Marchione KE, Fletcher JM, Lyon GR, Gore JC (2002): Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol Psychiatry 52: 101–110. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM (1999): Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain 122: 2033–2046. [DOI] [PubMed] [Google Scholar]
- Stapleton SR, Kiriakipoulos E, Mikulis D, Drake JM, Hoffman HJ, Humphreys R, Hwang P, Otsubo H, Holowka S, Logan W, Rutka JT (1997): Combined utility of functional MRI, cortical mapping, and frameless stereotaxy in the resection of lesions in eloquent areas of brain in children. Pediatr Neurosurg 26: 68–82. [DOI] [PubMed] [Google Scholar]
- Staudt M, Grodd W, Niemann G, Wildgruber D, Erb M, Krageloh‐Mann I (2001): Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology 57: 122–125. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical. [Google Scholar]
- Thompson‐Schill SL, D'Esposito M, Aguirre GK, Farah MJ (1997): Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proc Natl Acad Sci USA 23: 14792–14797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargha‐Khadem F, Isaacs E, van der Werf S, Robb S, Wilson J (1992): Development of intelligence and memory in children with hemiplegic cerebral palsy. The deleterious consequences of early seizures. Brain 115: 315–329. [DOI] [PubMed] [Google Scholar]
- Warburton E, Wise RJS, Price CJ, Weiller C, Hadar U, Ramsay S, Frackowiak RSJ (1996): Noun and verb retrieval by normal subjects. Brain 119: 159–179. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Mattay V, Callicott J, Kotrla K, Santha A, Van Gelderen P, Duyn J, Moonen C, Frank J (1996): fMRI applications in schizophrenia research. Neuroimage 4: S118–126. [DOI] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R (1991): Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain 114: 1803–1817. [DOI] [PubMed] [Google Scholar]
- Wood AG, Saling MM, Abbot DF, Jackson GD (2001): A neurocognitive account of frontal lobe involvement in orthographic lexical retrieval: an fMRI study. Neuroimage 14: 162–169. [DOI] [PubMed] [Google Scholar]
- Yakovlev PI, Lecours AR (1967): The myelenetic cycles of regional maturation of the brain In: Minkowski A, editor. Regional development of the brain in early life. Philadelphia: FA Davis; p 3–70. [Google Scholar]
- Yetkin FZ, Swanson S, Fischer M, Akansel G, Morris G, Mueller W, Haughton V (1998): Functional MR of frontal lobe activation: comparison with Wada language results. AJNR Am J Neuroradiol 19: 1095–1098. [PMC free article] [PubMed] [Google Scholar]


